Hong Kong Med J 2024;30:Epub 18 Dec 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Atypical imaging manifestations in non-alcoholic
Wernicke’s encephalopathy: a potentially reversible neurological condition not to be missed
Cherry CY Chan, MB, ChB, FRCR (Radiology)1; Kevin KF Fung, FHKCR, FHKAM (Radiology)1,2; Elaine YL Kan, FHKCR, FHKAM (Radiology)2
1 Department of Diagnostic and Interventional Radiology, Kwong Wah Hospital, Hong Kong SAR, China
2 Department of Radiology, Hong Kong Children’s Hospital, Hong Kong SAR, China
Corresponding author: Dr Cherry CY Chan (chancherrycy@gmail.com)
An 18-year-old female with good past health
was diagnosed with right tibial osteosarcoma in
February 2019. She underwent wide excision of
the right proximal tibia and distal femur with total
knee replacement. Postoperatively, her adjuvant
chemotherapy was complicated by multiple episodes
of opportunistic infection, acute renal impairment
due to drug toxicity and electrolyte disturbance. She
was hospitalised for >6 months with suboptimal oral
intake.
Over the course of a week, the patient had
two episodes of seizure. Her general consciousness
deteriorated acutely to a Glasgow Coma Scale
score of 8/15 (E4V1M3). Physical examination
revealed decorticate posture, generalised flaccidity
and areflexia. Serum sodium level and urea were
markedly elevated (154 mmol/L and 17.0 mmol/L,
respectively), in keeping with hypernatraemic
dehydration. Electroencephalogram showed diffuse
slow-wave encephalopathy. Her Glasgow Coma
Scale score did not improve following correction of
hypernatraemia.
Magnetic resonance imaging of the brain
revealed an abnormal high T2-weighted signal
and restricted diffusion in bilateral frontal lobe
cortices, dorsomedial thalami, periaqueductal grey,
tectal plate of the midbrain and mammillary bodies
(Fig 1a-d). Based on these findings, the patient
was diagnosed with Wernicke’s encephalopathy
and high-dose intravenous thiamine (vitamin B1)
was initiated. Although her level of consciousness
improved rapidly, there was poor recovery of limb
power. Follow-up magnetic resonance imaging of
the brain demonstrated cortical laminar necrosis
and haemorrhage at bilateral frontal cortices (Figs 1e and 2). After 2 years of intensive rehabilitation, she regained most of her upper limb power, but lower
limb power remained impaired.
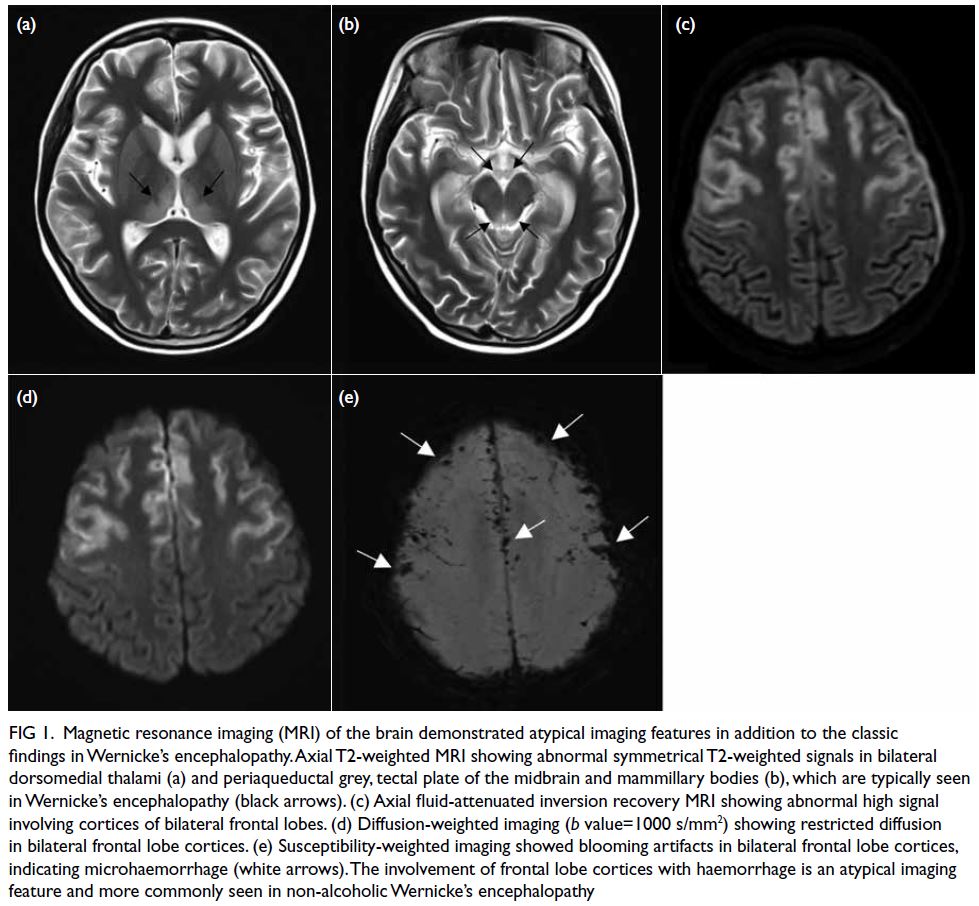
Figure 1. Magnetic resonance imaging (MRI) of the brain demonstrated atypical imaging features in addition to the classic findings in Wernicke’s encephalopathy. Axial T2-weighted MRI showing abnormal symmetrical T2-weighted signals in bilateral dorsomedial thalami (a) and periaqueductal grey, tectal plate of the midbrain and mammillary bodies (b), which are typically seen in Wernicke’s encephalopathy (black arrows). (c) Axial fluid-attenuated inversion recovery MRI showing abnormal high signal involving cortices of bilateral frontal lobes. (d) Diffusion-weighted imaging (b value=1000 s/mm2) showing restricted diffusion in bilateral frontal lobe cortices. (e) Susceptibility-weighted imaging showed blooming artifacts in bilateral frontal lobe cortices, indicating microhaemorrhage (white arrows). The involvement of frontal lobe cortices with haemorrhage is an atypical imaging feature and more commonly seen in non-alcoholic Wernicke’s encephalopathy
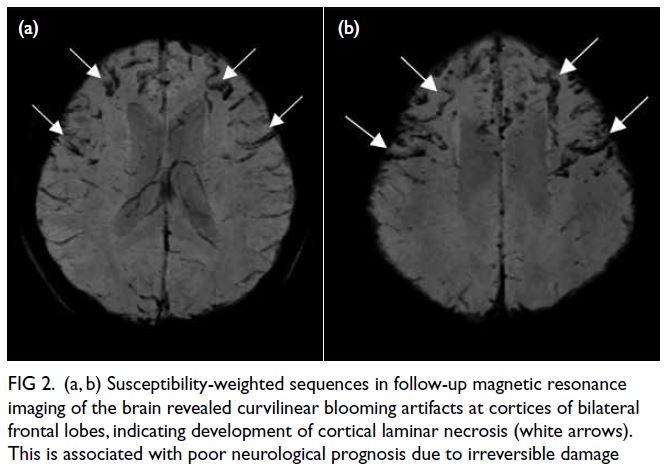
Figure 2. (a, b) Susceptibility-weighted sequences in follow-up magnetic resonance imaging of the brain revealed curvilinear blooming artifacts at cortices of bilateral frontal lobes, indicating development of cortical laminar necrosis (white arrows). This is associated with poor neurological prognosis due to irreversible damage
Wernicke’s encephalopathy is an acute
neurological syndrome caused by depletion of
intracellular thiamine in neurons that is essential for
production of neurotransmitters. The bodily reserve
of thiamine in a healthy individual is exhausted within
4 to 6 weeks in the absence of dietary thiamine.1
Wernicke’s encephalopathy is most commonly
associated with chronic alcoholism but can result
from any condition that causes malnutrition or
malabsorption.1 The classic clinical triad consists of confusion, ataxia and ophthalmoplegia, although
only a small proportion of patients exhibits all three.2
Left untreated, Wernicke’s encephalopathy carries
significant neurological morbidity and death. The
condition is potentially reversible if recognised and
treated early with intravenous thiamine replacement.
Classic imaging features of alcohol-associated
Wernicke’s encephalopathy include abnormal signal
involving deep periventricular and periaqueductal
grey matter in basal ganglia and brainstem,
most notably in the mamillary bodies.3 Atypical
findings are more frequently seen in non-alcoholic
Wernicke’s encephalopathy. These include abnormal
signal in other locations such as the cerebral cortex,
splenium, caudate nuclei, red nuclei, cranial nerve
nuclei, cerebellum and vermis.4 Further progression
to cortical laminar necrosis and haemorrhage,
as seen in our case, is rare and associated with a
poor prognosis due to irreversible neurological
damage.5
In patients with a poor nutritional state
who present with reduced consciousness, a high
index of clinical suspicion and prompt imaging are
important to establish the diagnosis of Wernicke’s
encephalopathy. Atypical imaging manifestations
are more commonly seen in non-alcoholic Wernicke’s
encephalopathy. Timely diagnosis is crucial since
the neurological impairment is potentially reversible
with intravenous thiamine replacement therapy.
Author contributions
Concept or design: CCY Chan, KKF Fung.
Acquisition of data: CCY Chan, KKF Fung.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: CCY Chan, KKF Fung.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: CCY Chan, KKF Fung.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: CCY Chan, KKF Fung.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of
Helsinki. Informed consent was obtained from the patient for
all treatments and procedures, and consent for publication.
References
1. Chandrakumar A, Bhardwaj A, ’t Jong GW. Review of
thiamine deficiency disorders: Wernicke encephalopathy
and Korsakoff psychosis. J Basic Clin Physiol Pharmacol 2018;30:153-62. Crossref
2. Harpe CG, Giles M, Finlay-Jones R. Clinical signs in the
Wernicke–Korsakoff complex: a retrospective analysis
of 131 cases diagnosed at necropsy. J Neurol Neurosurg
Psychiatry 1986;49:341-5. Crossref
3. Zuccoli. G, Pipitone N. Neuroimaging findings in acute
Wernicke’s encephalopathy: review of the literature. AJR
Am J Roentgenol 2009;192:501-8. Crossref
4. Bae SJ, Lee HK, Lee JH, Choi CG, Suh DC. Wernicke’s
encephalopathy: atypical manifestation at MR imaging.
AJNR Am J Neuroradiol 2001;22:1480-2.
5. Pereira DB, Pereira ML, Gasparetto EL. Nonalcoholic
Wernicke encephalopathy with extensive cortical
involvement: cortical laminar necrosis and hemorrhage
demonstrated with susceptibility-weighted MR phase
images. AJNR Am J Neuroradiol 2011;32:E37-8. Crossref

