Hong Kong Med J 2024 Oct;30(5):417.e1-2 | Epub 15 Oct 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PICTORIAL MEDICINE
Marchiafava–Bignami disease
F Ren, MD1; Q Wang, MD2
1 Department of Radiology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
2 Department of Ultrasound, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Corresponding author: Dr Q Wang (444028177@qq.com)
A 67-year-old female was admitted to the neurology
department in October 2020 with abnormal
behaviour and cognitive impairment. Her memory
and numeracy had declined, and symptoms
progressed over the preceding week. She had a
30-year history of chronic alcohol abuse with an
average daily intake of 500-mL liquor.
Routine biochemistry including electrolytes,
liver function, and vitamin B12 were within
normal limits. Magnetic resonance imaging of the
brain showed areas of hyperintensity of the corpus
callosum (splenium, body, and genu), bilateral middle
cerebellar peduncles, periventricular white matter,
and subcortical white matter of the frontal lobe on
T2-weighted and fluid-attenuated inversion recovery
images. Diffusion-weighted imaging revealed prominent high-intensity signal lesions involving
the splenium, and these corresponding lesions were
hypointense on the apparent diffusion coefficient
map (Fig). Based on her history, physical examination
and magnetic resonance imaging features, the patient
was diagnosed with Marchiafava–Bignami disease
(MBD). Despite a normal level of serum vitamins, the
patient was prescribed vitamin B and neurotrophic
treatment. Symptoms improved and she made a good
recovery over the next 30 days.
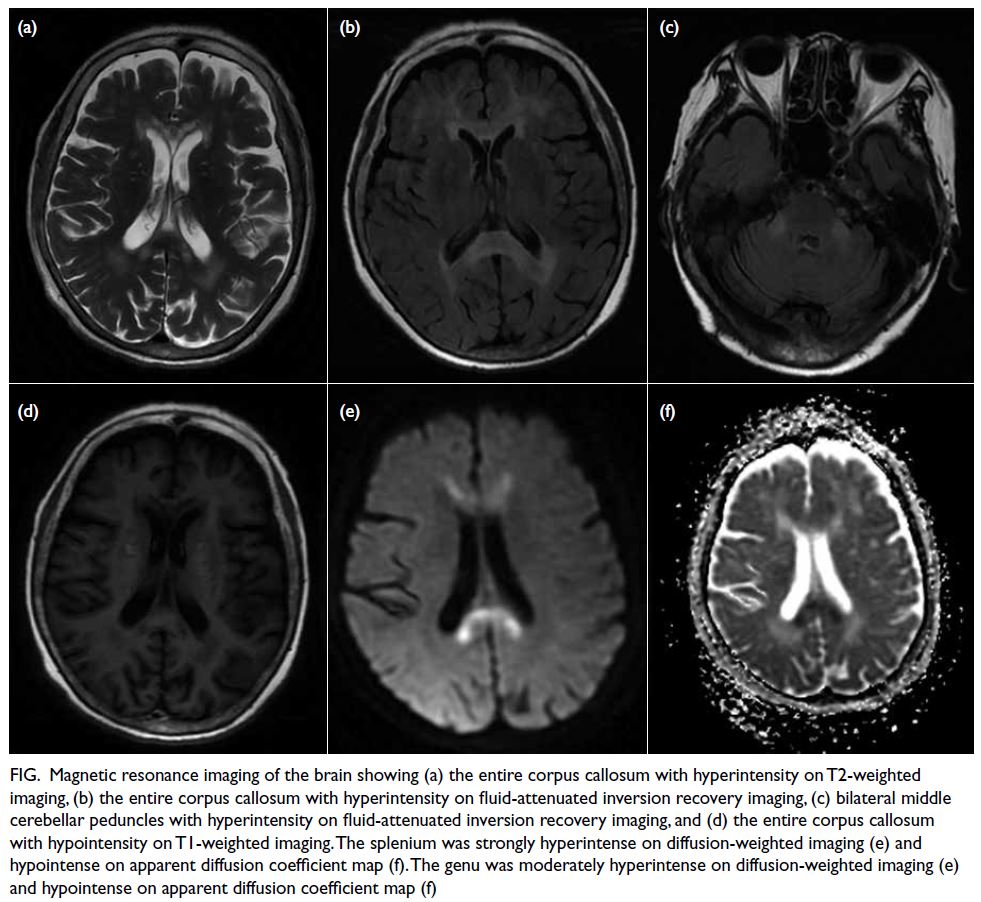
Figure. Magnetic resonance imaging of the brain showing (a) the entire corpus callosum with hyperintensity on T2-weighted imaging, (b) the entire corpus callosum with hyperintensity on fluid-attenuated inversion recovery imaging, (c) bilateral middle cerebellar peduncles with hyperintensity on fluid-attenuated inversion recovery imaging, and (d) the entire corpus callosum with hypointensity on T1-weighted imaging. The splenium was strongly hyperintense on diffusion-weighted imaging (e) and hypointense on apparent diffusion coefficient map (f). The genu was moderately hyperintense on diffusion-weighted imaging (e) and hypointense on apparent diffusion coefficient map (f)
Marchiafava–Bignami disease is a rare
neurological syndrome characterised by
primary degeneration and necrosis of the corpus
callosum associated with chronic alcoholism and
malnutrition. The clinical manifestations of MBD
are severe and nonspecific and include an altered mental state, impaired walking, deficient memory,
and dysarthria. Symptoms and imaging findings
may improve following thiamine treatment.1 2 The
role of magnetic resonance imaging is essential to
confirm the diagnosis. Chronic alcohol abuse plays
an important role in its development although MBD
has been occasionally diagnosed in patients with no
history of alcohol abuse, in particular individuals
with poorly controlled diabetes mellitus or following
surgery.3 4 The aetiology and pathophysiology of MBD remain unclear. Possible mechanisms include
cytotoxic oedema, blood-brain barrier breakdown,
demyelination, and necrosis. Early pathological
manifestations are mainly intramyelinic or cytotoxic
oedema. In the later stages, demyelination and
necrosis of the corpus callosum (especially in the
genu and the body) may follow5 with necrosis leading to atrophy and cavitation in chronic stages,
and a decreased number of oligodendrocytes.
Occasionally, similar lesions can involve extracallosal
regions, such as the anterior and posterior
commissures, subcortical white matter, middle
cerebellar peduncle, optic chiasm, putamen, internal
capsules, hippocampus, and frontal cortex.2
The recent advances in neuroradiology
techniques help understand the pathophysiological
processes of MBD. Studies with diffusion-weighted
imaging have shown a low apparent diffusion
coefficient, which has been interpreted as irreversible
cytotoxic oedema, and may precede the development
of demyelination and necrosis and predict a
poor or partial recovery.2 5 Nonetheless the high
apparent diffusion coefficient showing reversible
signal changes favoured an underlying vasogenic
oedema-related process. In magnetic resonance
spectroscopy studies, an increased choline/creatine
ratio indicates demyelination during the acute phase
of MBD, while a reduced N-acetylaspartate/creatine
ratio suggests secondary axonal injury. In addition,
decreased cerebral blood flow and cerebral blood
volume in magnetic resonance perfusion suggest
hypoperfusion. Recognition of the neuroradiological
features is crucial to establish a diagnosis.
Author contributions
Concept or design: Both authors.
Acquisition of data: Q Wang.
Analysis or interpretation of data: F Ren.
Drafting of the manuscript: F Ren.
Critical revision of the manuscript for important intellectual content: Q Wang.
Acquisition of data: Q Wang.
Analysis or interpretation of data: F Ren.
Drafting of the manuscript: F Ren.
Critical revision of the manuscript for important intellectual content: Q Wang.
Both authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
Both authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Hospital of Chengdu University of Traditional Chinese Medicine Research Ethics Committee, China. Informed consent for all treatments and
procedures, and consent for publication were obtained from the patient.
References
1. Tsai CY, Huang PK, Huang P. Simultaneous acute
Marchiafava–Bignami disease and central pontine
myelinolysis: a case report of a challenging diagnosis.
Medicine (Baltimore) 2018;97:e9878. Crossref
2. Hillbom M, Saloheimo P, Fujioka S, Wszolek ZK, Juvela S,
Leone MA. Diagnosis and management of Marchiafava–Bignami disease: a review of CT/MRI confirmed cases. J
Neurol Neurosurg Psychiatry 2014;85:168-73. Crossref
3. Pérez Álvarez AI, Ramón Carbajo C, Morís de la Tassa G,
Pascual Gómez J. Marchiafava–Bignami disease triggered
by poorly controlled diabetes mellitus [in English, Spanish].
Neurologia 2016;31:498-500. Crossref
4. Bachar M, Fatakhov E, Banerjee C, Todnem N.
Rapidly resolving nonalcoholic Marchiafava–Bignami
disease in the setting of malnourishment after gastric
bypass surgery. J Investig Med High Impact Case Rep
2018;6:2324709618784318. Crossref
5. Ménégon P, Sibon I, Pachai C, Orgogozo JM, Dousset V.
Marchiafava–Bignami disease: diffusion-weighted MRI
in corpus callosum and cortical lesions. Neurology
2005;65:475-7. Crossref

