Hong Kong Med J 2025 Feb;31(1):48–57 | Epub 17 Feb 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE
Practice recommendations for respiratory
syncytial virus prophylaxis among children in Hong Kong
KL Hon, MB, BS, MD1; Eddie WY Cheung, MB, BS, MMedSc2; Albert Martin Li, MB, BCh, MD3; Genevieve PG Fung, FHKAM (Paediatrics), FRCPCH3; David SY Lam, FHKAM (Paediatrics), FRCPCH4; Maria SH Lee, FHKAM (Paediatrics), FRCPCH5; Robert SY Lee, FHKCP, FHKAM6; Maurice Ping Leung, MB, BS, MD7; Daniel KK Ng, MB, BS, MD8
1 Department of Paediatrics, CUHK Medical Centre, The Chinese University of Hong Kong, Hong Kong SAR, China
2 Department of Paediatrics, Hong Kong Adventist Hospital, Hong Kong SAR, China
3 Department of Paediatrics, The Chinese University of Hong Kong, Hong Kong SAR, China
4 Department of Paediatrics and Adolescent Medicine, Tuen Mun Hospital, Hong Kong SAR, China
5 Department of Paediatrics and Adolescent Medicine, Queen Elizabeth Hospital, Hong Kong SAR, China
6 Department of Paediatrics and Adolescent Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
7 Division of Paediatrics, Premier Medical Centre, Hong Kong SAR, China
8 Department of Paediatrics, Hong Kong Sanatorium & Hospital, Hong Kong SAR, China
Corresponding author: Prof KL Hon (ehon@hotmail.com)
Abstract
Hong Kong has a high burden of hospitalisations
associated with respiratory syncytial virus (RSV)
infection in young children. Most international
guidelines concerning RSV prophylaxis are based
on studies conducted in temperate climates and
may not fully apply to subtropical locations such
as Hong Kong. In July 2022, a group of nine
experts in neonatology, paediatric intensive care,
paediatric respiratory medicine, and paediatric
cardiology in Hong Kong convened to formulate
recommendations for RSV prophylaxis. The
recommendations were based on literature review
and expert discussion. Each expert reviewed
evidence specific to a particular area and formulated
consensus statements. The expert panel reached a
consensus on 11 statements, which addressed the
epidemiology of RSV infection in Hong Kong, the
goals and outcomes of RSV prophylaxis in preterm
infants and infants with congenital heart disease
or bronchopulmonary dysplasia, safety, and cost.
Because there is no clear seasonality pattern for RSV
infection in Hong Kong, panel members emphasised
using gestational age, rather than season, to guide
prophylaxis recommendations. The experts agreed
that RSV prophylaxis should be considered for 5 to
6 months after hospital discharge among preterm
infants born at <29 weeks gestational age; it should
also be considered for children aged <1 year with haemodynamically significant congenital heart
disease or bronchopulmonary dysplasia.
Introduction
Acute lower respiratory tract infections associated
with respiratory syncytial virus (RSV) are a common
cause of hospitalisation among young children.1 2 In
Hong Kong, RSV infection is the leading reason for
hospitalisation among children aged <5 years with
respiratory viral infections, causing 50% of deaths in
this age-group.3 A study conducted at a paediatric
intensive care unit (ICU) in Hong Kong revealed
that paramyxovirus infections, predominantly
RSV, caused 5% of all paediatric ICU admissions
and were associated with significant morbidity.4
Among the RSV-infected patients, 39.4% needed
mechanical ventilation and 21.1% needed inotropic
support.4 Treatment for viral bronchiolitis is mainly
supportive because no pharmacological treatment or novel therapy has been shown to improve outcomes
compared with supportive care.5
Although numerous vaccines, therapeutic
antibodies, and antiviral drugs for the prevention
and treatment of RSV infection are in development,6
the only available prophylactic agent is palivizumab,7
a humanised immunoglobulin G1 monoclonal
antibody that targets the fusion protein of RSV.8
Palivizumab is effective in reducing the rate of RSV
hospitalisation (RSVH) among high-risk children.9
International guidelines recommend palivizumab
prophylaxis in groups such as preterm infants,
former preterm infants with chronic lung disease/bronchopulmonary dysplasia (BPD), and children
aged <2 years with haemodynamically significant
congenital heart disease (hsCHD).7 10
In Hong Kong, local healthcare practices
regarding palivizumab prophylaxis are informed
by data from international studies and guidance
statements.10 11 12 However, these international
publications do not reflect the local treatment
landscape. Palivizumab is reimbursed by the
government for preterm infants born at <34
weeks gestational age (wGA) who have chronic
lung disease requiring home oxygen therapy or
medication at discharge, up to a chronological age
of 6 to 9 months (maximum of five doses). The
perception among clinicians is that palivizumab use
varies across hospitals. Furthermore, international
guidance is predominantly derived from studies in
regions with temperate climates and may not fully
apply to subtropical locations such as Hong Kong,
particularly with respect to the seasonality of RSV
infection.13 14 In this article, we summarise RSV
prophylaxis recommendations developed by a group
of experts in Hong Kong, with the aim of assisting
physicians engaged in treating children at risk of
RSV infection, both locally and internationally.
Methods
A meeting was convened in July 2022 to formulate
evidence-based recommendations for RSV
prophylaxis among children in Hong Kong. The
panel comprised experts in neonatology, paediatric
intensive care, paediatric respiratory medicine, and
paediatric cardiology, representing both private
and public healthcare sectors. A set of clinical
questions was established, and selected panel
members screened the results of a series of focused
literature reviews. These reviews were centred
around the following topics: the epidemiology of
RSV infection (including seasonality), the burden of RSV infection in vulnerable paediatric populations,
international guidance concerning RSV prophylaxis,
and the efficacy and safety of prophylaxis. Literature
searches were performed using PubMed to identify
relevant English-language publications, with an
emphasis on studies published in the past 10 years
(up to April 2022). Proposed statements were drafted
and evaluated during the meeting using a modified
Delphi method. Panel members rated the statements
using a Likert scale (1–Agree completely; 2–Agree
with reservation; 3–Disagree with reservation; 4–Disagree completely). Consensus was defined as ≥75%
of panel members responding ‘1–Agree completely’
or ‘2–Agree with reservation’. In the absence of
consensus, the relevant statements were revised and
re-evaluated until consensus was reached. Where
applicable, the quality of evidence supporting each
statement was evaluated according to the Oxford
Centre for Evidence-Based Medicine’s 2011 Levels
of Evidence.15 Treatment recommendations were
assigned a subjective strength (strong, moderate, or
weak) based on the level of evidence and degree of
consensus.
This manuscript was prepared in accordance
with the AGREE (Appraisal of Guidelines for
Research and Evaluation) Reporting Checklist.16
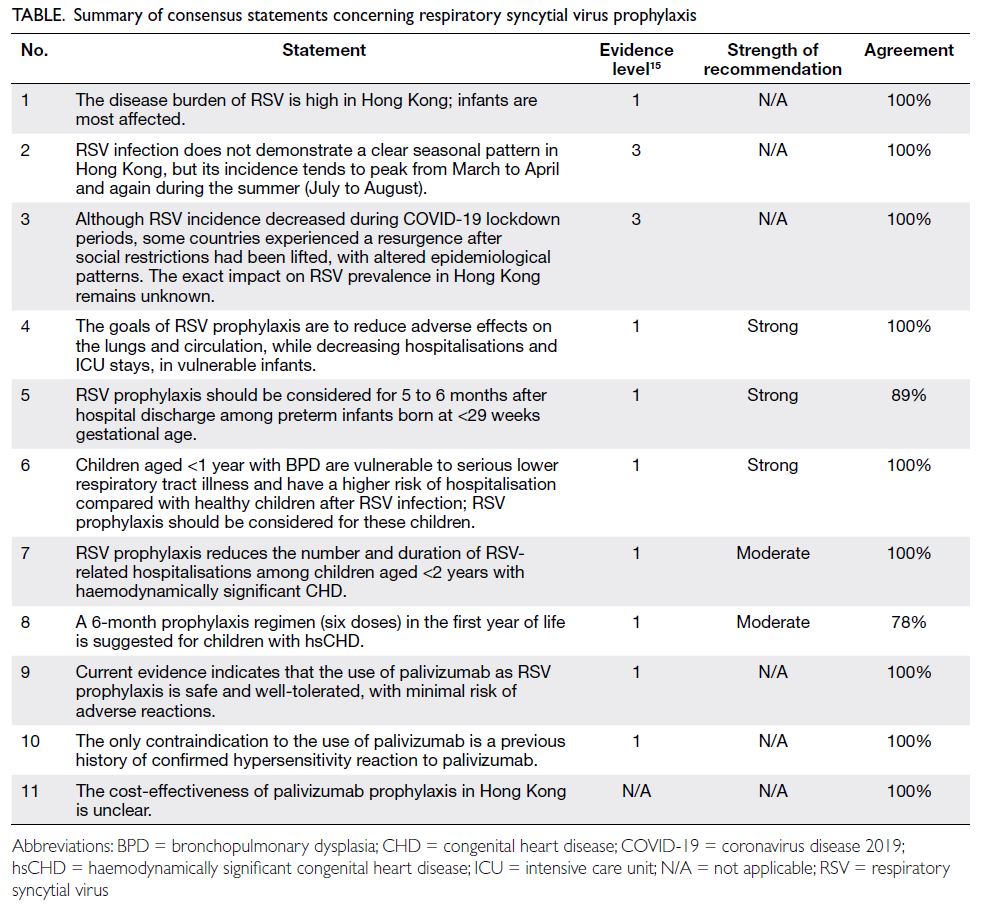
Consensus statements
Eleven statements were formulated and met the
consensus criteria during the meeting. These
statements, including their level of evidence, strength
of recommendation and agreement, are summarised
in the Table.15
Statement 1: The disease burden of respiratory syncytial virus is high in Hong Kong; infants are
most affected.
Data concerning RSV epidemiology in
Hong Kong are scarce, but two studies provided
important insights. A single-centre study conducted
from 1998 to 2012 revealed that the annual rate of
RSVH among children aged <5 years was 157.7 per
10 000; most hospitalisations involved infants aged
<1 year.3 This RSVH rate was higher than the rates
reported in a 2015 systematic review and modelling
study, which estimated that RSVH rates in high-income
countries were 26.3, 11.3, and 1.4 per 1000
in children aged ≤5 months, 6 to 11 months, and
12 to 59 months, respectively2; corresponding
mortality rates were 0.2%, 0.9%, and 0.7%.2 Almost
half of the hospitalisations and hospital deaths
attributed to RSV-associated acute lower respiratory
tract infection occurred in children aged <6
months.2 More recent local epidemiological data
were provided by a multicentre case-control study
conducted in four hospitals from 2013 to 2015,
which included 3538 admissions for paediatric RSV infection.17 The mortality rate was 0.14%, and
44.6% of hospitalisations involved infants aged ≤12
months17; this rate is lower than comparable data
from Western countries (ie, 75%-90% in infants
aged ≤12 months).18 Meta-analysis data from China
indicate that RSV is the leading cause of viral acute
respiratory tract infections, present in 18.7% of
cases overall and 26.5% of cases among infants aged
≤1 year.19 The actual burden of RSV infection in
China may be higher, due to the limited sensitivity
of diagnostic methods used during studies included
in the meta-analysis.19 Although differences in study
designs may explain the discrepancies between
international and local data, there is no doubt that
RSV is associated with a substantial disease burden
among infants in Hong Kong.
Statement 2: Respiratory syncytial virus infection does not demonstrate a clear seasonal pattern in
Hong Kong, but its incidence tends to peak from March to April and again during the summer (July to August).
In Western European countries, laboratory-confirmed
RSV infections generally exhibit a well-defined
seasonal pattern, with peaks in winter and
spring; few cases occur in summer and autumn.20
In Hong Kong, an analysis of RSVH across all age-groups
at a single centre over 15 years showed annual
peaks of approximately 12 cases per week occurring
around March and September; moderate levels of
cases (5-10 cases per week) were observed from May
to August, and the lowest rate of hospitalisation (<5
cases per week) occurred from October to February.3
A multicentre study of paediatric RSV admissions
across four Hong Kong hospitals from 2013 to 2015
revealed a similar pattern of peaks in hospitalisation
from March to April and July to August, separated by
moderate inter-peak levels during the summer; the
lowest levels of hospitalisation were observed from
October to February.17 Similar seasonality patterns were observed both overall and among a subset of
patients with heart disease.17 Most infections (87.7%
in the entire cohort and 91.1% in the heart disease
group) occurred between January and September.17
The same study showed that RSV incidence was
positively correlated with relative humidity, whereas
it was negatively correlated with wind speed and
atmospheric pressure.17 Despite differences in
populations, the pattern of RSVH seasonality
was consistent between these two studies; both
demonstrated that RSVH in Hong Kong mainly
occurs in warmer months.3 17
Statement 3: Although respiratory syncytial virus
incidence decreased during coronavirus disease
2019 lockdown periods, some countries experienced
a resurgence after social restrictions had been lifted,
with altered epidemiological patterns. The exact
impact on respiratory syncytial virus prevalence in
Hong Kong remains unknown.
Data from Australia,21 France22 and Japan23
show that strict infection control measures
implemented during the coronavirus disease
2019 (COVID-19) pandemic led to a substantial
reduction—up to 98%—in RSV cases during 2019
and 2020. In Australia, after the relaxation of
physical distancing measures in late 2020, the usual
incidence peak in autumn was replaced by a peak
in summer21; RSV incidence was higher in the 2020
summer peak than it had been in winter peaks from
2012 to 2019.21 Furthermore, the median patient age
after COVID-19 restrictions were lifted in 2019 to
2020 was 18.4 months, significantly older compared
with previous years (7.3-12.5 months from 2012
to 2019; P<0.001)21; this shift likely resulted from
decreased prior exposure and declining collective
immunity. Data from Hong Kong suggest that
measures adopted during the COVID-19 pandemic
(eg, social distancing, face masks, and enhanced
personal hygiene) reduced the incidence of RSV
infection; the surge in RSV cases during late 2021
coincided with the relaxation of these measures.24
However, the overall impacts of measures adopted
during the COVID-19 pandemic on RSVH rates,
affected populations, and seasonality in Hong Kong
are unclear.
Statement 4: The goals of respiratory syncytial virus
prophylaxis are to reduce adverse effects on the lungs
and circulation, while decreasing hospitalisations
and intensive care unit stays, in vulnerable infants.
Various international studies have
demonstrated that the risk of severe illness from
RSV infection increased among at-risk children,
namely preterm infants and those with CHD or
BPD. A retrospective cohort study in the US (1989-1993; 248 652 child-years) showed that children with BPD had a higher rate of RSVH in the first
year of life compared with children who lacked
underlying medical conditions (388 vs 30 per
1000, respectively).25 The same study also revealed
that preterm infants with CHD had a RSVH rate
of 120.8 per 1000 from 0 to 6 months after birth
(vs 44.1 in low-risk infants); this rate declined in
the second year of life to 18.2 per 1000 (vs 3.7 for
low-risk infants).25 A multicentre study in Korea
(n=1140) demonstrated that BPD increased the risk
of re-admission to neonatal ICUs among preterm
infants born at <34 wGA compared with similar
preterm infants who did not exhibit BPD (odds ratio
[OR]=2.95, 95% confidence interval [CI]=1.44-6.04;
P=0.003).26 The results of a retrospective database
study in Australia (2001-2010; n=870 314) indicated
that BPD had the largest effect on RSVH risk among
various risk factors.27 Furthermore, a meta-analysis
of 29 studies by Chaw et al28 assessed RSVH risk and
other measures of severe illness from RSV infection
among young children with BPD. In addition
to an increased risk of hospitalisation (OR=2.6,
95% CI=1.7-4.2; P<0.001), children with BPD
had an increased risk of ICU admission (OR=2.9,
95% CI=2.3-3.5; P<0.001), increased need for oxygen
supplementation (OR=4.2, 95% CI=0.5-33.7) and
mechanical ventilation (OR=8.2, 95% CI=7.6-8.9;
P<0.001), and longer median length of stay (7.2
days vs 2.5 days) compared with children who did
not exhibit BPD.28 Overall, these studies have shown
that children with BPD experience higher risks of
hospitalisation and severe illness from RSV infection
relative to children without BPD.
Illustrative data concerning the impact of
prematurity on RSV infection burden were provided
by SENTINEL1, an observational cohort study
conducted in the US involving preterm infants
(29-35 wGA, <12 months old) who did not receive
prophylaxis and were hospitalised for RSV during
peak season (2014-2015).29 Infants aged <6 months
experienced 78% of hospitalisations and 87% of ICU
admissions; they comprised 92% of cases requiring
invasive mechanical ventilation.29 Among infants
aged <3 months who had been born at 29 to 32
wGA, the ICU admission rate was 68%; 44% of these
infants required invasive mechanical ventilation.29
Regression analysis demonstrated that earlier
gestational age at birth and younger chronological age
at the time of RSV infection were factors associated
with ICU admission and the need for invasive
mechanical ventilation.29 A pooled analysis of seven
prospective observational studies conducted in the
Northern Hemisphere (2000-2014) assessed the
burden of RSV infection in preterm infants who had
been born at 33 to 35 wGA, lacked co-morbidities,
and were not receiving immunoprophylaxis
(n=7820).30 The pooled incidence rate of RSVH was
3.41%; among the infants, 22.2% required neonatal ICU admission and 70.4% required supplemental
oxygen.30 Although these two studies are not
directly comparable due to differences in design
and population—notably the infants’ gestational
age at birth—they consistently demonstrate a high
burden of severe illness in preterm infants with RSV
infection.29 30
Similar to preterm infants, infants with CHD
have an increased risk of severe disease.12 The local
burden of RSV infection among children with CHD
is unclear, but the multicentre study by Lee et al17
assessing paediatric RSVHs included a subset of
children with heart disease (not limited to CHD).
Relative to children without heart disease, children
with heart disease had a longer median hospital stay
(4 days vs 2 days; P<0.001), higher complication rate
(28.6% vs 9.8%; P<0.001), and higher rates of intensive
care (11.6% vs 1.4%; P<0.001) and mechanical
ventilation (3.6% vs 0.4%; P=0.003).17 Based on the
local and international data summarised above, and
in alignment with guidelines from the American
Academy of Pediatrics (AAP),11 we recommend
that RSV prophylaxis should focus on reducing
the disease burden in preterm infants and young
children with BPD or heart disease.
Statement 5: Respiratory syncytial virus prophylaxis
should be considered for 5 to 6 months after hospital
discharge among preterm infants born at <29 weeks
gestational age.
The AAP published guidance in 2009
recommending palivizumab prophylaxis at the start
of RSV season among infants born at <31 wGA, as
well as among infants born at 32 to 35 wGA who have
risk factors for increased exposure (eg, attending a
day-care facility or living with young siblings).7 The
AAP made a substantial change to the guidance
in 2014 by narrowing the intended population to
infants aged <12 months who had been born at
<29 wGA.11 An observational study in Italy compared
the RSVH rates among infants aged <2 years before
(up to 2016) and after changes in palivizumab
reimbursement criteria that aligned with the
changes in AAP recommendations.31 The study
identified a reduction in RSVH rates from 6.3 per
1000 (95% CI=6.0-6.7) to 5.5 per 1000 (95% CI=5.0-5.9) after the change.31 These data suggest that 29
wGA is an appropriate age cut-off for palivizumab
prophylaxis; our recommendation for this age
threshold concerning prophylaxis in preterm infants
aligns with the AAP’s 2014 guideline.11
As noted above, the seasonality of RSV
incidence is less distinct in Hong Kong than in
Europe,3 17 20 but local data indicate that gestational
age is a key determinant of RSVH risk. One study
showed that the cost-effectiveness of palivizumab
prophylaxis was higher among infants born at
<27 wGA than among infants born at <29 wGA, regardless of the season.32 Therefore, gestational age,
rather than season, should be a primary factor guiding
prophylaxis recommendations in Hong Kong.
Statement 6: Children aged <1 year with
bronchopulmonary dysplasia are vulnerable to
serious lower respiratory tract illness and have a
higher risk of hospitalisation compared with healthy
children after respiratory syncytial virus infection;
respiratory syncytial virus prophylaxis should be
considered for these children.
In the IMpact-RSV study, preterm children
aged ≤6 months who had been born at ≤35 wGA or
children aged ≤24 months with BPD were randomly
assigned to receive five monthly doses of palivizumab
or placebo.9 Overall, RSVH rates were reduced by
55% in the palivizumab group compared with the
placebo group (P<0.001); palivizumab treatment also
led to a 39% reduction in RSVH (vs placebo) among
children with BPD.9 These results were subsequently
reinforced by a meta-analysis of three randomised
studies (n=2831) showing favourable efficacy of
palivizumab, with a 51% reduction in RSVH (vs
placebo) among preterm children and children born
with BPD.33 In the US, a registry study of infants
receiving palivizumab (n=2116, predominantly
born at ≤35 wGA) demonstrated an RSHV rate
of 2.9%,34 which compares favourably to the 4.8%
hospitalisation rate observed in the pivotal trial.9
Based on these data, we recommend palivizumab
prophylaxis for 5 to 6 months after hospital discharge
among children aged <12 months who are receiving
medication for BPD, irrespective of prematurity.
Statement 7: Respiratory syncytial virus prophylaxis
reduces the number and duration of respiratory
syncytial virus–related hospitalisations among
children aged <2 years with haemodynamically
significant congenital heart disease.
Statement 8: A 6-month prophylaxis regimen (six
doses) in the first year of life is suggested for children
with haemodynamically significant congenital heart
disease.
We define hsCHD based on the population
included in the study by the Cardiac Synagis
Study Group.35 This included cyanotic patients
(oxygen saturation <85%, either unoperated or
partially corrected by surgery or interventional
catheterisations), patients with hypercyanotic
episodes (paroxysmal hypoxic events characterised
by severe reductions in pulmonary blood flow lasting
from minutes to several hours), patients receiving
cardiac medications, patients with congestive heart
failure (requiring treatment with two medications),
patients with pulmonary hypertension (mean
pulmonary artery pressure >25 mm Hg for >3-4
months of life) and patients with increased pulmonary blood flow.35
Prophylaxis for children aged ≤12 months
with hsCHD is widely supported by international
guidelines, but recommendations for prophylaxis
among children aged 12 to 24 months vary.12 In Hong
Kong, an individualised approach should be taken;
prophylaxis should be considered for children aged
≤12 months with hsCHD, congestive heart failure,
or pulmonary hypertension, especially at the start of
the local RSV season. Prophylaxis for children aged
12 to 24 months may be considered after corrective
surgery if residual defects are present, but prophylaxis
beyond 6 months post-surgery should be carefully
considered on a case-by-case basis. Currently, there
are insufficient data to recommend prophylaxis for
children aged >24 months with hsCHD.
The evidence supporting these statements was
collected from randomised clinical trials and real-world
studies. A placebo-controlled randomised
clinical trial of palivizumab prophylaxis, delivered as
five monthly injections, among young children (aged
≤24 months; n=1287) with hsCHD demonstrated
a 45% relative reduction in RSVH (P=0.003) and
a 56% reduction in total days of RSVH per 100
children (P=0.003), compared with placebo.35 The
same study revealed a 73% reduction in total RSVH
days requiring increased supplemental oxygen
per 100 children (P=0.014).35 The efficacy of six
doses of palivizumab prophylaxis among children
aged ≤12 months with hsCHD is also supported
by findings from an observational study in Taiwan
(n=1556), which showed a 49% reduction in RSVH
and a 57% reduction in admission days compared
with propensity-matched controls.36 A database
study from the US that included 2518 children with
hsCHD demonstrated a decline of 36% in RSVH
among children with hsCHD between pre- and
post-palivizumab guideline eras, compared with
an 8% decline among children without hsCHD
(P<0.001).37 Additional data confirming the efficacy
of palivizumab prophylaxis against RSVH among
children with hsCHD have been acquired through
real-world studies in Spain38 and Australia.39 In
Spain, a prospective, multicentre study of children
aged ≤24 months with hsCHD (n=2613) showed
that those with adequate palivizumab prophylaxis
(n=2366) had a lower rate of RSVH than those
with inadequate prophylaxis (n=247; 3.3% vs
7.9%, respectively).38 An observational cohort
study in Australia compared RSHV rates among
infants aged ≤12 months with haemodynamically
significant cardiac disease between 2008-2009,
when palivizumab prophylaxis was administered in a
coordinated manner, to the rates during 2005-2007,
when prophylaxis was given on an ad hoc basis.39
Admission rates for RSV bronchiolitis in 2008-2009
(2% per year) were significantly reduced compared
with the rates in 2005-2007 (5%-9% per year; P<0.03).39 These findings support our recommendation for
prophylaxis among children aged ≤24 months with
hsCHD; our suggested duration of dosing is based
on the above studies and the limited seasonality of
RSV observed in Hong Kong.
Clinical experience regarding palivizumab
prophylaxis for other special populations in Hong
Kong (eg, immunocompromised children and
children with Down syndrome, cystic fibrosis, or
neuromuscular disorder) is extremely limited. For
cases involving these children, clinicians should
refer to international recommendations.11
Statement 9: Current evidence indicates that the
use of palivizumab as respiratory syncytial virus
prophylaxis is safe and well-tolerated, with minimal
risk of adverse reactions.
Statement 10: The only contraindication to the use
of palivizumab is a previous history of confirmed
hypersensitivity reaction to palivizumab.
The favourable safety profile of palivizumab
has been demonstrated in clinical trials and
observational studies. In the pivotal IMpact-RSV
trial, which involved premature infants with BPD,
adverse event rates were similar in the palivizumab
and placebo groups (10%-11%).9 Discontinuations
due to palivizumab-related adverse events were rare
(0.3%), as were reports of injection site reactions
(1.8% [placebo] vs 2.7% [palivizumab]) and fever
(3.0% vs 2.8%).9 Observational data from several
studies suggest that palivizumab is well-tolerated
in at-risk children. The prospective observational
CARESS study from Canada included 13 025
infants treated with palivizumab (63.1% born
at ≤35 wGA, 11.1% aged <2 years with hsCHD,
and 7.5% exhibiting BPD) and monitored serious
adverse events from 2008 to 2013.40 Hospitalisations
for respiratory illness unrelated to palivizumab
were reported in 915 patients.40 Other than these
hospitalisations, 62 serious adverse events were
reported in 52 patients.40 Of these 62 adverse events,
14 hypersensitivity episodes in six patients (2.8
per 10 000 patient-months) were deemed possibly
or probably related to palivizumab.40 The events
experienced by these six patients included erythema
or urticaria, difficulty swallowing, vomiting, nasal
congestion, bronchospasm, and acute respiratory
distress; two patients required hospitalisation.40 All
six patients discontinued palivizumab, and their
symptoms resolved after 30 days of monitoring
with no immediate life-threatening consequences.40
In a prospective study involving 100 high-risk
children in Russia, 94 children completed their
palivizumab dosing schedule; there were no
reported RSV-related hospitalisations or deaths.41
Three non-serious adverse events were considered
palivizumab-related: rhinitis and acute intermittent rhinitis (both occurring in one patient) and atopic
dermatitis.41 Data concerning palivizumab use
in immunocompromised children (n=167) and
children with Down syndrome (n=138) were
obtained during a post-marketing surveillance study
in Japan.42 Adverse drug reactions occurred in 25
patients (8.22%), including 11 patients (3.62%) who
experienced palivizumab-related serious adverse
drug reactions.42 Further support for palivizumab
safety in immunocompromised children was
presented in a Japanese study of children aged ≤2
years; of the 30 included participants, 26 (92.9%)
completed the study.43 Most adverse events were mild
to moderate; only two patients experienced serious
adverse events, none of which were considered
palivizumab-related.43 Overall, these data indicate
that in routine clinical practice, palivizumab-related
adverse effects and hypersensitivity reactions are
rare; palivizumab is well-tolerated in various patient
populations.
Statement 11: The cost-effectiveness of palivizumab
prophylaxis in Hong Kong is unclear.
International studies regarding the costeffectiveness
of palivizumab prophylaxis yielded
mixed results. For example, a systematic review
of 28 studies suggested that the incremental costeffectiveness
ratio for preterm infants (born at
29-35 wGA) ranged from US$5188 to US$791 265
per quality-adjusted life-year, with 90% of estimates
below US$50 000 per quality-adjusted life-year.44
The authors concluded that prophylaxis was cost-effective
for preterm infants and infants born with
lung complications.44 However, another systematic
review (also comprising 28 studies) by Hussman
et al45 concluded that the overall cost-effectiveness
of palivizumab prophylaxis was inconsistent: some
studies showed favourable outcomes, whereas others
showed unfavourable outcomes or inconclusive
results. A cost-effectiveness study conducted in
Hong Kong concluded that palivizumab was more
cost-effective among preterm infants born at
<27 wGA than among those born at <29 wGA, but
the authors advised careful interpretation of the
results because patient selection was biased towards
individuals with more severe lung disease.32 Another
Hong Kong study, a retrospective analysis by Chen
et al,46 assessed the cost-effectiveness of palivizumab
prophylaxis using data from 236 patients aged <12
months with hsCHD, 26 of whom had RSVH. The
study, which assumed no local seasonality of RSV,
concluded that palivizumab prophylaxis was not
cost-effective for this population in Hong Kong46;
this result contrasts with our suggested regimen in
Statement 8. This study, identified after our main
literature review and consensus meetings, provides
an alternative local opinion. For this reason,
Statement 8 is presented as a suggestion with moderate strength, rather than a recommendation.
As noted above, the study by Chen et al46 assumed
no local RSV seasonality, despite the existence
of peaks in March to April and July to August; its
applicability is limited by its reliance on relative
risk reductions in RSVH from studies conducted
in temperate regions.46 Our opinion is that further
cost-effectiveness studies of palivizumab in Hong
Kong and other tropical locations are required.
Furthermore, because reimbursement policies and
healthcare costs considerably vary among locations,
and because we aim to provide consensus statements
that are useful to healthcare professionals elsewhere
in Asia, decisions regarding the cost-effectiveness of
prophylaxis must be guided by local data.
Conclusion
The burden of RSVH in Hong Kong is high, and
children aged <1 year experienced more than half
of all hospitalisations.3 Respiratory syncytial virus
infections generally peak in the summer months
in Hong Kong, although the seasonality pattern is
less distinct compared with temperate regions.3 17 20
Therefore, our recommendations place greater
emphasis on patient populations, rather than
seasonality.
Our criteria for prophylaxis would lead to a
substantial increase in the number of infants eligible
for palivizumab prophylaxis in Hong Kong, relative
to current practice. Consistent with guidance from
the AAP, we recommend prophylaxis for preterm
infants born at <29 wGA.11 Although the <29 wGA
cut-off may appear to be more restrictive than the
current Hospital Authority limit (<34 wGA), most
premature infants are discharged without oxygen or
medication and therefore do not meet the existing
criteria for palivizumab prophylaxis.
Our guidance statements aim to identify
the populations for which RSV prophylaxis is
appropriate and to summarise the efficacy and safety
data supporting palivizumab prophylaxis. Although
a high level of consensus was reached for these
statements, all recommendations should be tailored
to the needs of individual patients, ideally using a
multidisciplinary clinical approach.
As of early 2025, three RSV vaccines have
been approved for medical use in the US.47 In
June 2024, the US Centers for Disease Control
and Prevention recommended that people aged
≥75 years and people aged 60 to 74 years who are
at increased risk of severe RSV receive the RSV
vaccine.48 One of the RSV vaccines, Abrysvo, is
indicated for active immunisation for the prevention
of lower respiratory infection caused by RSV in
people ≥60 years of age, high-risk individuals aged
18 years through 59 years, and pregnant individuals
at 32 through 36 weeks gestational age to prevent
severe disease in their infants from birth through 6 months of age.49 However, currently in Hong Kong,
the Scientific Committee on Vaccine Preventable
Diseases under the Centre for Health Protection does
not recommend universal RSV vaccination for elderly
persons or pregnant women.50 Recommendations
about childhood RSV immunisation by local expert
panel should be called for in the not-so-remote future.
Author contributions
Concept or design: GPG Fung, KL Hon, AM Li, MSH Lee, DKK Ng.
Acquisition of data: EWY Cheung, KL Hon, DSY Lam, MSH Lee.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: EWY Cheung, KL Hon, RSY Lee, AM Li, MSH Lee, DKK Ng.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: EWY Cheung, KL Hon, DSY Lam, MSH Lee.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: EWY Cheung, KL Hon, RSY Lee, AM Li, MSH Lee, DKK Ng.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
MP Leung, AM Li and DKK Ng have received an honorarium
for this consensus meeting from AstraZeneca Hong Kong.
EWY Cheung has received an honorarium for lectures from
AstraZeneca Hong Kong. As an editor of the journal, KL Hon
was not involved in the peer review process. Other authors
have disclosed no conflicts of interest.
Acknowledgement
The authors thank Mr Mika Mok, Ms Magdalene Chu and Dr
Alister Smith of MIMS Hong Kong for their assistance in the
planning and coordination of the consensus meeting, as well
as the medical writing of this manuscript, with funding from
AstraZeneca Hong Kong.
Funding/support
The development of this manuscript and the meeting it
documents were funded by an unrestricted grant from
AstraZeneca Hong Kong to the Hong Kong Society of
Paediatric Respirology and Allergy. The funder had no role in
the formulation of clinical questions or consensus statements,
data collection/analysis/interpretation, or manuscript
preparation.
References
1. Ruangnapa K, Kaeotawee P, Surasombatpattana P, et al.
Viral and atypical bacterial infection in young children
hospitalized due to acute lower respiratory tract infection
in Southern Thailand. Pediatr Respirol Crit Care Med
2019;3:67-71. Crossref
2. Shi T, McAllister DA, O’Brien KL, et al. Global, regional,
and national disease burden estimates of acute lower
respiratory infections due to respiratory syncytial virus in
young children in 2015: a systematic review and modelling
study. Lancet 2017;390:946-58. Crossref
3. Chan PK, Tam WW, Lee TC, et al. Hospitalization
incidence, mortality, and seasonality of common respiratory viruses over a period of 15 years in a developed
subtropical city. Medicine (Baltimore) 2015;94:e2024. Crossref
4. Tong AS, Hon KL, Tsang YC, et al. Paramyxovirus
infection: mortality and morbidity in a pediatric intensive
care unit. J Trop Pediatr 2016;62:352-60. Crossref
5. Hon KL, Leung AK, Wong AH, Dudi A, Leung KK.
Respiratory syncytial virus is the most common causative
agent of viral bronchiolitis in young children: an updated
review. Curr Pediatr Rev 2023;19:139-49. Crossref
6. Mazur NI, Martinón-Torres F, Baraldi E, et al. Lower
respiratory tract infection caused by respiratory syncytial
virus: current management and new therapeutics. Lancet
Respir Med 2015;3:888-900. Crossref
7. Luna MS, Manzoni P, Paes B, et al. Expert consensus on
palivizumab use for respiratory syncytial virus in developed
countries. Paediatr Respir Rev 2020;33:35-44. Crossref
8. Johnson S, Oliver C, Prince GA, et al. Development of a
humanized monoclonal antibody (MEDI-493) with potent
in vitro and in vivo activity against respiratory syncytial
virus. J Infect Dis 1997;176:1215-24. Crossref
9. Palivizumab, a humanized respiratory syncytial virus
monoclonal antibody, reduces hospitalization from
respiratory syncytial virus infection in high-risk infants.
The IMpact-RSV Study Group [editorial]. Pediatrics
1998;102:531-7. Crossref
10. Zhang XL, Zhang X, Hua W, et al. Expert consensus on
the diagnosis, treatment, and prevention of respiratory
syncytial virus infections in children. World J Pediatr 2024;
20:11-25. Crossref
11. American Academy of Pediatrics Committee on Infectious
Diseases; American Academy of Pediatrics Bronchiolitis
Guidelines Committee. Updated guidance for palivizumab
prophylaxis among infants and young children at increased
risk of hospitalization for respiratory syncytial virus
infection. Pediatrics 2014;134:415-20. Crossref
12. Tulloh RM, Medrano-Lopez C, Checchia PA, et al. CHD
and respiratory syncytial virus: global expert exchange
recommendations. Cardiol Young 2017;27:1504-21. Crossref
13. Suryadevara M, Domachowske JB. Epidemiology and
seasonality of childhood respiratory syncytial virus
infections in the tropics. Viruses 2021;13:696. Crossref
14. Hon KL, Leung TF, Cheng WY, et al. Respiratory syncytial
virus morbidity, premorbid factors, seasonality, and
implications for prophylaxis. J Crit Care 2012;27:464-8. Crossref
15. Oxford Centre for Evidence-Based Medicine. Oxford
Centre for Evidence-Based Medicine 2011 Levels of
Evidence. Available from: https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf. Accessed 3 May 2022.
16. Brouwers MC, Kerkvliet K, Spithoff K; AGREE Next Steps
Consortium. The AGREE Reporting Checklist: a tool to
improve reporting of clinical practice guidelines. BMJ
2016;352:i1152. Crossref
17. Lee SH, Hon KL, Chiu WK, Ting YW, Lam SY.
Epidemiology of respiratory syncytial virus infection and
its effect on children with heart disease in Hong Kong: a
multicentre review. Hong Kong Med J 2019;25:363-71. Crossref
18. Bont L, Checchia PA, Fauroux B, et al. Defining the
epidemiology and burden of severe respiratory syncytial
virus infection among infants and children in western
countries. Infect Dis Ther 2016;5:271-98. Crossref
19. Zhang Y, Yuan L, Zhang Y, Zhang X, Zheng M, Kyaw MH.
Burden of respiratory syncytial virus infections in China: systematic review and meta-analysis. J Glob Health 2015;5:020417. Crossref
20. Broberg EK, Waris M, Johansen K, Snacken R, Penttinen P;
European Influenza Surveillance Network. Seasonality
and geographical spread of respiratory syncytial virus
epidemics in 15 European countries, 2010 to 2016. Euro
Surveill 2018;23:17-00284. Crossref
21. Foley DA, Yeoh DK, Minney-Smith CA, et al. The
interseasonal resurgence of respiratory syncytial virus in
Australian children following the reduction of coronavirus
disease 2019–related public health measures. Clin Infect
Dis 2021;73:e2829-30. Crossref
22. Casalegno J, Javouhey E, Ploin D, et al. Delayed start of
the respiratory syncytial virus epidemic at the end of the
20/21 Northern Hemisphere winter season, Lyon, France.
medRxiv 2021 Mar 12. Available from: https://www.medrxiv.org/content/10.1101/2021.03.12.21253446v1. Accessed 3 May 2022.
23. Ujiie M, Tsuzuki S, Nakamoto T, Iwamoto N. Resurgence
of respiratory syncytial virus infections during COVID-19
pandemic, Tokyo, Japan. Emerg Infect Dis 2021;27:2969-70. Crossref
24. Centre for Health Protection, Department of Health,
Hong Kong SAR Government. Detection of other
respiratory viruses in respiratory specimens in 2021. 2022.
Available from: https://www.chp.gov.hk/en/statistics/data/10/641/642/6933.html. Accessed 3 May 2022.
25. Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF,
Griffin MR. Rates of hospitalization for respiratory
syncytial virus infection among children in medicaid. J
Pediatr 2000;137:865-70. Crossref
26. Lee JH, Kim CS, Chang YS, Choi JH; Committee on
Data Collection and Statistical Analysis of the Korean
Society of Neonatology. Respiratory syncytial virus–related readmission in preterm infants less than 34 weeks’
gestation following discharge from a neonatal intensive
care unit in Korea. J Korean Med Sci 2015;30 Suppl 1(Suppl
1):S104-10. Crossref
27. Homaira N, Oei JL, Mallitt KA, et al. High burden of RSV
hospitalization in very young children: a data linkage study.
Epidemiol Infect 2016;144:1612-21. Crossref
28. Chaw PS, Hua L, Cunningham S, et al. Respiratory syncytial
virus–associated acute lower respiratory infections in
children with bronchopulmonary dysplasia: systematic
review and meta-analysis. J Infect Dis 2020;222 (Suppl
7):S620-7. Crossref
29. Anderson EJ, Krilov LR, DeVincenzo JP, et al. SENTINEL1:
an observational study of respiratory syncytial virus
hospitalizations among U.S. infants born at 29 to 35 weeks’
gestational age not receiving immunoprophylaxis. Am J
Perinatol 2017;34:51-61. Crossref
30. Anderson EJ, Carbonell-Estrany X, Blanken M, et al.
Burden of severe respiratory syncytial virus disease among
33-35 weeks’ gestational age infants born during multiple
respiratory syncytial virus seasons. Pediatr Infect Dis J
2017;36:160-7. Crossref
31. Belleudi V, Trotta F, Pinnarelli L, Davoli M, Addis A.
Neonatal outcomes following new reimbursement
limitations on palivizumab in Italy. Arch Dis Child
2018;103:1163-7. Crossref
32. Lee SR, Kwok KL, Ng DK, Hon KL. Palivizumab for infants
<29 weeks in Hong Kong without a clear-cut season for
respiratory syncytial virus infection—a cost-effectiveness
analysis. J Trop Pediatr 2018;64:418-25. Crossref
33. Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD,
Bacic Vrca V, Barsic B. Monoclonal antibody for reducing
the risk of respiratory syncytial virus infection in children.
Cochrane Database Syst Rev 2013:CD006602. Crossref
34. Parnes C, Guillermin J, Habersang R, et al. Palivizumab
prophylaxis of respiratory syncytial virus disease in 2000-2001: results from The Palivizumab Outcomes Registry.
Pediatr Pulmonol 2003;35:484-9. Crossref
35. Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab
prophylaxis reduces hospitalization due to respiratory
syncytial virus in young children with hemodynamically
significant congenital heart disease. J Pediatr 2003;143:532-40. Crossref
36. Chiu SN, Wang JN, Fu YC, et al. Efficacy of a novel
palivizumab prophylaxis protocol for respiratory syncytial
virus infection in congenital heart disease: a multicenter
study. J Pediatr 2018;195:108-14.e1. Crossref
37. Chu PY, Hornik CP, Li JS, Campbell MJ, Hill KD.
Respiratory syncytial virus hospitalisation trends in
children with haemodynamically significant heart disease,
1997-2012. Cardiol Young 2017;27:16-25. Crossref
38. Medrano López C, García-Guereta L; CIVIC Study Gorup.
Community-acquired respiratory infections in young
children with congenital heart diseases in the palivizumab
era: the Spanish 4-season civic epidemiologic study. Pediatr
Infect Dis J 2010;29:1077-82. Crossref
39. Alexander PM, Eastaugh L, Royle J, Daley AJ,
Shekerdemian LS, Penny DJ. Respiratory syncytial virus
immunoprophylaxis in high-risk infants with heart disease.
J Paediatr Child Health 2012;48:395-401. Crossref
40. Chen JJ, Chan P, Paes B, et al. Serious adverse events in
the Canadian registry of children receiving palivizumab
(CARESS) for respiratory syncytial virus prevention. PLoS
One 2015;10e0134711. Crossref
41. Turti TV, Baibarina EN, Degtiareva EA, et al. A prospective,
open-label, non-comparative study of palivizumab
prophylaxis in children at high risk of serious respiratory
syncytial virus disease in the Russian Federation. BMC Res
Notes 2012;5:484. Crossref
42. Kashiwagi T, Okada Y, Nomoto K. Palivizumab prophylaxis
against respiratory syncytial virus infection in children with
immunocompromised conditions or Down syndrome: a
multicenter, post-marketing surveillance in Japan. Paediatr
Drugs 2018;20:97-104. Crossref
43. Mori M, Onodera M, Morimoto A, et al. Palivizumab use in
Japanese infants and children with immunocompromised
conditions. Pediatr Infect Dis J 2014;33:1183-5. Crossref
44. Mac S, Sumner A, Duchesne-Belanger S, Stirling R,
Tunis M, Sander B. Cost-effectiveness of palivizumab for
respiratory syncytial virus: a systematic review. Pediatrics
2019;143:e20184064. Crossref
45. Hussman JM, Li A, Paes B, Lanctôt KL. A review of costeffectiveness
of palivizumab for respiratory syncytial virus.
Expert Rev Pharmacoecon Outcomes Res 2012;12:553-67. Crossref
46. Chen RH, Chiu SS, Lee SL, Yung TC. Population-based
respiratory syncytial virus hospitalization disease burden
and cost effectiveness of palivizumab prophylaxis in
infants with hemodynamically significant congenital heart
diseases. J Pediat Infants 2021;4:48-55. Crossref
47. United States Centers for Disease Control and Prevention.
FDA has approved vaccines and monoclonal antibodies to
protect against RSV. Available from: https://www.fda.gov/consumers/covid-19-flu-and-rsv/respiratory-syncytial-virus-rsv. Accessed 12 Feb 2025.
48. United States Centers for Disease Control and Prevention.
RSV vaccines. 2024 Aug 30. Available from: https://www.cdc.gov/rsv/vaccines/index.html. Accessed 12 Feb 2025.
49. Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the
Pfizer respiratory syncytial virus vaccine during pregnancy
for the prevention of respiratory syncytial virus–associated
lower respiratory tract disease in infants: recommendations
of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep
2023;72:1115-22.Crossref
50. Centre for Health Protection, Department of Health,
Hong Kong SAR Government. Scientific Committee on
Vaccine Preventable Diseases issues interim consensus
on respiratory syncytial virus vaccines [press release].
2025 Jan 17. Available from: https://www.info.gov.hk/gia/general/202501/17/P2025011700541.htm. Accessed 12 Feb 2025.