Hong Kong Med J 2024;30:Epub 16 Dec 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Consolidated and updated ultrasonographic fetal biometry and estimated fetal weight references for the Hong Kong Chinese population
Fangzi Liu, MB, ChB, MRCOG1; Jing Lu, MD2; Angel HW Kwan, MB, ChB, FHKAM (Obstetrics and Gynaecology)1; YK Yeung, MB, BS1; Lo Wong, MB, BS, FHKAM (Obstetrics and Gynaecology)1; Christopher PH Chiu, MB, BS, FHKAM (Obstetrics and Gynaecology)1; Liona CY Poon, MB, BS, MD3; Daljit Singh Sahota, BEng, PhD3
1 Department of Obstetrics and Gynaecology, Prince of Wales Hospital, Hong Kong SAR, China
2 Department of Obstetrics and Gynaecology, The First Affiliated Hospital of Xiamen University, Xiamen, China
3 Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Prof Daljit Singh Sahota (daljit@cuhk.edu.hk)
Abstract
Introduction: This study aimed to construct
consolidated and updated ultrasonographic
fetal biometry and estimated fetal weight (EFW)
references for the Hong Kong Chinese population
and evaluate the extent of under- and overdiagnosis
of small-for-gestational-age (SGA) and large-for-gestational-age (LGA) using these new references.
Methods: Fetal biometry and EFW references were
constructed using the Generalised Additive Model
for Location, Scale, and Shape, based on data from
1679 singleton pregnancies in non-smoking Chinese
women. Ultrasound scans were performed at 12 to
40 weeks of gestation to measure biparietal diameter,
head circumference, abdominal circumference (AC),
and femur length, following standardised protocols.
The rates of SGA and LGA diagnoses using the
existing and updated Hong Kong fetal biometry
references were compared in an independent cohort
of 10 229 pregnancies.
Results: The median number of scans per gestational
week between 20 and 39 weeks was 75 (interquartile
range=67-83). Compared with existing references,
the new AC reference would significantly (P<0.001)
increase the proportions of SGA fetuses with AC
measurements at <3rd and <10th percentiles from
1.7% and 6.1% to 3.4% and 10.0%, respectively.
Conversely, it would significantly decrease (P<0.001) the proportions of LGA fetuses with AC at >90th
and >97th percentiles from 15.0% and 4.9% to 11.5%
and 3.5%, respectively.
Conclusion: Adoption of the new references,
particularly for AC, may lead to increased
identification of SGA cases and decreased
identification of LGA cases. The proportions of these
cases will be more consistent with their intended
diagnostic thresholds. Further studies are needed to
determine how these references impact pregnancy
outcomes.
New knowledge added by this study
- Updated biometry and estimated fetal weight (EFW) references were constructed for antenatal assessment of fetal size.
- Improved detection of small-for-gestational-age (SGA) fetuses was achieved.
- Reduced identification of fetuses classified as large-for-gestational-age was noted.
- The updated biometry and EFW references were implemented in clinical practice by hospitals managed by the Hospital Authority in the second quarter of 2023.
- There is a need for clinicians to prepare for an increase in the number of cases requiring closer monitoring and potentially earlier interventions for SGA fetuses and a need for clear guidelines to manage the increased number of potential SGA pregnancies without overtreatment.
Introduction
Fetal biometry and estimated fetal weight (EFW)
are routinely documented by sonographers and
ultrasound providers during the antenatal period
as early indicators of suspected or actual abnormal fetal growth. At a given gestational age (GA), small
or large fetal size is often suspected when biometry
measurements are below or above the reference
extremes. Small for gestational age (SGA), typically
defined as a fetus with an abdominal circumference (AC) or EFW <10th percentile, is associated with
increased risks of stillbirth, preterm delivery, and
neonatal morbidity and mortality1 2; this diagnosis
requires more frequent ultrasound monitoring.
In contrast, large for gestational age (LGA) refers
to a fetus with AC or EFW >90th percentile and
is associated with increased risks of macrosomia,
shoulder dystocia, neonatal hypoglycaemia,
caesarean delivery, and postpartum haemorrhage.3 4
Management of an LGA fetus may include strict
maternal glycaemic control in cases of gestational
diabetes, early induction of labour, or scheduled
caesarean delivery. Therefore, reliable reference
charts for fetal biometry and size are essential in
obstetric practice to optimise the use of antenatal
surveillance resources, especially in public medical
institutions.
The current fetal biometry references adopted
by obstetricians and ultrasound providers in Hong
Kong were constructed using a cohort of Hong Kong
Chinese women from 1999 to 2000, based on best
practices available at that time, and were published
in 2008.5 However, the clinical utility of these 2008
biometry references for identifying SGA and LGA
was not evaluated until 2016 by Cheng et al,6 who
found that the percentile thresholds used to classify
fetuses as SGA and LGA led to underdiagnosis
of SGA and overdiagnosis of LGA. Specifically,
only 4.6% of fetuses had an AC <10th percentile,
whereas 13.3% had an AC >90th percentile,6 raising concerns about the validity of the measurements
in 20085 and whether they still reflect current fetal
size, considering changes in population and socio-demographic
characteristics.
The aims of the current study were to construct
revised ultrasonographic fetal biometry and EFW
references for the Hong Kong Chinese population,
using statistical methods recommended by the
World Health Organization (WHO), and to compare
the rates of SGA and LGA diagnoses based on the
new and existing references.
Methods
This study utilised fetal biometry data from three
population cohort studies previously conducted at
Prince of Wales Hospital, The Chinese University
of Hong Kong.5 6 7 Fetal biometry data from two of
the cohorts5 7 were used to construct the revised
biometry and EFW references, while the remaining
cohort6 was used to assess the clinical utility of
specific percentiles from the updated biometry
references. This study followed the TRIPOD
(Transparent Reporting of a multivariable prediction
model for Individual Prognosis Or Diagnosis)
reporting guideline.8
Derivation of biometry and estimated fetal
weight references
The new fetal biometry references were developed
using data collected from non-smoking Chinese
women with viable, spontaneously conceived
singleton pregnancies, recruited at 11 to 13 weeks
of gestation from the general obstetric population
in the years 1999-20005 and 2015-2016.7 Women
who consented to participate in either cohort were
randomly selected to undergo a study-specific
ultrasound examination of fetal size by a maternal-fetal
medicine specialist at GAs ranging from
12 to 40 weeks. Gestational age at recruitment
was calculated based on the first date of the last
menstrual period if it corresponded to the crown-rump
length measurement within a 4-day margin;
otherwise, the GA was adjusted using a crown-rump
length formula specific to the Chinese population.9
Pregnancies with fetal anomalies were excluded
from both cohorts.
Transabdominal ultrasounds were performed
using standard commercially available transducers
and machines present in the hospital, as
described in the original studies.5 7 Fetal biometric
measurements, including head circumference (HC),
biparietal diameter (BPD) measured in an outer-inner
manner, AC, and femur length (FL) were
obtained using identical standardised protocols,
as previously described.5 7 Estimated fetal weight
was derived from biometric data using the formula
EFW=10(1.326+0.0107×HC+0.0438×AC+0.158×FL−0.00326×AC×FL), as
previously published by Hadlock et al10 and adopted by the WHO.11
Biometry reference models for HC, BPD, AC,
FL and EFW according to GA were constructed
using the Generalised Additive Model for Location,
Scale, and Shape (GAMLSS) package (version 5.0) in
R statistical software (version 3.3.2). Best-fit models
were developed in a stepwise manner, beginning
with models based on the normal distribution
and considering alternatives such as the Box—Cox
power exponential, as appropriate. Gestational
age was included as a polynomial term, and all
measurements were transformed to their natural
logarithm equivalent before model construction.
Goodness of fit was assessed by inspecting residuals
using quantile—quantile plots and worm plots to
determine whether kurtosis adjustments were
necessary.12
Biometry models were constructed for 12 to
40 weeks of gestation, whereas EFW models were
constructed for 20 to 40 weeks. Final smoothing
models were chosen by balancing smoothness of
percentiles, goodness of fit, and model simplicity.
These final models were used to calculate smoothed
values for the 50th, 10th, and 90th percentiles
(Zα= ±1.281), as well as the 3rd and 97th percentiles
(Zα= ±1.881). Percentiles were determined using the
expression μ × (1+υσZα)1/υ, where Zα represents the percentile of interest and μ, υ, and σ are dependent
on the time covariate (ie, GA).
Standard errors (SEs) of the 50th
percentile were estimated using the expression 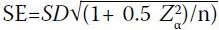 , assuming that the SE of the
percentile of interest can be expressed as a multiple
of the standard deviation (SD).13 14
, assuming that the SE of the
percentile of interest can be expressed as a multiple
of the standard deviation (SD).13 14
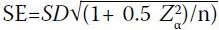 , assuming that the SE of the
percentile of interest can be expressed as a multiple
of the standard deviation (SD).13 14
, assuming that the SE of the
percentile of interest can be expressed as a multiple
of the standard deviation (SD).13 14Clinical utility of the revised biometry
references
The expected clinical performance of the revised
references was evaluated based on the same cohort
of second- and third-trimester fetal ultrasound scans
previously used to assess the INTERGROWTH-21st standards.6 This cohort consisted of biometry
measurements from 10 229 fetuses, with respective
median birthweight and GA at delivery of 3140 g
(interquartile range [IQR]=2850-3412) and
275 days (IQR=268-281); of these fetuses, 5419
(53.0%) were male.6 All fetal scans were performed
transabdominally by either maternal-fetal medicine
specialists or midwives who had passed the American
Registry for Diagnostic Medical Sonography
certification, using standard commercially available
transducers and ultrasound machines.
To compare the relative performances of the
revised and existing biometry references, Z-scores
were calculated as recommended by Salomon et al.15
Expected median and SD values were determined
for each gestational week. Z-scores for each fetal
parameter were then calculated using the formula: (observed value − expected median) / expected SD. These fetal parameter Z-scores were used to
determine the proportion of biometry measurements
in the cohort that were <10th or >90th percentiles
and <3rd or >97th percentiles, with ±1.282 and
±1.881 as respective thresholds.
Results
Updated biometry references were constructed from
a combined cohort of 1679 pregnancies. The median
maternal age at expected date of delivery, as well
as weight and height at recruitment, were 32 years
(IQR=28-34), 53 kg (IQR=38.5-58.1), and 157 cm
(IQR=154-161), respectively. Of the pregnancies,
892 (53.1%) were nulliparous women. Birth details
were unavailable for 115 (6.8%) pregnancies, all from
the cohort recruited by Leung et al,5 which was used
to construct the existing biometry reference. In the
1564 (93.2%) pregnancies with documented birth
details, the median birthweight, GA at delivery,
and male sex proportion were 3160 g (IQR=2900-3405), 277 days (IQR=270-283), and 830 (53.1%),
respectively. The median number of scans per
gestational week between 20 and 39 weeks was 75 (IQR=67-83).
The best-fitting GAMLSS for fetal biometry
and EFW are reported in online supplementary Tables 1 and 2, respectively. The distribution of
residuals from the fitted models approximated that
of a normal standard distribution, with means of 0,
variances of 1, skewness ranging from 0 to 0.1, and
kurtosis ranging from 3.22 to 3.69. The Figure shows
the fitted 50th, 3rd/97th, and 10th/90th smoothed
percentiles.
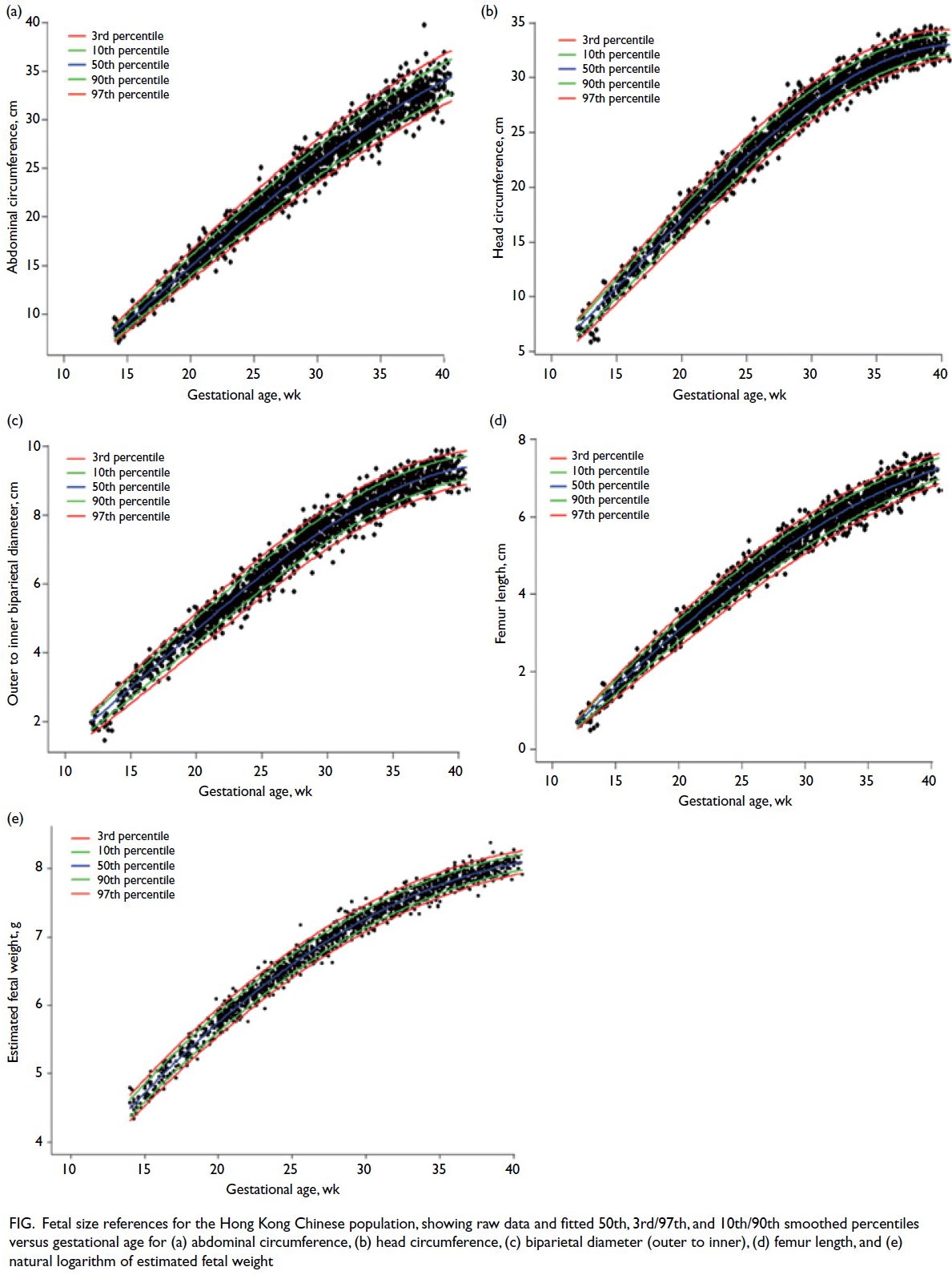
Figure. Fetal size references for the Hong Kong Chinese population, showing raw data and fitted 50th, 3rd/97th, and 10th/90th smoothed percentiles versus gestational age for (a) abdominal circumference, (b) head circumference, (c) biparietal diameter (outer to inner), (d) femur length, and (e) estimated fetal weight
The Table summarises the comparison of the
proportions of fetuses whose biometry was assessed
for fetal size above and below specific percentiles
across the 10 229 pregnancies. The proportions of
fetuses identified <3rd and >97th percentiles, as
well as <10th and >90th percentiles, by the revised
biometry references were approximately 3% and
10%, respectively, except for the FL reference.
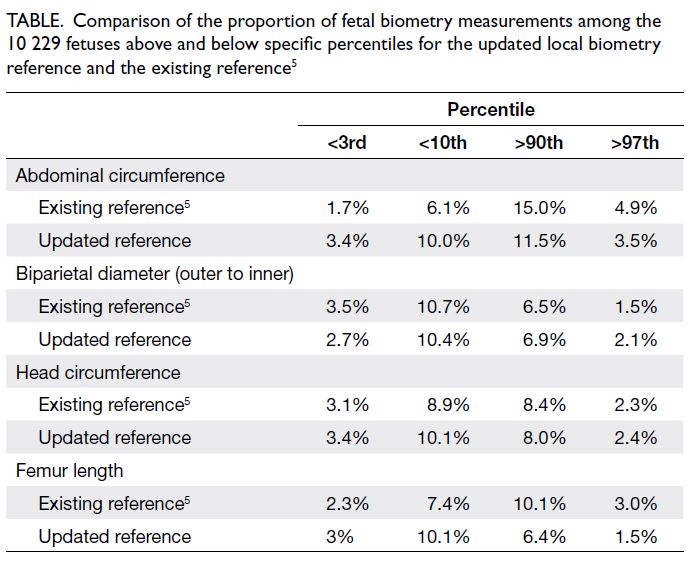
Table. Comparison of the proportion of fetal biometry measurements among the 10 229 fetuses above and below specific percentiles for the updated local biometry reference and the existing reference5
The analysis showed that, compared with the
existing AC biometry reference,5 the revised AC
biometry reference would significantly increase
the proportions of fetuses with AC measurements
at <3rd and <10th percentiles from 1.7% and 6.1%
to 3.4% and 10.0%, respectively (both P<0.001). It
would also significantly decrease the proportions of
fetuses with AC measurements at >90th and >97th
percentiles from 15.0% and 4.9% to 11.5% and 3.5%,
respectively (both P<0.001). Compared with the
existing biometry references,5 the revised biometry
references would identify greater numbers of fetuses
with short FL (<3rd percentile P=0.002; <10th
percentile P<0.001) and smaller HC (<3rd percentile
P=0.23; <10th percentile P=0.003) at the extreme
lower percentile limits.
Discussion
Principal findings
In this study, we developed updated biometry and
EFW references, then assessed how they compare
with existing references created over 20 years ago.5
These new references serve as a guide for local
obstetricians and ultrasound providers, both in
public institutions and private practice, to assess
relative and absolute fetal sizes.
Results in the context of current knowledge
In recent years, both the INTERGROWTH-21st project16 and the WHO11 have published
biometry and EFW charts according to GA. The
INTERGROWTH-21st reference was proposed
as a universal standard, based on the premise that
fetuses of well-nourished mothers, irrespective
of ethnicity or parental characteristics, grow at
similar rates.16 Thus, a single INTERGROWTH-21st standard was recommended for assessing
fetal size and growth worldwide. In contrast,
the WHO suggested that its references could be
customised to accommodate local populations,
adjusting diagnostic thresholds for SGA and LGA
to reflect population-specific characteristics.11
Local studies assessing the suitability and impact
of adopting the INTERGROWTH-21st and WHO
charts have indicated that these approaches would
lead to substantial misclassification of fetuses as
small.6 7 17 Similar concerns about the potential for
inaccurate classification have been reported by
other research groups that assessed either or both
the INTERGROWTH-21st and WHO biometry
charts.18 19 20 Customisation of the WHO charts to fit
the Hong Kong population would be comparable to
developing a locally tailored biometry reference, the
approach we have taken in this study.
Implications for clinical practice
The revised references had minimal impact on
measurements of bony structures, such as HC,
BPD, and FL. However, AC, which reflects fetal
subcutaneous fat mass and nutritional status,21
plays a greater role in calculating EFW, particularly
in the third trimester.10 The revised references
should reduce the misdiagnosis of SGA and LGA,
given that they are mainly based on AC and EFW.
However, this change might increase the workload
for obstetricians because additional scans will be
needed to distinguish constitutional smallness from
growth restriction.
The revised biometry and newly developed
EFW references replaced the existing Leung et al’s
biometry references5 previously used for antenatal
management in hospitals managed by the Hospital
Authority starting from the second quarter of 2023.
The major clinical impact of the revised biometry references was expected to be an increase in the
proportion of fetuses classified as SGA and a
decrease in those classified as LGA, such that the
proportions become more consistent with their
intended diagnostic thresholds at the 3rd and 10th
percentiles. By definition, the smallest 10% of fetuses
are regarded as SGA,1 2 and the largest 10% are
considered LGA.3 4 Although not all of these fetuses
exhibit restricted growth, these classifications carry
prognostic importance because they predict risks
of perinatal morbidity and mortality, especially
for SGA. Furthermore, fetuses classified as LGA
are more likely to require induction of labour
or caesarean delivery. Fetal biometry and EFW
references can serve as screening tools to detect
fetuses at both extremes of the growth spectrum.
Further evaluation, such as assessments of growth
velocity, performance of Doppler studies, and use of
biophysical profiles, can help differentiate between
those at high risk and those who are constitutionally
small or large.1
One key purpose of biometry references is
to reduce obstetric complications such as shoulder
dystocia, stillbirth, and neonatal morbidity and
mortality by improving the identification of SGA
and LGA fetuses. Further studies will be needed
to determine whether revision of the percentiles,
particularly the AC reference, and development
of a local EFW reference will show significant
correlations with perinatal outcomes. However,
such studies will need to be conducted over several
years and require support from a funding body,
considering the generally low incidence of adverse
perinatal outcomes in Hong Kong pregnancies.22
In a review of stillbirth rates from 2000 to 2020, Wong et al23 concluded that although stillbirth
rates had declined from approximately 3.3 to
2.9 per 1000 births between the first and second
decades, further improvements remained necessary
regarding early identification of early fetal growth
restriction. This analysis indicated that 16% of all
stillbirths were related to fetal growth restriction of
unknown cause.23 Whether the revised references, by
classifying an increased number of fetuses as SGA,
lead to improved early detection of fetal growth
restriction requires prospective investigation. One
approach could involve using information obtained
during first-trimester Down syndrome screening to
identify fetuses at increased risk of being considered
SGA, followed by either longitudinal or cross-sectional
assessments later in pregnancy. Leung
et al24 previously reported that low serum levels
of pregnancy associated plasma protein-A and
smaller fetal crown-rump length at 11 to 13 weeks
of gestation were independent predictors of SGA
status. More recently, Papastefanou et al25 proposed
a model for predicting SGA classification using
a combination of maternal factors and the same
biomarkers included in preeclampsia screening to
identify potential fetuses at risk of SGA status.
Strengths and limitations
The revised biometry and newly developed EFW
references were derived from a larger cohort,
improving the precision of the estimated percentiles,
specifically those used for clinical decision-making.
By combining two cohorts with similar inclusion
and exclusion criteria and using standardised
ultrasound measurement protocols,5 7 the precision
of the estimated percentiles has been enhanced.
The existing biometry references were based on
706 pregnancies, yielding SEs of 0.05 SD for the
10th and 90th percentiles and 0.06 SD for the 3rd
and 97th percentiles. By developing the revised
references from 1679 cases, we have improved the
precision; the abovementioned SEs are now 0.03 SD
and 0.04 SD, respectively. Additionally, consistent
with biometry references reported by other groups,
we used the semi-parametric GAMLSS method to
concurrently model the mean, variance, skew, and
kurtosis; conversely, the approach by Leung et al5
utilised a simpler mean±k×SD model and assumed
no kurtosis or skewness. The GAMLSS method is
recommended by the WHO,11 26 27 which adopted this
approach during the development of its biometry
and EFW references because the GAMLSS enabled
more accurate prediction and smoother curves
compared with earlier modelling approaches.26
Finally, we avoided a common limitation, identified
in a previous review,28 by not retrospectively using
routinely collected fetal measurements to derive
biometry references—this could lead to skewed
charts and inaccurate percentile limits.
A limitation of the newly revised references
is that they are monoethnic because they were
derived from pregnancies in Chinese women at
a single hospital, which provides medical care to
approximately 18% of the territory’s population.29
Hong Kong is a largely homogenous society in which
approximately 92% of individuals are Han Chinese.30
However, considering possible ethnic differences,
especially when comparing East and Southeast
Asians with other groups, caution may be needed
when interpreting biometry and EFW measurements
in other ethnic populations.31 32
Conclusion
We have constructed and updated ultrasonographic
fetal biometry and EFW reference percentiles
for the antenatal assessment of fetal size in Hong
Kong Chinese singleton pregnancies. The adoption
of these updated biometry percentile references,
particularly regarding AC, is expected to result in
an increased proportion of fetuses classified as SGA
and a decreased proportion of fetuses considered
LGA. The proportions of SGA and LGA cases will
be more consistent with the intended diagnostic
thresholds. Further prospective studies are needed
to determine whether the introduction of these
revised biometry and EFW reference percentiles by
the hospitals of the Hospital Authority will lead to
improved perinatal outcomes.
Author contributions
Concept or design: F Liu, DS Sahota.
Acquisition of data: F Liu, J Lu, AHW Kwan, L Wong.
Analysis or interpretation of data: F Liu, YK Yeung, CPH Chiu, DS Sahota.
Drafting of the manuscript: F Liu, DS Sahota.
Critical revision of the manuscript for important intellectual content: LCY Poon, DS Sahota.
Acquisition of data: F Liu, J Lu, AHW Kwan, L Wong.
Analysis or interpretation of data: F Liu, YK Yeung, CPH Chiu, DS Sahota.
Drafting of the manuscript: F Liu, DS Sahota.
Critical revision of the manuscript for important intellectual content: LCY Poon, DS Sahota.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank the pregnant women in this study, as well
as the Fetal Medicine team, midwives, and research assistants
at the Prince of Wales Hospital who recruited participants
and performed fetal scans in the primary study cohorts used
to construct the updated biometry references.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This is a retrospective analysis of data that were collected as part of approved studies conducted by the Joint Chinese University of Hong Kong–New Territories Cluster Clinical
Research Ethics Committee, Hong Kong, for the same use
and purpose (Ref Nos.: CRE-9019, CRE-2012.538, and CRE
2014.507). Informed consent was obtained from patients
when the data was originally collected.
Supplementary material
The supplementary material was provided by the authors and
some information may not have been peer reviewed. Accepted
supplementary material will be published as submitted by the
authors, without any editing or formatting. Any opinions
or recommendations discussed are solely those of the
author(s) and are not endorsed by the Hong Kong Academy
of Medicine and the Hong Kong Medical Association.
The Hong Kong Academy of Medicine and the Hong Kong
Medical Association disclaim all liability and responsibility
arising from any reliance placed on the content.
References
1. Lees CC, Stampalija T, Baschat A, et al. ISUOG Practice
Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction.
Ultrasound Obstet Gynecol 2020;56:298-312. Crossref
2. ACOG Practice Bulletin No. 227: Fetal Growth Restriction: correction [editorial]. Obstet Gynecol 2021;137:754. Crossref
3. Evidence review for large-for-gestational age baby:
intrapartum care for women with existing medical
conditions or obstetric complications and their babies.
Evidence review Q. NICE Guideline No. 121. National
Institute for Health and Care Excellence: London; 2019.
4. Macrosomia: ACOG Practice Bulletin, Number 216.
Obstet Gynecol 2020;135:e18-35. Crossref
5. Leung TN, Pang MW, Daljit SS, et al. Fetal biometry in
ethnic Chinese: biparietal diameter, head circumference,
abdominal circumference and femur length. Ultrasound
Obstet Gynecol 2008;31:321-7. Crossref
6. Cheng YK, Leung TY, Lao TT, Chan YM, Sahota DS. Impact
of replacing Chinese ethnicity-specific fetal biometry
charts with the INTERGROWTH-21(st) standard. BJOG
2016;123 Suppl 3:48-55. Crossref
7. Cheng YK, Lu J, Leung TY, Chan YM, Sahota DS.
Prospective assessment of INTERGROWTH-21st and
World Health Organization estimated fetal weight
reference curves. Ultrasound Obstet Gynecol 2018;51:792-8. Crossref
8. Collins GS, Reitsma JB, Altman DG, Moons KG.
Transparent Reporting of a multivariable prediction model
for Individual Prognosis Or Diagnosis (TRIPOD): the
TRIPOD statement. BMC Med 2015;13:1. Crossref
9. Sahota DS, Leung TY, Leung TN, Chan OK, Lau TK. Fetal
crown-rump length and estimation of gestational age in
an ethnic Chinese population. Ultrasound Obstet Gynecol
2009;33:157-60. Crossref
10. Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK.
Estimation of fetal weight with the use of head, body, and
femur measurements—a prospective study. Am J Obstet
Gynecol 1985;151:333-7. Crossref
11. Kiserud T, Piaggio G, Carroli G, et al. The World Health
Organization fetal growth charts: a multinational longitudinal study of ultrasound biometric measurements
and estimated fetal weight. PLoS Med 2017;14:e1002220. Crossref
12. van Buuren S, Fredriks M. Worm plot: a simple diagnostic
device for modelling growth reference curves. Stat Med
2001;20:1259-77. Crossref
13. Healy MJ. Notes on the statistics of growth standards. Ann
Hum Biol 1974;1:41-6. Crossref
14. Royston P. Constructing time-specific reference ranges.
Stat Med 1991;10:675-90. Crossref
15. Salomon LJ, Bernard JP, Duyme M, Buvat I, Ville Y. The
impact of choice of reference charts and equations on the
assessment of fetal biometry. Ultrasound Obstet Gynecol
2005;25:559-65. Crossref
16. Papageorghiou AT, Ohuma EO, Altman DG, et al.
International standards for fetal growth based on serial
ultrasound measurements: the Fetal Growth Longitudinal
Study of the INTERGROWTH-21st Project. Lancet
2014;384:869-79. Crossref
17. Lok IW, Kong MC, To WW. Updated gestational age
specific birthweight reference of Hong Kong Chinese
newborns and comparison with local and international
growth charts. Open J Obstet Gynecol 2021;11:940-54. Crossref
18. Liu S, Metcalfe A, León JA, et al. Evaluation of the
INTERGROWTH-21st project newborn standard for use
in Canada. PLoS One 2017;12:e0172910. Crossref
19. Jakubowski D, Salloum D, Maciejewski M, et al. Comparison
of application of Fenton, Intergrowth-21st and WHO
growth charts in a population of Polish newborns. Clin Exp
Obstet Gynecol 2021;48:949-54. Crossref
20. Huang TM, Tsai CH, Hung FY, Huang MC. A novel
reference chart and growth standard of fetal biometry
in the Taiwanese population. Taiwan J Obstet Gynecol
2022;61:794-9. Crossref
21. Gardeil F, Greene R, Stuart B, Turner MJ. Subcutaneous fat
in the fetal abdomen as a predictor of growth restriction.
Obstet Gynecol 1999;94:209-12. Crossref
22. Hong Kong College of Obstetricians and Gynaecologists.
Territory-Wide Audit in Obstetrics and Gynaecology.
2014. Available from: https://www.hkcog.org.hk/hkcog/Download/Territory-wide_Audit_in_Obstetrics_Gynaecology_2014.pdf. Accessed 1 Apr 2023.
23. Wong ST, Tse WT, Lau SL, Sahota DS, Leung TY. Stillbirth
rate in singleton pregnancies: a 20-year retrospective study
from a public obstetric unit in Hong Kong. Hong Kong
Med J 2022;28:285-93. Crossref
24. Leung TY, Sahota DS, Chan LW, et al. Prediction of birth
weight by fetal crown-rump length and maternal serum
levels of pregnancy-associated plasma protein-A in the
first trimester. Ultrasound Obstet Gynecol 2008;31:10-4. Crossref
25. Papastefanou I, Wright D, Nicolaides KH. Competing-risks
model for prediction of small-for-gestational-age
neonate from maternal characteristics and medical history.
Ultrasound Obstet Gynecol 2020;56:196-205. Crossref
26. Stasinopoulos DM, Rigby RA. Generalized Additive
Models for Location, Scale and Shape (GAMLSS) in R. J
Stat Softw 2007;23:1-46. Crossref
27. Borghi E, de Onis M, Garza C, et al. Construction of
the World Health Organization child growth standards:
selection of methods for attained growth curves. Stat Med
2006;30:247-65. Crossref
28. Ioannou C, Talbot K, Ohuma E, et al. Systematic review of
methodology used in ultrasound studies aimed at creating charts of fetal size. BJOG 2012;119:1425-39. Crossref
29. Census and Statistics Department, Hong Kong SAR
Government. District profiles (population and households).
2023. Available from: https://www.censtatd.gov.hk/en/map_ghs.html. Accessed 1 Apr 2023.
30. Race Relations Unit, Home Affairs Department, Hong
Kong SAR Government. The demographics: ethnic groups.
Available from: https://www.had.gov.hk/rru/english/info/demographics.htm. Accessed 1 Apr 2023.
31. Shiono PH, Klebanoff MA, Graubard BI, Berendes HW,
Rhoads GG. Birth weight among women of different ethnic
groups. JAMA 1986;255:48-52. Crossref
32. Kierans WJ, Joseph KS, Luo ZC, Platt R, Wilkins R,
Kramer MS. Does one size fit all? The case for ethnicspecific
standards of fetal growth. BMC Pregnancy
Childbirth 2008;8:1. Crossref

