Hong Kong Med J 2024 Aug;30(4):281–90 | Epub 16 Jul 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Changes in the epidemiology and clinical manifestations of human immunodeficiency virus–associated tuberculosis in Hong Kong
Alan CK Chan, MRCP, FHKAM (Medicine)1; SS Huang, FHKCP, FHKAM (Medicine)1; KH Wong, FFPH, FHKAM (Medicine)2; CC Leung, FFPH, FHKAM (Medicine)3; MP Lee, MB, BS, FHKAM (Medicine)4; TY Tsang, MSc (LON), FRCP (Lond)5; WS Law, FHKCP, FHKAM (Medicine)1; LB Tai, MRCP, FHKAM (Medicine)1
1 Tuberculosis and Chest Service, Department of Health, Hong Kong SAR, China
2 Special Preventive Programme, Department of Health, Hong Kong SAR, China
3 Hong Kong Tuberculosis, Chest and Heart Diseases Association, Hong Kong SAR, China
4 Department of Medicine, Queen Elizabeth Hospital, Hong Kong SAR, China
5 Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong SAR, China
Corresponding author: Dr Alan CK Chan (chikuen_chan@dh.gov.hk)
Abstract
Introduction: Human immunodeficiency virus
(HIV)–associated tuberculosis (TB) remains an
important health challenge worldwide. Although
TB prevalence has decreased in the general
population, there is limited information regarding
temporal trends in the incidence of HIV-associated
TB in Hong Kong. There are also insufficient data
regarding changes in clinical manifestation patterns
among HIV-associated TB patients over time. This
study aimed to describe temporal trends in the
epidemiology and clinical manifestations of HIV-associated
TB in Hong Kong.
Methods: We retrospectively reviewed data
regarding HIV-associated TB patients that were
reported to the TB-HIV Registry of the Department
of Health during the period 2007 to 2020. Trends
of TB as a primary acquired immunodeficiency
syndrome (AIDS)–defining illness, as well as changes
in demographic features and clinical manifestations
of HIV-associated TB during this period were
examined using Cochran–Armitage trend test.
Results: A decreasing trend was observed in the
proportion of all reported cases of AIDS in which
TB was a primary AIDS-defining illness during the
study period. The proportions of female patients
and patients with extrapulmonary involvement
significantly increased, whereas the proportions
of ever-smokers and patients with sputum smear
positivity significantly decreased during the same
period. A decreasing trend was observed in the
proportion of patients with pulmonary TB in
which the lower zone was the predominant site of
lung parenchymal lesions. Among patients with a diagnosis of HIV infection before TB, an increasing
trend was observed in the proportion of patients
receiving antiretroviral therapy.
Conclusion: Important temporal changes
were observed in the epidemiology and clinical
manifestations of HIV-associated TB. These results
highlight the need for continued surveillance
regarding the patterns of demographic features
and clinical manifestations to inform policymakers
when planning control strategies for HIV-associated
TB.
New knowledge added by this study
- Tuberculosis (TB) has assumed a less important role as a primary acquired immunodeficiency syndrome–defining illness in Hong Kong over the 14 years of the study period.
- Significant temporal changes were observed in the clinical manifestations of human immunodeficiency virus (HIV)–associated TB.
- Knowledge of the changing patterns of demographic features and clinical manifestations will help policymakers plan control strategies for HIV-associated TB.
- Recognition of changes in clinical manifestations will also help optimise TB and HIV management and improve treatment outcome.
Introduction
The advent of highly active antiretroviral therapy
(HAART) in 1996 led to a substantial decrease in the
incidence of opportunistic infections among people
living with human immunodeficiency virus (HIV)
in many regions.1 2 3 Nonetheless, HIV-associated
tuberculosis (TB) remains an important global
health challenge. The World Health Organization
estimated that 10.6 million people were living with
TB worldwide in 2021, 6.7% of whom were living
with HIV.4 In the same year, 1.6 million people died
of TB, including 187 000 people who were living with
HIV.4 The burden of HIV-associated TB considerably
varies across countries and regions.5 6 The prevalence
of HIV-associated TB in individual areas reportedly
changes over time.7 8 9 Awareness of changes in the
epidemiology and clinical manifestations of patients
with HIV-associated TB can help policymakers
formulate timely relevant prevention and control
measures. It can also help improve treatment
outcomes for patients with HIV-associated TB.
In Hong Kong, the TB case notification rate has
exhibited an overall decreasing trend over the past
few decades.10 In 2021, the provisional number of
TB cases reported to the Department of Health was
3741.11 The corresponding TB notification rate was 50.5 per 100 000 inhabitants, a substantial decrease
from 84.1 per 100 000 inhabitants in 2006.11 The
overall prevalence of HIV infection in the general
adult population has remained low (<0.1%).12 The
epidemiology and clinical manifestations of HIV-associated
TB during the period 1996 to 2006 in
Hong Kong have been reported.13 Notably, the
report showed that TB had become an increasingly
important acquired immunodeficiency syndrome
(AIDS)–defining illness in Hong Kong, surpassing
Pneumocystis jirovecii pneumonia as the most
common primary AIDS-defining illness in 2005; the
two illnesses represented 39% and 31% of all such
illnesses, respectively, in 2005.13 The presentation of
HIV-associated TB is often atypical.13 Considering
the declining prevalence of TB in the general
population and accompanying decrease in TB
transmission in Hong Kong, the implementation
of strategies to enhance screening for latent TB
infection, the increased use of molecular tests for TB
diagnosis, and the expansion of HAART in recent
years, we conducted a retrospective review of data
regarding patients with HIV-associated TB that were
reported to the TB-HIV Registry of the Department
of Health during the period 2007 to 2020. We aimed
to identify temporal trends in the epidemiology and
clinical manifestations of HIV-associated TB during
that period.
Methods
We retrospectively reviewed data contained within
the TB-HIV Registry, which captured information
regarding nearly all cases of HIV-associated TB
diagnosed in the Tuberculosis and Chest Service
and Special Preventive Programme (SPP) under
the Department of Health, as well as cases referred
from regional hospitals of the Hong Kong Hospital
Authority, during the period 2007 to 2020. The
details of data contained in the TB-HIV Registry,
along with the criteria for TB as a primary AIDS-defining
illness, were described in a previous
report.13 There have been no changes in the criteria
for TB as a primary AIDS-defining illness since the
last report. Where necessary, both clinic records
and hospital discharge records were reviewed. All
data were imported into Epi Info14 and exported to
statistical software SPSS (Windows version 26.0;
IBM Corp, Armonk [NY], US) for analysis. The
Cochran–Armitage trend test in XLSTAT software
(Lumivero, Denver [CO], US) was used to identify
trends in the proportion of reported AIDS cases
with TB as a primary AIDS-defining illness during
the study period. The Cochran–Armitage trend test
was also used to examine changes in demographic
features and clinical manifestations during the
same period. Where relevant, we compared the
demographic features of patients reported to the
TB-HIV Registry during the study period with the features of a historical cohort from the period 1996
to 200613 using the Chi squared test. P values <0.05
were considered statistically significant.
This study was an extension of a previous
study designed to evaluate the public health
programme for HIV-associated TB in Hong Kong15;
it did not constitute research on human participants.
Throughout the review process, we implemented all
reasonable precautions to protect the confidentiality
of personal data and excluded personally identifiable
information from the electronic database.
Results
Tuberculosis as a primary acquired immunodeficiency syndrome–defining illness
All 390 cases reported to the TB-HIV Registry from
1 January 2007 to 31 December 2020 were included
in this retrospective analysis. Information about
whether TB constituted a primary AIDS-defining
illness was available for 363 of 390 (93.1%) patients,
where TB was listed as a primary AIDS-defining
illness in 251 of those cases (69.1%). Overall, TB
as a primary AIDS-defining illness represented a
decreasing trend of 18.3% of 1375 reported AIDS
cases during the period 2007 to 202012 (Cochran–Armitage trend test, P<0.001) [Fig], compared with
28.2% (192/680; Chi squared test, P<0.001) among
the historical cohort of patients reported during the
period 1996 to 2006.13
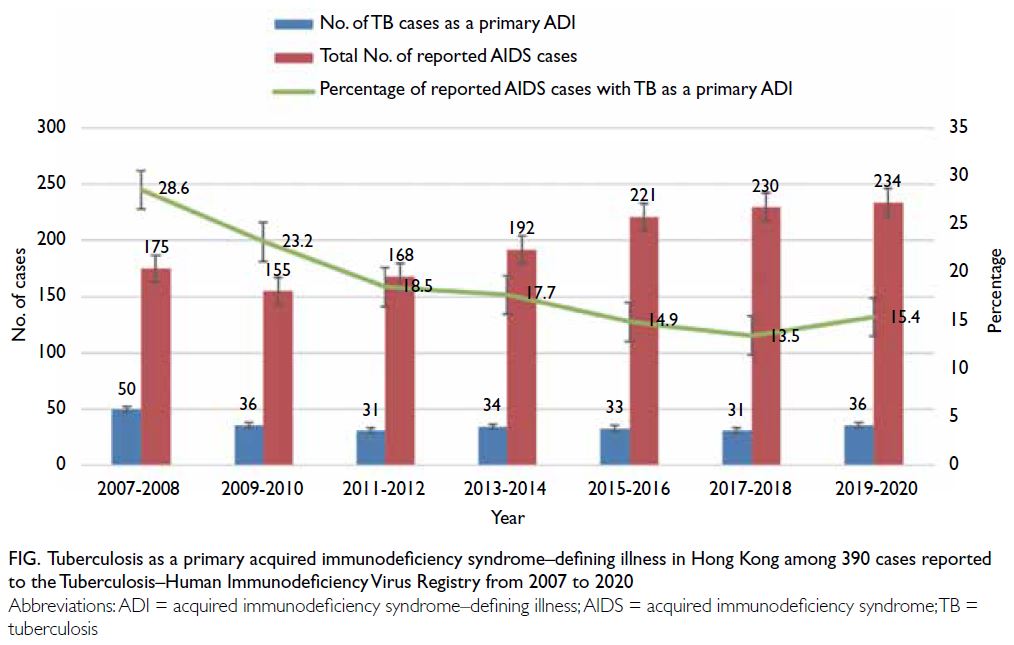
Figure. Tuberculosis as a primary acquired immunodeficiency syndrome–defining illness in Hong Kong among 390 cases reported to the Tuberculosis–Human Immunodeficiency Virus Registry from 2007 to 2020
Demographic features
Trends in demographic features, including age, sex,
case category, ethnicity, residence, primary source
of care (first presentation), and smoking status,
for the 390 HIV-associated TB cases reported to
the TB-HIV Registry during the study period are
shown in Table 1. The proportion of female patients
significantly increased whereas that of ever-smokers
significantly decreased (Cochran–Armitage trend
test, P=0.035 and P=0.029, respectively). No
significant trends were detected in other variables
examined. Additionally, the proportions of Chinese
individuals and permanent residents were lower in
the present cohort than in the historical cohort of
1996 to 200613 (260/390, 66.7% vs 152/190, 80.0%;
Chi squared test, P=0.001 and 258/390, 66.2%
vs 144/190, 75.8%; Chi squared test, P=0.018,
respectively). The proportion of female patients was
significantly higher in the present cohort than in the
historical cohort13 (82/390, 21.0% vs 21/190, 11.1%; Chi squared test, P=0.003).
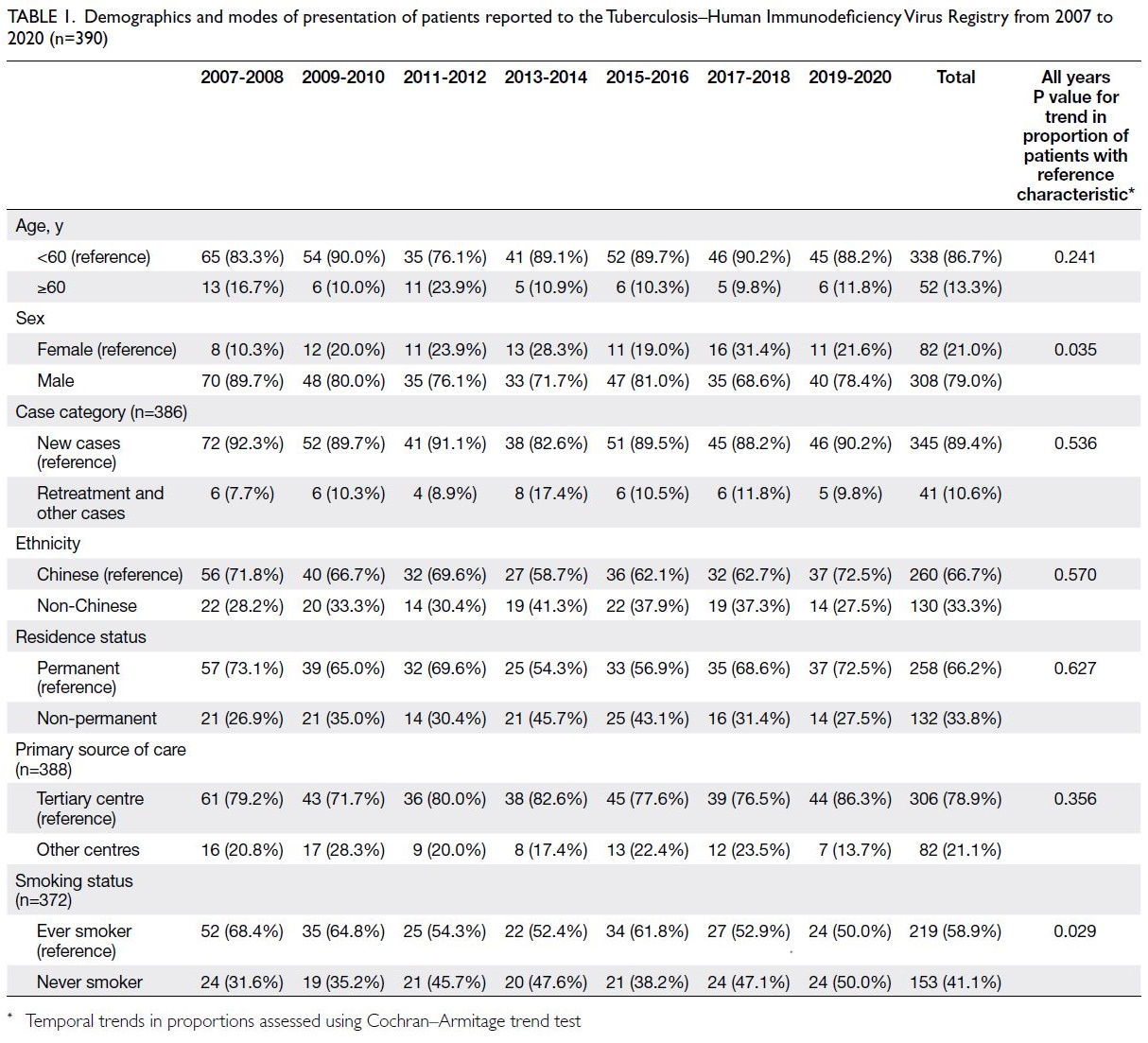
Table 1. Demographics and modes of presentation of patients reported to the Tuberculosis–Human Immunodeficiency Virus Registry from 2007 to 2020 (n=390)
Clinical manifestations
Trends in clinical manifestations, including
symptoms, presence of pulmonary TB, radiographic
features (for cases with abnormalities on chest
radiographs), presence of extrapulmonary TB
(EPTB), most common EPTB sites, sputum smear
positivity status, drug susceptibility patterns,
presence of TB risk factors, CD4 cell count at TB diagnosis, presence of other AIDS-defining illnesses
at the time of co-infection, and antiretroviral therapy
(ART) status (among patients with a diagnosis of
HIV infection before TB), among the 390 TB cases
are presented in Tables 2 and 3. The proportions
of patients presenting with site-specific symptoms
(other than chest-related symptoms) and with EPTB
both significantly increased during the period 2007
to 2020 (Cochran–Armitage trend test, P=0.029 and
P=0.008, respectively) [Table 2]. The most common
EPTB sites were lymph nodes (42.8%), pleura
(21.5%), and abdomen (13.8%). Among patients who underwent sputum smear tests, the proportion of
patients with sputum smear positivity significantly
decreased (Cochran–Armitage trend test, P=0.006)
[Table 2]. Among patients with lung parenchymal
lesions on chest radiographs, a decreasing trend
was observed in the proportion of patients in which
the lower zone was the predominant lesion site
(Cochran–Armitage trend test, P=0.045) [Table 3].
Among patients with a diagnosis of HIV infection
before TB, the proportion of patients receiving ART
at TB diagnosis significantly increased (Cochran–Armitage trend test, P=0.003) [Table 2].
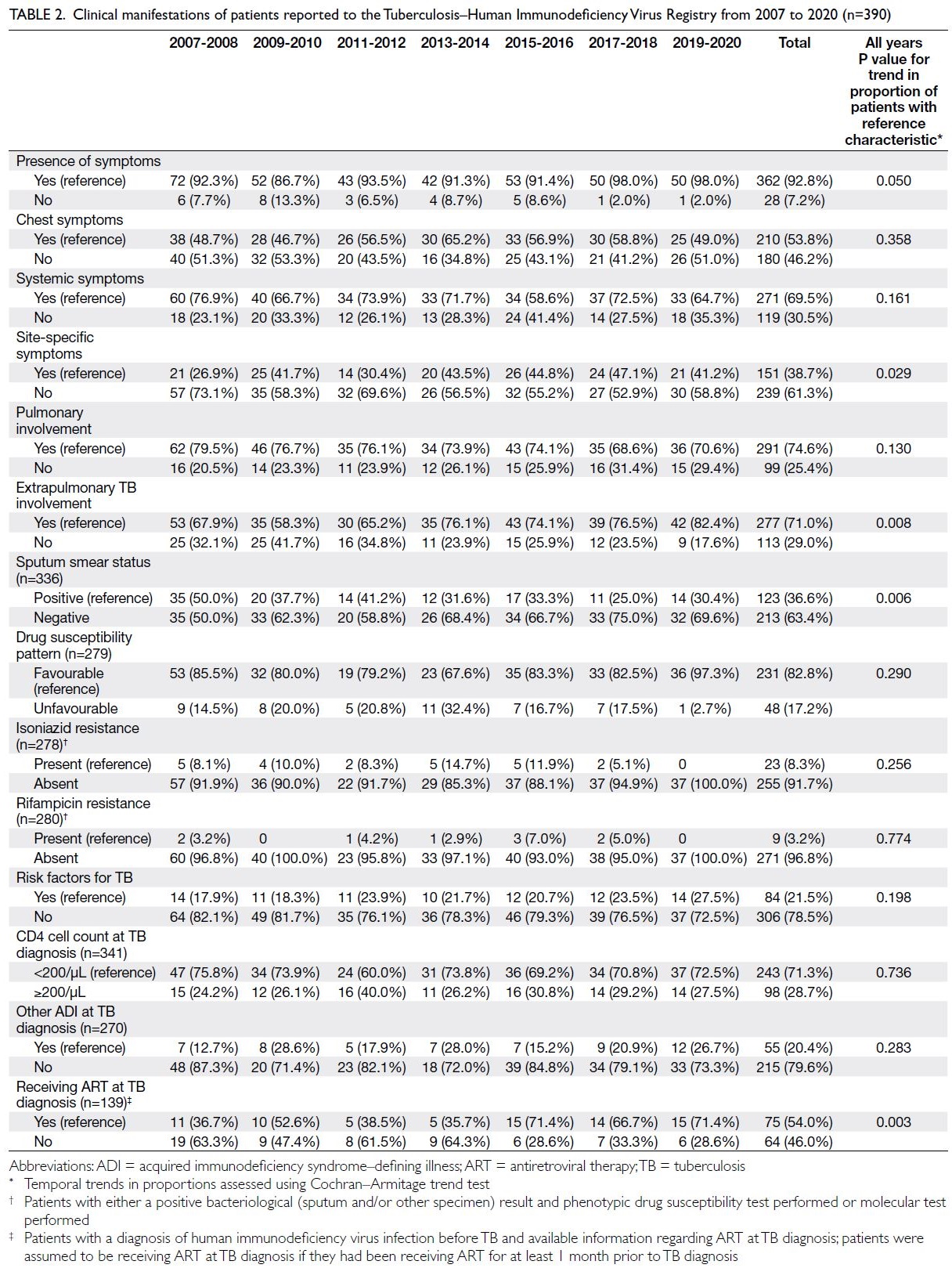
Table 2. Clinical manifestations of patients reported to the Tuberculosis–Human Immunodeficiency Virus Registry from 2007 to 2020 (n=390)
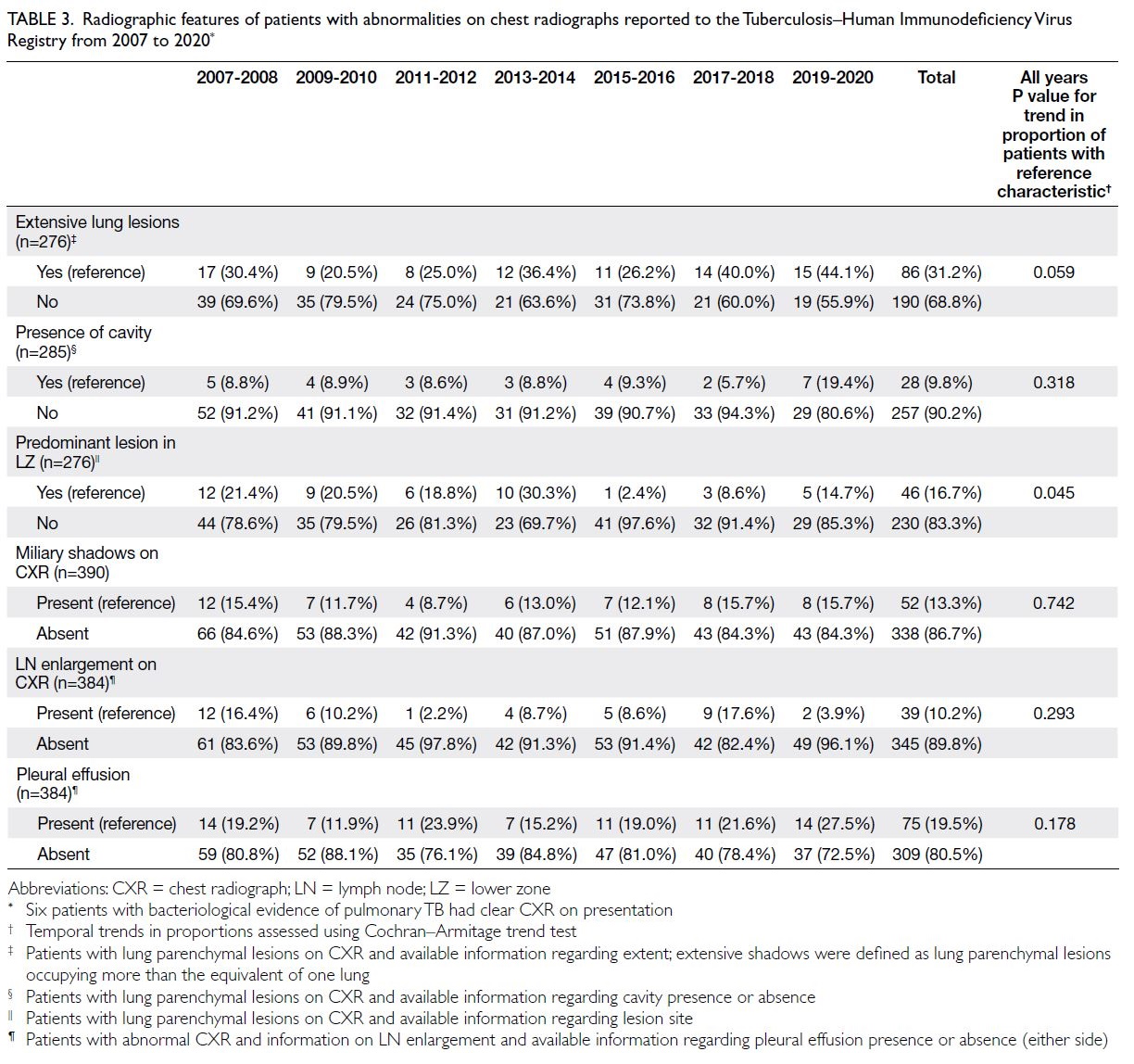
Table 3. Radiographic features of patients with abnormalities on chest radiographs reported to the Tuberculosis–Human Immunodeficiency Virus Registry from 2007 to 2020
Discussion
This study revealed a decreasing trend in the
proportion of reported AIDS cases with TB as a
primary AIDS-defining illness during the period
2007 to 2020. The overall proportion (18.3%) was also
lower than the proportion (28.2%) in the historical
cohort of cases reported during the period 1996
to 2006.13 The proportions of Chinese individuals
and permanent residents were lower, whereas
the proportion of female patients was higher, in
our cohort compared with the historical cohort.13 The proportions of female patients and patients
with extrapulmonary involvement significantly
increased, whereas the proportions of ever-smokers
and the proportion with sputum smear positivity
among pulmonary TB cases significantly decreased
during the period 2007 to 2020. A decreasing
trend was observed in the proportion of patients
with pulmonary TB in which the lower zone was
the predominant site of lung parenchymal lesions.
Among patients with a diagnosis of HIV infection
before TB, an increasing trend was observed in the
proportion of patients receiving ART.
Decreasing trend of tuberculosis as an
acquired immunodeficiency syndrome–defining illness
In Hong Kong, TB is considered an AIDS-defining
illness when the disease is extrapulmonary.
Pulmonary TB and cervical lymph node TB are
considered AIDS-defining illnesses only when
the CD4 cell count at the time of TB diagnosis is
<200/μL, as recommended by the Scientific
Committee of the Advisory Council on AIDS in
1994.16 Since then, there have been no changes in the
criteria for TB as an AIDS-defining illness among
individuals infected with HIV. The decreasing trend
in Hong Kong regarding the proportion of reported
AIDS cases with TB as a primary AIDS-defining illness
might be due to decreased community transmission
of TB through better TB control, the expansion of
HAART since its introduction in 1997, and (perhaps
to a lesser extent) increased acceptance of testing
for latent TB infection and preventive treatment
for TB among HIV-infected individuals since the
early 2000s. Similar decreasing trends related to
HAART-induced improvements in immune status
among HIV-infected individuals have also been
identified during studies conducted in some other
countries.7 9 17 In an observational, retrospective
study of AIDS cases included in the Barcelona AIDS
register between 1994 and 2005, decreases were
observed regarding the incidence of TB as an AIDS-defining
illness among both native and immigrant
populations.7 Another study examining trends in the
incidence of AIDS-defining opportunistic illnesses
over a 25-year period in Brazil showed a reduction
in TB incidence from 1991-1993 to 2009-2012.9 A
prospective cohort study of participants in the HIV
Outpatient Study at 12 HIV clinics within the US
indicated that TB incidence decreased after HAART
introduction.17 Conversely, among participants in
the HOMER cohort study (HAART Observational
Medical Evaluation and Research) conducted during
the period 1996 to 2007 in Canada, no statistically
significant trends were observed in the proportion of
cases with TB as the AIDS-defining illness, probably
because the small number of TB cases reported
in each time period limited the ability to detect
significant changes in the reported cases.18 Another
study examining AIDS notification data in Australia
during the period 1993 to 2000 revealed that the
proportion of AIDS cases with TB as the AIDS-defining
illness was higher during 1996 to 2000
(post-HAART era) than during 1993 to 1995 (pre-HAART era); the authors attributed this difference
to the increasing proportion of Australian patients
with AIDS who had been born in sub-Saharan Africa
and Asia during the 1990s, among whom the risk
of TB was considerably higher.19 Further studies
examining trends in TB as an AIDS-defining illness,
as well as location-specific factors that may influence such trends in the post-HAART era, are warranted
to facilitate control strategies for HIV-associated TB.
Changes in demographic features and their
implications
Further attention is needed regarding the observation
of higher proportions of non-Chinese individuals
(mostly Asians and Africans, who have much higher
TB incidence than the rate among Hong Kong
Chinese individuals) and non-permanent residents
of Hong Kong in the 2007-2020 cohort compared
with the historical 1996-2006 cohort. Similar
findings of higher incidences of AIDS-associated
TB in foreign-born populations from countries with
much higher TB incidence compared with the native
population have been reported on the basis of some
studies conducted in developed countries.7 20 21 These
observations highlight the need for TB screening
and prophylaxis for people living with HIV who were
born in countries with a high background prevalence of TB.
Intriguingly, we observed an increasing trend
in the proportion of women during the period 2007
to 2020. The reason for this increase is unclear; it
may be related to changes in the societal roles of
men and women that influence exposure risk. The
role of an increased proportion of EPTB (reportedly
associated with female sex and observed throughout
our cohort, as discussed below) requires further
investigation.
The proportion of ever-smokers significantly
decreased during the period 2007 to 2020, consistent
with the findings of some studies conducted in
the US.22 23 In an analysis of patients from a HIV
surveillance system in the US, the prevalence of
current smoking declined from 37.6% in 2009 to
33.6% in 2014.22 In another prospective cohort study
that examined smoking trends among HIV-positive
patients in the US, a decline in the annual prevalence
of current smoking from 1984 to 2012 was also
reported; however, disparities were noted according
to race, ethnicity, and education.23 Nonetheless,
because smoking increases HIV-related and non–HIV-related morbidity and mortality among people
living with HIV, smoking cessation interventions
remain an essential component of routine care for
such individuals.
Changes in clinical manifestations
The predominance of the lower zone as the site of
lung parenchymal lesions on chest radiographs
is a relatively common feature among patients
with HIV-associated pulmonary TB, according to
our previous report on the 1996 to 2006 cohort13
and some other reports.24 25 The present study
showed that the lower zone was less frequently
the predominant site of lung parenchymal lesions
during the period 2007 to 2020. The proportion of patients in the 2007 to 2020 cohort with the lower
zone as the predominant site (16.7%) was also
lower compared with the proportion of patients in
the historical 1996 to 2006 cohort (32.4%).13 This
difference may be related to the higher CD4 cell
count at TB diagnosis among patients in the current
cohort compared with patients in the historical
cohort (median CD4 cell counts at TB diagnosis:
100/μL and 78/μL, respectively). Nonetheless, lower
zone involvement was present in approximately one-sixth
of pulmonary TB cases reported during 2007
and 2020. A high index of suspicion is required for
the accurate and timely diagnosis of pulmonary TB
in people living with HIV.
A decreasing trend was observed in the
proportion of patients with sputum smear positivity
during the period 2007 to 2020. The overall
proportion of patients with sputum smear positivity
in the 2007 to 2020 cohort (36.6%) was also lower
than the proportion in the historical cohort of 1996
to 2006 (42.2%)13; it was similar to the proportion
identified during a population database study in
South Korea (36.4%).26 These results suggest that
TB cases have been diagnosed at increasingly
earlier stages due to enhanced active TB screening
efforts among people living with HIV, as well as the
increased use of molecular testing that enhanced the
diagnosis of smear-negative cases in Hong Kong.
These results highlight the need for continued TB
screening efforts and early detection of TB among
people living with HIV.
Our findings indicate that EPTB became more
common among HIV-associated TB patients during
the period 2007 to 2020. The overall proportion of
patients with EPTB was also higher in the present
cohort compared to that reported in a local study
that examined risk factors for mortality in an earlier
cohort (2006 to 2015) and that in the historical
1996 to 2006 cohort (71.0%, 64.9%,15 and 62.6%,13
respectively). These differences may have arisen
through enhanced diagnosis of EPTB with the
increased use of molecular testing in Hong Kong.
An increasing trend of extrapulmonary involvement
among TB patients has also been reported in some
other studies, although such studies are mostly
population-based.27 28 29 Few reports have been
published regarding temporal trends in EPTB
specifically among HIV-associated TB patients.30
Further studies are needed to examine these trends
and associated factors.
Strengths and limitations
Strengths of this study included its use of cohort
data from the TB-HIV Registry covering a relatively
long period (14 years) to study temporal changes
in the epidemiology and clinical manifestations
of HIV-associated TB. However, some limitations should be considered when interpreting the results
of this study. First, the TB-HIV Registry may not
capture all HIV-associated TB cases—some patients
encountered in the SPP were not referred to a chest
clinic but underwent anti-TB treatment in private
clinics or other countries. The total number of
HIV-associated TB cases in the HIV Surveillance
Report of SPP was approximately 10% higher than
the number in the TB-HIV Registry; the difference
mostly comprised non-permanent residents
temporarily staying in Hong Kong. Nonetheless, data
from the HIV Surveillance Report showed a similar
decreasing trend in TB as a primary AIDS-defining
illness during the study period (data not shown).
Second, this study utilised a retrospective design, and
data present in the database of the TB-HIV Registry
may be incomplete. Information regarding some
parameters such as case category, co-morbidities,
and CD4 cell count was unavailable for some
patients. To overcome this limitation, we traced and
reviewed relevant clinical records from chest clinics
and hospitals when necessary. Therefore, we expect
minimal bias due to missing data. Finally, the sample
size may have led to insufficient statistical power for
detecting temporal changes in some less common
parameters.
Conclusion
This study showed that TB has become less important
as a primary AIDS-defining illness in Hong Kong
over the 14 years of the study period. Nonetheless,
it remains the second most common primary
AIDS-defining illness after P jirovecii pneumonia.
Important temporal changes were also observed in
the patterns of demographic features and clinical
manifestations. Continued surveillance regarding
the patterns of demographic features and clinical
manifestations is needed to inform policymakers
during the formulation of TB control strategies to
improve patient care and treatment outcomes among
people living with HIV. This surveillance is especially
important in situations such as the coronavirus
disease 2019 era, during which resources from TB
programmes may be diverted to management of the
global pandemic.
Author contributions
Concept or design: ACK Chan, SS Huang.
Acquisition of data: ACK Chan, SS Huang.
Analysis or interpretation of data: ACK Chan, SS Huang.
Drafting of the manuscript: ACK Chan, SS Huang.
Critical revision of the manuscript for important intellectual content: KH Wong, CC Leung, MP Lee, TY Tsang, WS Law, LB Tai.
Acquisition of data: ACK Chan, SS Huang.
Analysis or interpretation of data: ACK Chan, SS Huang.
Drafting of the manuscript: ACK Chan, SS Huang.
Critical revision of the manuscript for important intellectual content: KH Wong, CC Leung, MP Lee, TY Tsang, WS Law, LB Tai.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank all of their colleagues in the Tuberculosis
and Chest Service and the Special Preventive Programme
of the Department of Health who provided assistance and
support to make this paper possible. The authors also thank Ms
Ida KY Mak, Research Officer at the Tuberculosis and Chest
Service of the Department of Health, for her dedicated efforts
in maintaining the Tuberculosis–Human Immunodeficiency
Virus Registry and assisting with the analysis.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This research was a retrospective analysis of observational
data routinely collected in a local Registry as part of the
ongoing evaluation of the public health programme for
human immunodeficiency virus–associated tuberculosis in
Hong Kong. Approval for the evaluation and exemption from
obtaining informed patient consent has been granted by the
Ethics Committee of the Department of Health of the Hong
Kong SAR Government (Ref No.: L/M 416/2017).
References
1. Low A, Gavriilidis G, Larke N, et al. Incidence of
opportunistic infections and the impact of antiretroviral
therapy among HIV-infected adults in low- and middleincome
countries: a systematic review and meta-analysis.
Clin Infect Dis 2016;62:1595-603. Crossref
2. B-Lajoie MR, Drouin O, Bartlett G, et al. Incidence and
prevalence of opportunistic and other infections and the
impact of antiretroviral therapy among HIV-infected
children in low- and middle-income countries: a systematic
review and meta-analysis. Clin Infect Dis 2016;62:1586-94. Crossref
3. Rubaihayo J, Tumwesigye NM, Konde-Lule J. Trends in
prevalence of selected opportunistic infections associated
with HIV/AIDS in Uganda. BMC Infect Dis 2015;15:187. Crossref
4. World Health Organization. Global Tuberculosis Report
2022. Geneva: World Health Organization; 2022. Available
from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022. Accessed 4 Jul 2024.
5. World Health Organization. WHO releases new global
lists of high-burden countries for TB, HIV-associated TB
and drug-resistant TB. 2021. Available from: https://www.who.int/news/item/17-06-2021-who-releases-new-global-lists-of-high-burden-countries-for-tb-hiv-associated-tb-and-drug-resistant-tb. Accessed 4 Jul 2024.
6. Benito N, Moreno A, Miro JM, Torres A. Pulmonary
infections in HIV-infected patients: an update in the 21st
century. Eur Respir J 2012;39:730-45. Crossref
7. Martin V, García de Olalla P, Orcau A, Caylà JA. Factors
associated with tuberculosis as an AIDS-defining disease
in an immigration setting. J Epidemiol 2011;21:108-13. Crossref
8. González-García A, Fortún J, Elorza Navas E, et al. The
changing epidemiology of tuberculosis in a Spanish tertiary
hospital (1995-2013). Medicine (Baltimore) 2017;96:e7219. Crossref
9. Coelho L, Cardoso SW, Amancio RT, et al. Trends in AIDS-defining
opportunistic illnesses incidence over 25 years in
Rio de Janeiro, Brazil. PLoS One 2014;9:e98666. Crossref
10. Centre for Health Protection, Department of Health, Hong Kong SAR Government. Annual Report. Tuberculosis and
Chest Service. 2020. Available from: https://www.info.gov.hk/tb_chest/doc/Annual_Report_2020.pdf. Accessed 16 Nov 2022.
11. Centre for Health Protection, Department of Health,
Hong Kong SAR Government. Notification & death rate
of tuberculosis (all forms), 1947-2022. Available from:
https://www.chp.gov.hk/en/statistics/data/10/26/43/88.html. Accessed 17 Nov 2022.
12. Centre for Health Protection, Department of Health, Hong
Kong SAR Government. HIV Surveillance Report—2020
Update. 2021. Available from: https://www.chp.gov.hk/
files/pdf/aids20.pdf. Accessed 16 Nov 2022.
13. Chan CK, Alvarez Bognar F, Wong KH, et al. The
epidemiology and clinical manifestations of human
immunodeficiency virus–associated tuberculosis in Hong
Kong. Hong Kong Med J 2010;16:192-8.
14. Centers for Disease Control and Prevention, United States
Government. Epi Info. Available from: https://www.cdc.gov/epiinfo/index.html. Accessed 4 Jul 2024.
15. Chan CK, Wong KH, Lee MP, et al. Risk factors associated
with 1-year mortality among patients with HIV-associated
tuberculosis in areas with intermediate tuberculosis burden
and low HIV prevalence. Hong Kong Med J 2018;24:473-83. Crossref
16. Scientific Committee of the Advisory Council on AIDS Hong Kong. Classification system for HIV infection and surveillance case definition for AIDS in adolescents and adults in Hong Kong. 1995. Available from: https://www.aids.gov.hk/pdf/g40.pdf. Accessed 16 Nov 2022.
17. Buchacz K, Baker RK, Palella FJ Jr, et al. AIDS-defining
opportunistic illnesses in US patients, 1994-2007: a cohort
study. AIDS 2010;24:1549-59. Crossref
18. Jafari S, Chan K, Aboulhosn K, et al. Trends in reported
AIDS defining illnesses (ADIs) among participants in a
universal antiretroviral therapy program: an observational
study. AIDS Res Ther 2011;8:31. Crossref
19. Dore GJ, Li Y, McDonald A, Ree H, Kaldor JM; National
HIV Surveillance Committee. Impact of highly active
antiretroviral therapy on individual AIDS-defining illness
incidence and survival in Australia. J Acquir Immune Defic
Syndr 2002;29:388-95. Crossref
20. Camoni L, Regine V, Boros S, Salfa MC, Raimondo M,
Suligoi B. AIDS patients with tuberculosis: characteristics
and trend of cases reported to the National AIDS Registry
in Italy—1993-2010. Eur J Public Health 2012;23:658-63. Crossref
21. Dore GJ, Li Y, McDonald A, Kaldor JM. Spectrum of AIDS-defining
illnesses in Australia, 1992 to 1998: influence of
country/region of birth. J Acquir Immune Defic Syndr
2001;26:283-90.
22. Frazier EL, Sutton MY, Brooks JT, Shouse RL, Weiser J.
Trends in cigarette smoking among adults with HIV
compared with the general adult population, United
States—2009-2014. Prev Med 2018;111:231-4. Crossref
23. Akhtar-Khaleel WZ, Cook RL, Shoptaw S, et al. Trends and
predictors of cigarette smoking among HIV seropositive
and seronegative men: the multicenter AIDS cohort study.
AIDS Behav 2016;20:622-32. Crossref
24. Affusim C, Abah V, Kesieme EB, et al. The effect of low
CD4+ lymphocyte count on the radiographic patterns
of HIV patients with pulmonary tuberculosis among
Nigerians. Tuberc Res Treat 2013;2013:535769. Crossref
25. Chamie G, Luetkemeyer A, Walusimbi-Nanteza M, et al.
Significant variation in presentation of pulmonary
tuberculosis across a high resolution of CD4 strata. Int J
Tuberc Lung Dis 2010;14:1295-302.
26. Kim JM, Kim NJ, Choi JY, Chin BS. History of acquired
immune deficiency syndrome in Korea. Infect Chemother
2020;52:234-44. Crossref
27. Ben Ayed H, Koubaa M, Marrakchi C, et al. Extrapulmonary
tuberculosis: update on the epidemiology, risk factors and
prevention strategies. Int J Trop Dis 2018;1:006.
28. Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR.
Epidemiology of extrapulmonary tuberculosis in the
United States, 1993-2006. Clin Infect Dis 2009;49:1350-7. Crossref
29. Sandgren A, Hollo V, van der Werf MJ. Extrapulmonary
tuberculosis in the European Union and European
Economic Area, 2002 to 2011. Euro Surveill 2013;18:20431. Crossref
30. Mohammed H, Assefa N, Mengistie B. Prevalence of
extrapulmonary tuberculosis among people living with
HIV/AIDS in sub-Saharan Africa: a systemic review and
meta-analysis. HIV AIDS (Auckl) 2018;10:225-37. Crossref

