Hong Kong Med J 2024 Aug;30(4):291–9 | Epub 16 Aug 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Glycaemic control and microvascular complications among paediatric type 2 diabetes mellitus patients in Hong Kong at 2 years after diagnosis
WI Yam, MB, ChB, FHKAM (Paediatrics)1; Shirley MY Wong, MB, BS, FHKAM (Paediatrics)1; PT Cheung, MB, BS, FHKAM (Paediatrics)2; Elaine YW Kwan, MB, BS, FHKAM (Paediatrics)3; YY Lam, MB, BS, FHKAM (Paediatrics)4; LM Wong, MB, BS, FHKAM (Paediatrics)5; KL Ng, MB, BS, FHKAM (Paediatrics)6; Sammy WC Wong, MB, ChB, FHKAM (Paediatrics)7; CY Lee, MB, BS, FHKAM (Paediatrics)8; MK Tay, LMCHK, FHKAM (Paediatrics)9; KT Chan, MB, BS, FHKAM (Paediatrics)3; Antony CC Fu, MB, ChB, FHKAM (Paediatrics)10; Joanna YL Tung, MB, BS, FHKAM (Paediatrics)11; Gloria SW Pang, MB, BS, FHKAM (Paediatrics)11; HC Yau, MB, ChB, FHKAM (Paediatrics)12; Queenie WS See, MB, BS, FHKAM (Paediatrics)2; Priscilla WC Lo, MB, BS, FHKAM (Paediatrics)6; Sharon WY To, MB, BS, FHKAM (Paediatrics)10; HW Yuen, MB, ChB, FHKAM (Paediatrics)4; Jacky YK Chung, MB, BS, FHKAM (Paediatrics)8; Eunice WY Wong, MB, BS, FHKAM (Paediatrics)5; Sarah WY Poon, MB, BS, FHKAM (Paediatrics)11; Charlotte HY Lam, MB, BS, FHKAM (Paediatrics)7; Suki SY Chan, LMCHK, MRCPCH12; Janez HC Tsui, MB, BS, MRCPCH3; Cindy SY Chan, MB, BS, MRCPCH9; Betty WM But, MB, BS, FHKAM (Paediatrics)1
1 Department of Paediatrics, Queen Elizabeth Hospital, Hong Kong SAR, China
2 Department of Paediatrics and Adolescent Medicine, Queen Mary Hospital, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
3 Department of Paediatrics and Adolescent Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
4 Department of Paediatrics and Adolescent Medicine, Kwong Wah Hospital, Hong Kong SAR, China
5 Department of Paediatrics and Adolescent Medicine, Tuen Mun Hospital, Hong Kong SAR, China
6 Department of Paediatrics and Adolescent Medicine, United Christian Hospital, Hong Kong SAR, China
7 Department of Paediatrics and Adolescent Medicine, Alice Ho Miu Ling Nethersole Hospital, Hong Kong SAR, China
8 Department of Paediatrics, Caritas Medical Centre, Hong Kong SAR, China
9 Department of Paediatrics, Tseung Kwan O Hospital, Hong Kong SAR, China
10 Department of Paediatrics and Adolescent Medicine, Princess Margaret Hospital, Hong Kong SAR, China
11 Department of Paediatrics, Hong Kong Children’s Hospital, Hong Kong SAR, China
12 Department of Paediatrics, Prince of Wales Hospital, Hong Kong SAR, China
Corresponding author: Dr WI Yam (ywi817@ha.org.hk)
Abstract
Introduction: Type 2 diabetes mellitus (T2DM) is
becoming increasingly common among children
and adolescents worldwide, including those in Hong
Kong. This study analysed the characteristics and
prevalence of microvascular complications among
paediatric T2DM patients in Hong Kong at diagnosis
and 2 years after diagnosis.
Methods: All patients aged <18 years who had been
diagnosed with DM at public hospitals in Hong
Kong were recruited into the Hong Kong Childhood
Diabetes Registry. Data collected at diagnosis and 2
years after diagnosis were retrospectively retrieved
from the Registry for patients diagnosed from 2014
to 2018.
Results: Median haemoglobin A1c (HbA1c) levels
were 7.5% (n=203) at diagnosis and 6.5% (n=135)
2 years after diagnosis; 59.3% of patients achieved
optimal glycaemic control (HbA1c level <7%) at
2 years. A higher HbA1c level at diagnosis was
associated with worse glycaemic control at 2 years
(correlation coefficient=0.39; P<0.001). The presence
of dyslipidaemia (adjusted odds ratio [aOR]=3.19;
P=0.033) and fatty liver (aOR=2.50; P=0.021) at 2
years were associated with suboptimal glycaemic
control. Diabetic neuropathy and retinopathy were
rare in our cohort, but 18.6% of patients developed
microalbuminuria (MA) within 2 years after
diagnosis. Patients with MA had a higher HbA1c
level at 2 years (median: 7.2% vs 6.4%; P=0.037).
Hypertension was a risk factor for MA at 2 years,
independent of glycaemic control (aOR=4.61; P=0.008).
Conclusion: These results highlight the importance
of early diagnosis and holistic management
(including co-morbidity management) for paediatric
T2DM patients.
New knowledge added by this study
- A total of 59.3% of paediatric type 2 diabetes mellitus patients in Hong Kong had achieved satisfactory glycaemic control at 2 years after diagnosis.
- Factors associated with suboptimal glycaemic control at 2 years after diagnosis were higher haemoglobin A1c level at diagnosis, fatty liver at 2 years, and dyslipidaemia at 2 years.
- Overall, 18.6% of patients had microalbuminuria at 2 years and exhibited hypertension as a risk factor, independent of glycaemic control.
- Early diagnosis of diabetes mellitus is important because initial disease severity predicts the risk of suboptimal glycaemic control at 2 years.
- Management of co-morbidities, including fatty liver, dyslipidaemia, and hypertension, is important for the maintenance of glycaemic control and prevention of microalbuminuria.
Introduction
Type 2 diabetes mellitus (T2DM) in children
and adolescents (hereinafter, youth) is becoming
increasingly common worldwide.1 2 A recent meta-analysis
estimated that approximately 41 600 new
cases of T2DM were identified among youth in
2021.3 Type 2 DM in youth exhibits relatively rapid
clinical progression with a sharp decline in beta-cell
function and high risk of complications.4 In
a study recently published by the TODAY (Type 2
Diabetes in Adolescents and Youth) Study Group,
which analysed 500 young adults with youth-onset T2DM, 60.1% of patients developed at least
one microvascular complication (diabetic kidney,
nerve, or retinal disease) and 28.4% of patients
developed at least two complications.5 In addition
to hyperglycaemia, the presence of co-morbidities
(eg, hypertension and dyslipidaemia) was associated
with an increased risk of complications.5
A similar increase in the incidence of T2DM
has been observed in Hong Kong. We previously
reported that the crude incidence rate increased
from 1.27 per 100 000 person-years in 1997-2007
to 3.42 per 100 000 person-years in 2008-2017.6
However, there have been limited data regarding
the outcomes of paediatric T2DM patients in Hong
Kong. In this study, we reviewed the glycaemic
control findings and microvascular complication
rates among recently diagnosed paediatric T2DM
patients in Hong Kong, with a focus on outcomes at
2 years after diagnosis; we sought to identify factors
associated with poor glycaemic control and the
development of microalbuminuria (MA).
Methods
Setting
Data analysed in this study were retrieved from the Hong Kong Childhood Diabetes Registry, a database
established in 2016. The Registry was approved
by the Research Ethics Committee of the Hospital
Authority of Hong Kong, which includes 11 public
hospitals. Investigators retrieved information from
medical records and entered relevant data into
the Registry. Standardised data entry forms for
recording baseline clinical characteristics and annual
entry forms were provided for investigators to enter
data into the Registry at diagnosis and annually
thereafter. Data were cross-checked by at least two
investigators.
Inclusion and exclusion criteria
All patients aged <18 years who had been diagnosed
with DM at public hospitals in Hong Kong were
recruited. All recruited patients met the diagnostic
criteria for DM according to the International
Society for Paediatric and Adolescent Diabetes Clinical Practice Consensus Guidelines: patients
were symptomatic and had either fasting blood
glucose level ≥7 mmol/L, 2-hour blood glucose
level ≥11.1 mmol/L during an oral glucose tolerance
test, random blood glucose level ≥11.1 mmol/L,
or haemoglobin A1c (HbA1c) level ≥6.5%.4
Asymptomatic patients underwent repeat testing
with a different test, as suggested in the Guidelines.4
The classification of DM was based on the attending
clinician’s assessment of clinical symptoms and
laboratory findings, including obesity status, family
history, autoimmunity, and clinical course. Patients
diagnosed with T2DM from 2014 to 2018 were
included in the analysis, including those who had an
initial diagnosis of type 1 DM that was subsequently
revised to T2DM. Patients who refused Registry
recruitment and patients whose diagnosis was
revised to type 1 DM or maturity-onset DM of the
young were not included in the analysis.
Data collection and definitions
The following data were retrieved from the Registry:
patient age, sex, family history of T2DM (in first- or
second-degree relatives), symptoms at presentation,
anti-islet cell antibody test results, body mass index
(BMI), HbA1c level, presence of co-morbidities
(non-alcoholic fatty liver disease, dyslipidaemia,
hypertension, and obstructive sleep apnoea),
presence of complications (MA, retinopathy, and
neuropathy), treatments received, and frequency
of blood glucose self-monitoring. Overweight and
obesity were defined using age- and sex-specific
cut-offs established by the International Obesity
Task Force, which predicted BMI values at 18 years
(25, 30, and 35 kg/m2) by the respective standard
deviations to define overweight, obesity and morbid
obesity, respectively; standard deviations of BMI
were calculated according to age- and sex-specific
reference data provided by the International Obesity
Task Force.7 In this study, weight loss was defined as
any decrease in BMI z-score, and improvement in
HbA1c level was defined as any decrease in HbA1c
level. Non-alcoholic fatty liver disease was defined
as an elevated alanine transferase level (based on
age- and sex-specific reference data) or compatible
ultrasound findings. Dyslipidaemia was defined as
an elevated low-density lipoprotein level of ≥2.6
mmol/L, a triglyceride level ≥1.7 mmol/L, or the
receipt of lipid-lowering agents. Hypertension was
defined as an elevated systolic blood pressure ≥95th
percentile for age, height, and sex—on at least two
occasions—or the receipt of anti-hypertensive
medication. Obstructive sleep apnoea was defined as
the presence of clinical symptoms indicating sleep-disordered
breathing, along with polysomnography
findings of obstructive apnoeas/hypopneas.
Microalbuminuria was defined as an elevated spot
urine albumin-creatinine ratio >2.5 mg/mmol for boys and >3.5 mg/mmol for girls in at least two
of three samples within a 6-month period, or as
the receipt of any treatment for MA. Retinopathy
(eg, non-proliferative and proliferative diabetic
retinopathy, as well as macula oedema) was identified
by digital fundus photography and confirmed via
referral to an ophthalmologist. Neuropathy was
clinically identified by the presence of symptoms
(numbness and paraesthesia) and through clinical
examinations including the 10-g monofilament
test, vibration sense assessment, and ankle reflex
evaluation. Suboptimal glycaemic control was
defined as HbA1c level ≥7%, as suggested by the
International Society for Paediatric and Adolescent
Diabetes Clinical Practice Consensus Guidelines.4
Data analysis
Statistical analyses were performed using SPSS
software (Windows version 23; IBM Corp,
Armonk [NY], United States). All available data
were included in the statistical analysis, and the
numbers of available values are listed in the tables.
Continuous variables, including age, HbA1c level,
and BMI z-score, were tested for normality using the
Shapiro–Wilk test. Data with skewed distributions
were expressed as medians and interquartile ranges
(IQRs), and comparisons were made using the
Mann-Whitney U test. Analyses of relationships
between two continuous variables were assessed by
Spearman rank correlation and expressed using the
Spearman correlation coefficient (ρ). Categorical
variables were expressed as exact numbers of
patients with percentages. Binary logistic regression
analysis was used to assess risk factors for suboptimal
glycaemic control at 2 years and MA at 2 years.
Univariate analyses were performed to determine
unadjusted odds ratios and 95% confidence
intervals. Multivariate analyses of factors associated
with suboptimal glycaemic control at 2 years were
performed while including HbA1c level at diagnosis
to adjust for initial disease severity. Multivariate
analyses of factors associated with MA at 2 years
were performed while including HbA1c level at 2
years to eliminate the effect of glycaemic control at 2
years; this approach was intended to independently
assess the effects of co-morbidities. Missing data
were not included in regression analyses. Statistical
tests were two-sided and were performed with a 5%
significance threshold (ie, alpha=0.05). The STROBE
(Strengthening the Reporting of Observational
Studies in Epidemiology) checklist for cohort studies
was used when reporting the study findings.
Results
Study population
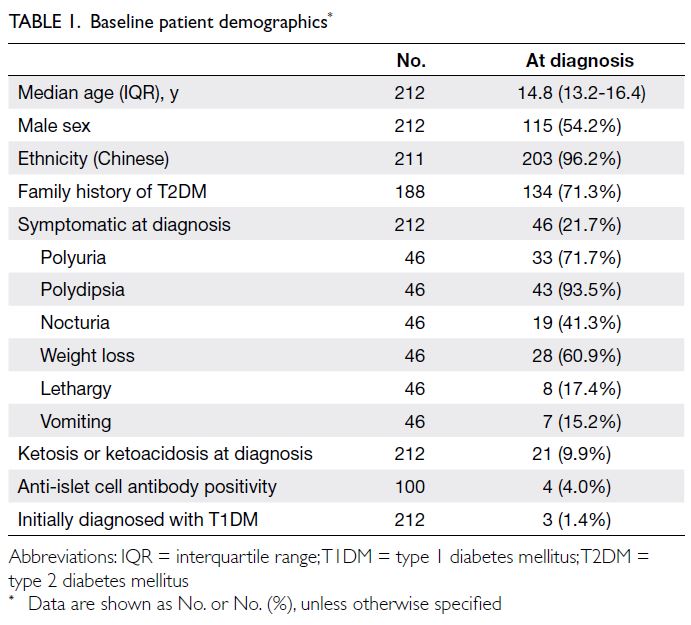
In total, 212 patients diagnosed with T2DM between 2014 and 2018 were recruited into the Registry. Their baseline demographics are summarised in Tables 1 and 2. Of these patients, 71.3% had a family history
of T2DM, and 21.7% were symptomatic at diagnosis
(Table 1). At 2 years after the diagnosis of DM,
143 patients (67.5%) continued attending follow-up
visits. There were no significant differences in
baseline characteristics between the groups with and
without follow-up at 2 years, except for a higher age
at diagnosis in the loss to follow-up group (online supplementary Table).
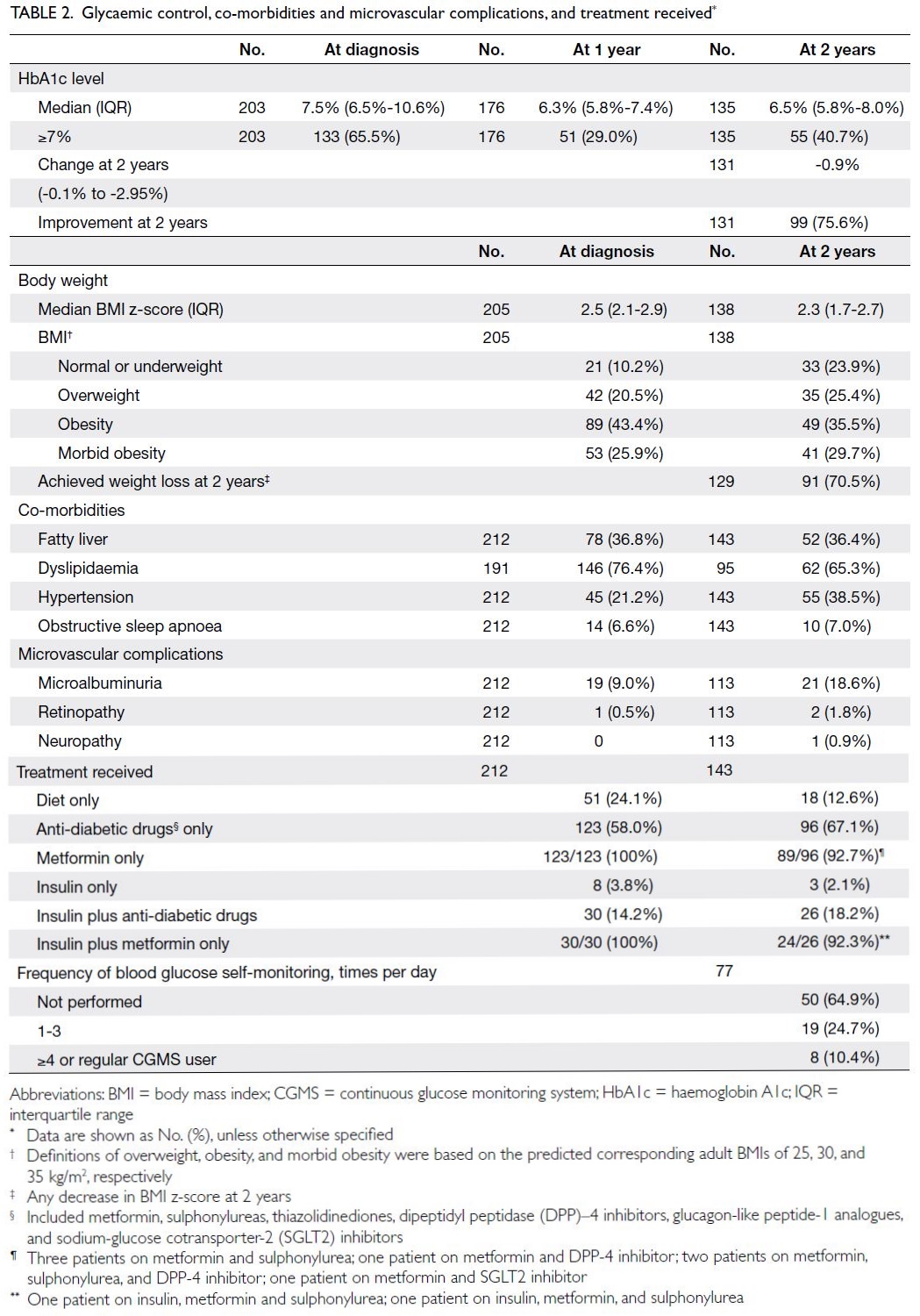
Glycaemic control
Haemoglobin A1c levels at diagnosis, 1 year after
diagnosis, and 2 years after diagnosis were available
for 203, 176, and 135 patients, respectively. The
median HbA1c levels at diagnosis, 1 year after
diagnosis, and 2 years after diagnosis were 7.5%
(IQR=6.5%-10.6%), 6.3% (IQR=5.8%-7.4%), and
6.5% (IQR=5.8%-8.0%), respectively; at these times,
65.5%, 29.0%, and 40.7% of patients had suboptimal
glycaemic control (ie, HbA1c level ≥7%), respectively
[Table 2].
Co-morbidities
There was an overall improvement in BMI z-score at 2
years after diagnosis (median BMI z-score decreased
from 2.5 at diagnosis to 2.3 at 2 years). Overall, 146 of
191 patients (76.4%) had dyslipidaemia at diagnosis,
whereas 62 of 95 patients (65.3%) had dyslipidaemia
at 2 years. However, more patients had hypertension at 2 years—the number increased from 45 of 212 patients (21.2%) at diagnosis to 55 of 143 patients (38.5%) at 2 years (Table 2).
Microvascular complications
Overall, 21 of 113 (18.6%) patients screened at 2 years after diagnosis developed MA, compared with
9.0% at diagnosis. Two patients (1.8%) developed
retinopathy, whereas one patient (0.9%) developed
neuropathy, at 2 years after diagnosis (Table 2).
Treatments received and monitoring
At diagnosis, 24.1% of patients were not receiving any
pharmacological treatment, 58.0% were receiving
anti-diabetic drugs, and 18% required insulin. At 2
years, only 12.6% of patients were not receiving any
medications. The proportions of patients requiring
insulin were similar at diagnosis and 2 years (2.1%).
In total, 64.9% of patients did not perform daily
blood glucose self-monitoring (Table 2).
Factors affecting glycaemic control at 2 years
A higher initial HbA1c level was associated with
suboptimal glycaemic level at 2 years (correlation
coefficient=0.39, P<0.001; n=130). There were no
significant correlations of HbA1c level at 2 years
with age at diagnosis (correlation coefficient=0.02,
P=0.852; n=135), BMI z-score at diagnosis
(correlation coefficient=-0.10, P=0.277; n=133), or
BMI z-score at 2 years (correlation coefficient=0.04,
P=0.638; n=131). Greater decline in BMI z-score
was associated with a lower HbA1c level at 2 years
(correlation coefficient=-0.22, P=0.011; n=129).
However, there was no correlation between the
change in BMI z-score and the change in HbA1c
level (correlation coefficient <0.01, P=0.973; n=126).
Table 3 shows factors associated with
suboptimal glycaemic control at 2 years. The effect
of a family history of T2DM was not statistically
significant after adjustment for initial HbA1c
level (adjusted odds ratio [aOR]=2.29; P=0.075).
A similar result was observed regarding the effect
of symptomatic disease at diagnosis (aOR=2.01;
P=0.174) and weight loss (aOR=0.53; P=0.128). The
presence of fatty liver (aOR=2.50; P=0.021) and
dyslipidaemia (aOR=3.19; P=0.033) at 2 years were
associated with suboptimal glycaemic control at 2
years, even after adjustment for initial HbA1c level.
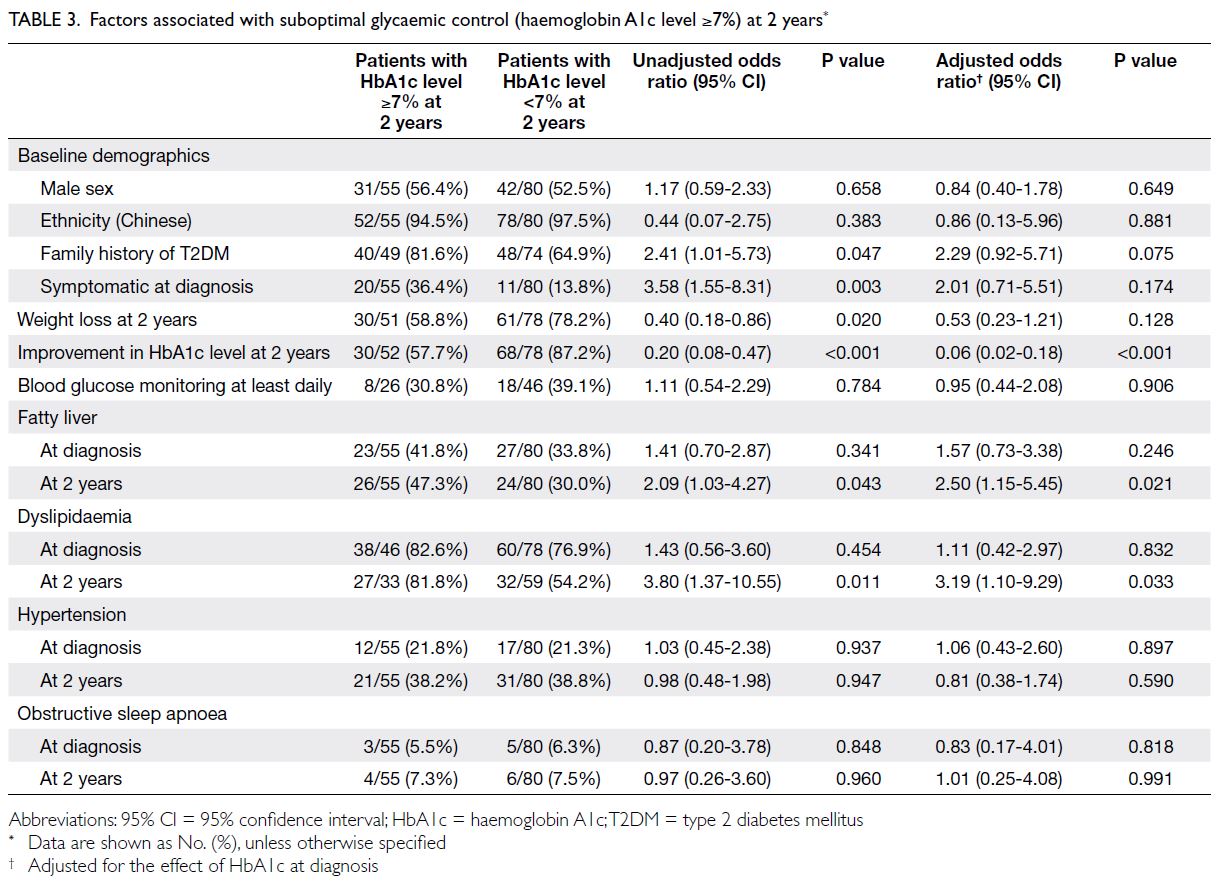
Table 3. Factors associated with suboptimal glycaemic control (haemoglobin A1c level ≥7%) at 2 years
Factors associated with the development of
microalbuminuria at 2 years
Patients with MA had higher HbA1c levels at 2 years
compared with patients who did not exhibit MA
(median HbA1c level: 7.2% vs 6.4%; P=0.037) [Table 4]. Dyslipidaemia at 2 years was associated with MA at 2 years in the univariate analysis (unadjusted odds ratio=5.51; P=0.030), but the effect did not remain statistically significant after adjusting for
glycaemic control at 2 years (Table 5). The presence
of hypertension at 2 years was a risk factor for MA at
2 years, independent of glycaemic control at 2 years
(aOR=4.61; P=0.008) [Table 5].
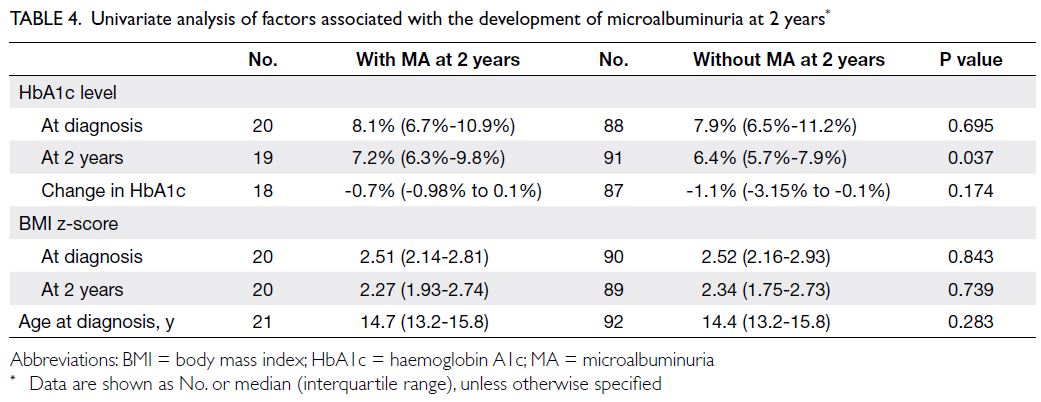
Table 4. Univariate analysis of factors associated with the development of microalbuminuria at 2 years
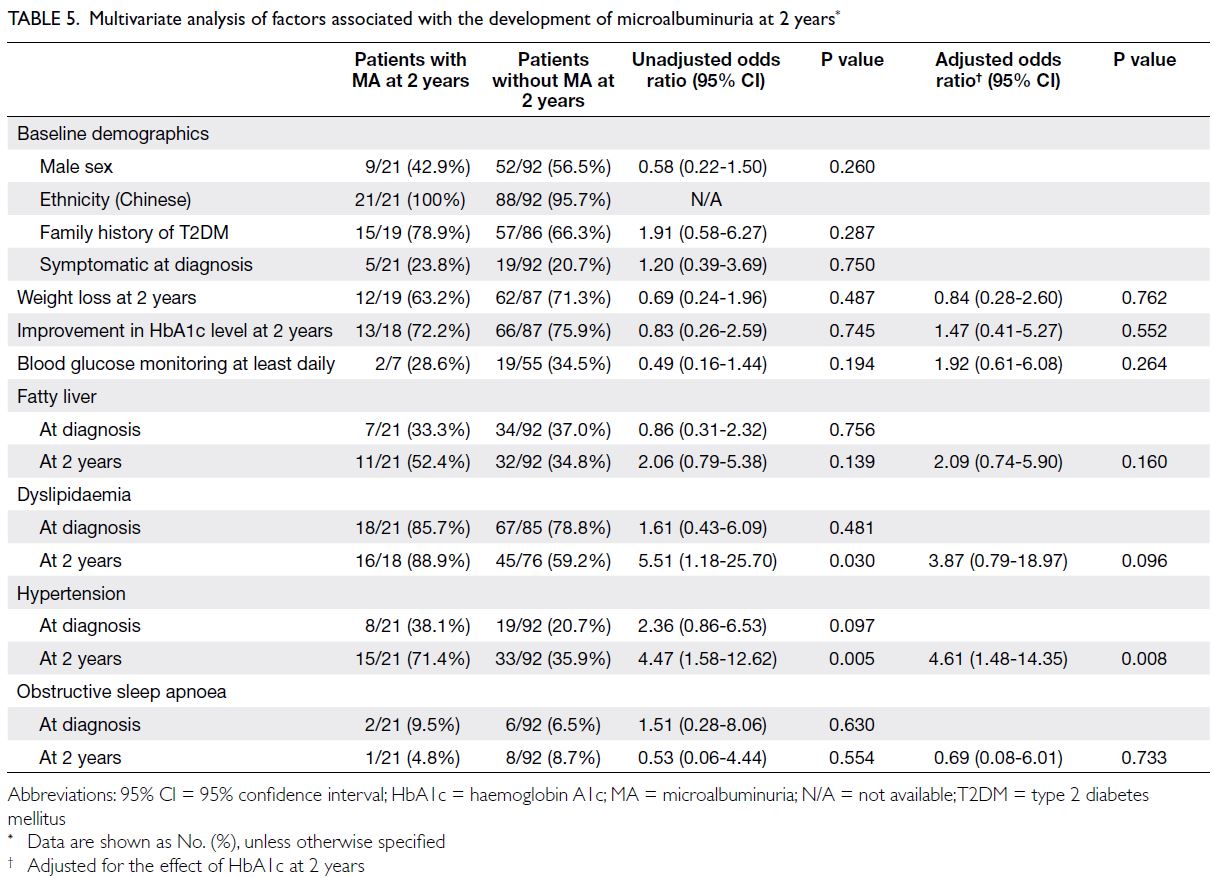
Table 5. Multivariate analysis of factors associated with the development of microalbuminuria at 2 years
Discussion
Glycaemic control
The results of this study provide insights into the
early post-diagnosis clinical course of T2DM among youth in Hong Kong. Nearly 60% of patients (59.3%)
in our cohort had optimal glycaemic control with
HbA1c level <7% at 2 years after diagnosis. Previous
studies regarding glycaemic control among youth
with T2DM showed variable results, presumably due
to heterogeneity in the study populations, follow-up
periods, and glycaemic targets.8 9 10 11 A clinical trial by
the TODAY Study Group8 followed up 234 youth
with DM, who were put on metformin and lifestyle
modifications and with initial HbA1c level <8%, for
3.86 years on average. It showed that 46.6% of youth
with DM exhibited loss of glycaemic control, defined
by HbA1c level >8%.8 In a study of 301 paediatric
T2DM patients with initial HbA1c level ≥7% in the
United States, Barr et al9 found that after 1 year,
37% of patients achieved optimal control (HbA1c
level ≤6.5%) and 58% achieved durable glycaemic
control (HbA1c level ≤8%). However, at 3 years,
only 26% of patients achieved HbA1c level ≤6.5%,
whereas 59% exhibited HbA1c level ≤8%.9 Candler et al10 followed 100 paediatric T2DM patients in the
United Kingdom; the median HbA1c level was 7%
after 1 year, and 38.8% of patients exhibited HbA1c
level <6.5%. Notably, no data regarding HbA1c levels at diagnosis were provided in the study.10 In
a study of 96 patients in Israel, Meyerovitch et al11
found that the median HbA1c level was 7.97%
after an average follow-up period of 3.11 years,
compared with 7.8% at diagnosis. Additionally,
>50% of patients required insulin at the end of the
follow-up period.11 Although our cohort appeared
to have better glycaemic control compared with the
previous studies, our patients might have had lower
initial HbA1c levels at diagnosis, considering that
most of them were asymptomatic (78.3%). Our study
also showed that patients with a higher initial HbA1c
level tended to have a persistently high HbA1c level
at 2 years. These findings emphasise the importance
of early diagnosis and treatment before patients
develop clinically significant hyperglycaemia,
which makes DM more difficult to control. Most
of our patients were overweight or obese (89.8%);
many of them also had co-morbidities including
hypertension, dyslipidaemia, and fatty liver (Table 2). Previous studies in Hong Kong showed a high
risk of metabolic syndrome (OR up to 32.2) in
overweight and obese children.12 13 Active screening
for metabolic syndrome would enable early diagnosis and treatment of DM and its co-morbidities.
Co-morbidities
Factors associated with suboptimal glycaemic
control were dyslipidaemia and fatty liver at 2 years
after diagnosis. A recent study showed that each 1%
increase in HbA1c level was associated with 13% and
20% increases in the risks of abnormal triglyceride
and low-density lipoprotein levels, respectively.14
The importance of weight loss has been emphasised
in various guidelines, for example, The American
Diabetes Association recommends weight loss of at
least 5% in adult overweight or obese DM patients.15
However, a specific weight loss target cannot be
established in growing children. The study by
Candler et al10 regarding youth with T2DM showed
that each one-unit increase in BMI z-score was
associated with a 34.9% increase in HbA1c level.
Although the present study indicated that a greater
drop in BMI z-score was associated with lower
HbA1c level at 2 years, the association between
weight loss and prevention of suboptimal glycaemic
control at 2 years was not significant after adjustment
for initial HbA1c level.
Microvascular complications
Diabetic retinopathy and neuropathy were rare
among youth with T2DM. However, the proportion
of patients with MA increased from 9.0% at diagnosis
to 18.6% at 2 years (Table 2). Previous studies
regarding the prevalence of diabetic complications
in youth have shown mixed results, probably due to
genetic variation and differences in DM duration.
High prevalences have been observed in cohorts with
long DM durations.16 The MA prevalence has been
approximately 20% to 30% in most studies of youth
with a short duration of T2DM. In the SEARCH for
Diabetes in Youth study, the MA prevalence in youth
with T2DM was 22.2%, and the average duration of
disease was 1.9 years.17 In an Australian population,
Eppens et al18 found that 28% of patients had MA,
with a median disease duration of 1.3 years. Candler
et al10 showed that the MA prevalence increased
from 4.2% to 16.4% within 1 year after diagnosis. Our
cohort showed a similar prevalence compared with
other cohorts. Nevertheless, the increasing trend
is concerning, particularly because MA has been
identified as an independent predictor of mortality
risk in adults.19 Thus, we conducted further analysis
of risk factors for MA, revealing the associations
of higher HbA1c level and hypertension at 2 years,
consistent with the SEARCH for Diabetes in Youth
study.17 The deleterious effects of hypertension on
the kidneys explain the additional increase in MA
risk, independent of glycaemic control.
Strengths and limitations
Strengths of this study included its provision of insights regarding the early outcomes of youth
with T2DM in Hong Kong, particularly concerning
glycaemic control and associated factors. First, our
findings supported the implementation of active
screening in overweight and obese individuals
to allow early diagnosis of DM, considering the
high prevalence of overweight or obesity and the
relationship of lower initial HbA1c level with better
glycaemic control at 2 years. Second, our findings
indicated that the presence of co-morbidities
at 2 years, rather than baseline, was associated
with suboptimal glycaemic control and MA,
demonstrating the reversibility of the risk factors
and highlighting the importance of co-morbidity
management. Third, our study identified challenges in
managing youth with T2DM, including a high loss to
follow-up rate (n=69, 23.5%) [online supplementary Table], suboptimal glycaemic control in >40% of
patients at 2 years, infrequent blood glucose self-monitoring
by the patients, and increasing trends in
MA and hypertension.
Indeed, the high loss to follow-up rate was a
major limitation of our study. Many patients did not
return for clinical assessment or were transferred to
an adult endocrinology clinic. Although the loss to
follow-up group had a higher age at diagnosis (online supplementary Table), this presumably did not have a
substantial impact on the results because age was not
a significant risk factor for poor glycaemic control or
the likelihood of MA onset. Although a high loss to
follow-up rate is a common phenomenon in studies
of children with T2DM,20 this obstacle hindered
the achievement of good glycaemic control and
prevention of complications. It also created difficulty
in acquiring long-term follow-up data. Another
limitation was that our patients were managed by
different doctors in different hospitals; there remains
no standardised protocol for the management of
paediatric T2DM patients in Hong Kong, which may
be a confounding factor for multicentre studies such
as ours.
Conclusion
Approximately 60% of youth with T2DM in Hong
Kong achieved HbA1c level <7% at 2 years after
diagnosis. A higher HbA1c level at diagnosis was
associated with worse glycaemic control at 2 years.
The presence of dyslipidaemia and fatty liver at 2 years
were factors associated with suboptimal glycaemic
control. Overall, 18.6% of patients developed MA
at 2 years; other microvascular complications were
rare. These results highlight the importance of early
diagnosis and holistic management, including co-morbidity
management. The high loss to follow-up
rate, high proportion of patients with suboptimal
glycaemic control, and increasing number of
patients with MA and hypertension are ongoing
challenges in the management of youth with DM. The establishment of a standardised protocol may
improve outcomes in our patient population. Future
research could include studies regarding the effects
of insulin resistance and beta-cell function on
metabolic outcomes in youth with DM.
Author contributions
Concept or design: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: WI Yam, SMY Wong.
Drafting of the manuscript: WI Yam.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: WI Yam, SMY Wong.
Drafting of the manuscript: WI Yam.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The research was conducted as a part of the Hong Kong
Childhood Diabetes Registry, which was approved by the
Kowloon Central / Kowloon East Cluster Research Ethics
Committee of Hospital Authority, Hong Kong (Ref No.: KC/KE-16-0087/ER-3). Written informed consent was obtained
from parents for patients aged <16 years and from both
parents and patients for patients aged between ≥16 and <18 years.
Supplementary material
The supplementary material was provided by the authors and
some information may not have been peer reviewed. Accepted
supplementary material will be published as submitted by the
authors, without any editing or formatting. Any opinions
or recommendations discussed are solely those of the
author(s) and are not endorsed by the Hong Kong Academy
of Medicine and the Hong Kong Medical Association.
The Hong Kong Academy of Medicine and the Hong Kong
Medical Association disclaim all liability and responsibility
arising from any reliance placed on the content.
References
1. Lawrence JM, Divers J, Isom S, et al. Trends in prevalence
of type 1 and type 2 diabetes in children and adolescents in
the US, 2001-2017. JAMA 2021;326:717-27. Crossref
2. Haynes A, Kalic R, Cooper M, Hewitt JK, Davis EA.
Increasing incidence of type 2 diabetes in indigenous and
non-indigenous children in Western Australia, 1990-2012.
Med J Aust 2016;204:303. Crossref
3. Wu H, Patterson CC, Zhang X, et al. Worldwide estimates
of incidence of type 2 diabetes in children and adolescents
in 2021. Diabetes Res Clin Pract 2022;185:109785. Crossref
4. Shah AS, Zeitler PS, Wong J, et al. ISPAD Clinical Practice Consensus Guidelines 2022: type 2 diabetes in children and adolescents. Pediatr Diabetes 2022;23:872-902. Crossref
5. TODAY Study Group; Bjornstad P, Drews KL, et al. Long-term complications in youth-onset type 2 diabetes. N Engl J Med 2021;385:416-26. Crossref
6. Tung JY, Kwan EY, But BW, et al. Incidence and clinical
characteristics of pediatric-onset type 2 diabetes in Hong
Kong: the Hong Kong Childhood Diabetes Registry 2008
to 2017. Pediatr Diabetes 2022;23:556-61. Crossref
7. Cole TJ, Lobstein T. Extended international (IOTF) body
mass index cut-offs for thinness, overweight and obesity.
Pediatr Obes 2012;7:284-94. Crossref
8. TODAY Study Group; Zeitler P, Hirst K, et al. A clinical
trial to maintain glycemic control in youth with type 2
diabetes. N Engl J Med 2012;366:2247-56. Crossref
9. Barr MM, Aslibekyan S, Ashraf AP. Glycemic control and
lipid outcomes in children and adolescents with type 2
diabetes. PLoS One 2019;14:e0219144. Crossref
10. Candler TP, Mahmoud O, Lynn RM, Majbar AA,
Barrett TG, Shield JP. Treatment adherence and BMI
reduction are key predictors of HbA1c 1 year after
diagnosis of childhood type 2 diabetes in the United
Kingdom. Paediatr Diabetes 2018;19:1393-9. Crossref
11. Meyerovitch J, Zlotnik M, Yackobovitch-Gavan M,
Phillip M, Shalitin S. Real-life glycemic control in children
with type 2 diabetes: a population-based study. J Paediatr
2017;188:173-80.e1. Crossref
12. Ozaki R, Qiao Q, Wong GW, et al. Overweight, family
history of diabetes and attending schools of lower
academic grading are independent predictors for metabolic
syndrome in Hong Kong Chinese adolescents. Arch Dis
Child 2007;92:224-8. Crossref
13. Kong AP, Ko GT, Ozaki R, Wong GW, Tong PC, Chan JC.
Metabolic syndrome by the new IDF criteria in Hong Kong
Chinese adolescents and its prediction by using body mass
index. Acta Paediatr 2008;97:1738-42. Crossref
14. Brady RP, Shah AS, Jensen ET, et al. Glycemic control is
associated with dyslipidemia over time in youth with type 2
diabetes: the SEARCH for Diabetes in Youth study. Pediatr
Diabetes 2021;22:951-9. Crossref
15. American Diabetes Association Professional Practice
Committee. 8. Obesity and weight management for the
prevention and treatment of type 2 diabetes: standards
of medical care in diabetes—2022. Diabetes Care
2022;45(Supp 1):S113-24. Crossref
16. Amutha A, Mohan V. Diabetes complications in childhood
and adolescent onset type 2 diabetes—a review. J Diabetes
Complications 2016;30:951-7. Crossref
17. Maahs DM, Snively BM, Bell RA, et al. Higher prevalence
of elevated albumin excretion in youth with type 2 than
type 1 diabetes: the SEARCH for Diabetes in Youth study.
Diabetes Care 2007;30:2593-8. Crossref
18. Eppens MC, Craig ME, Cusumano J, et al. Prevalence of
diabetes complications in adolescents with type 2 compared
with type 1 diabetes. Diabetes Care 2006;29:1300-6. Crossref
19. Chronic Kidney Disease Prognosis Consortium;
Matsushita K, van der Velde M, et al. Association of
estimated glomerular filtration rate and albuminuria
with all-cause and cardiovascular mortality in general
population cohorts: a collaborative meta-analysis. The
Lancet 2010;375:2073-81. Crossref
20. Shoemaker A, Cheng P, Gal RL, et al. Predictors of loss to
follow-up among children with type 2 diabetes. Horm Res
Paediatr 2017;87:377-84. Crossref