Hong Kong Med J 2024 Apr;30(2):173–5 | Epub 16 Apr 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Multisystem inflammatory syndrome in adults in Hong Kong: two case reports
Abram JY Chan, MB, BS, FHKAM (Medicine)1; Judianna SY Yu, MB, BS, FHKAM (Medicine)2; Alwin WT Yeung, MB, BS, FRCP2; HP Shum, MB, BS, MD3; KC Lung, MB, BS, FRCP1
1 Department of Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
2 Department of Medicine and Geriatrics, Ruttonjee and Tang Shiu Kin Hospitals, Hong Kong SAR, China
3 Department of Intensive Care, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
Corresponding author: Dr Abram JY Chan (cjy548@ha.org.hk)
Case presentations
Case 1
A 51-year-old Chinese woman presented to Pamela
Youde Nethersole Eastern Hospital on 29 September
2022 with a 1-week history of intermittent fever and
confusion. She enjoyed good past health and had
received three doses of Comirnaty vaccine with the
last dose administered on 18 February 2022. She was
first symptomatic and with a positive rapid antigen
test for coronavirus disease 2019 (COVID-19) on 2
September 2022. She recovered after 8 days without
the need for antiviral therapy. Respiratory samples
over the initial 4 days of admission were negative
for severe acute respiratory syndrome coronavirus
2 (SARS-CoV-2) polymerase chain reaction (PCR).
Magnetic resonance imaging of the brain on 30
September 2022 revealed focal cytotoxic oedema
in the splenium of the corpus callosum, possibly
indicative of encephalitis or encephalopathy (Fig a). Chest X-ray on admission revealed diffuse right
lung opacities (Fig b). The chest symptoms of the
patient deteriorated with bilateral involvement
and increased need for oxygen support, and she
was transferred to the intensive care unit with use
of a high-flow nasal cannula. She was intubated on
2 October 2022 as oxygenation was suboptimal.
Lumbar puncture was unremarkable. Chest X-ray
on 5 October 2022 showed dense bilateral opacities
(Fig c), and computed tomography of the thorax
on 5 October 2022 showed bilateral pulmonary
consolidations and diffuse ground glass opacities.
She also developed anaemia (haemoglobin level: 7.9
g/dL) and thrombocytopenia (platelet count: 78 × 109/L). She was in a hyperinflammatory state with
ferritin level of 14057 pmol/L, C-reactive protein
level of 273 mg/L, lactate dehydrogenase level of
1081 IU/L, and persistently elevated D-dimer level
of >8000 ng/mL. Immunoglobulin G antibody level
against SARS-CoV-2 receptor-binding domain
on 3 October 2022 was 34 225.34 AU/mL. The
patient was otherwise haemodynamically stable.
Electrocardiogram showed sinus rhythm and high-sensitivity
troponin I level was only mildly elevated (19.7-119 ng/L).
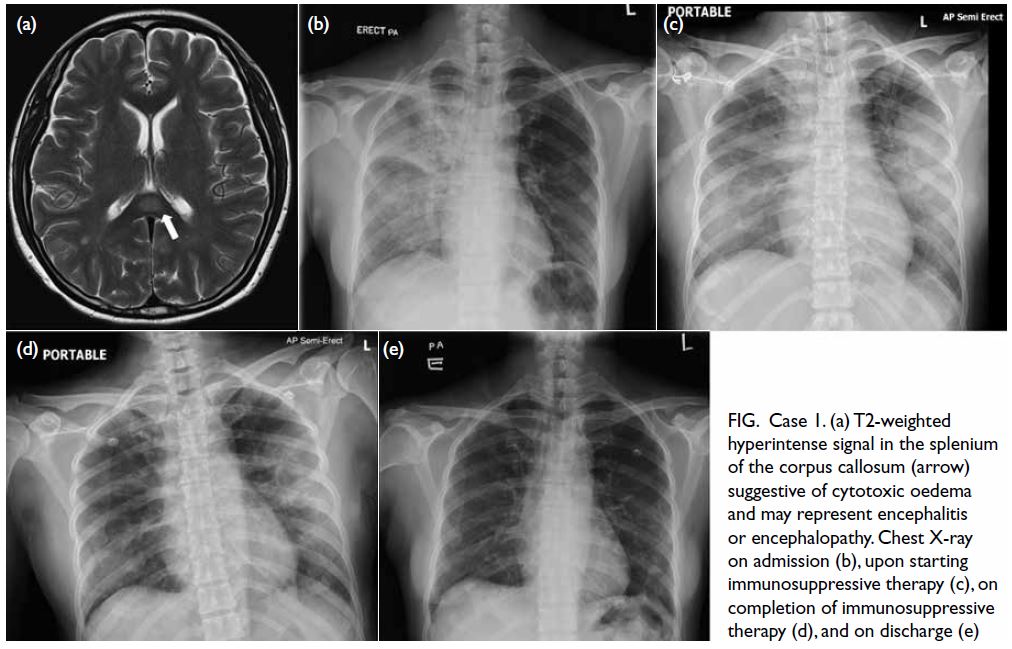
Figure. Case 1. (a) T2-weighted hyperintense signal in the splenium of the corpus callosum (arrow) suggestive of cytotoxic oedema and may represent encephalitis or encephalopathy. Chest X-ray on admission (b), upon starting immunosuppressive therapy (c), on completion of immunosuppressive therapy (d), and on discharge (e)
BioFire FilmArray Pneumonia Panel for
endotracheal aspirate detected 105 copies/mL of
Staphylococcus aureus while mecA/C gene was not
detected, and culture also grew scanty methicillin-sensitive
S aureus. Cytomegalovirus DNA PCR
was negative. Repeated respiratory samples
including nasopharyngeal/throat swabs, sputum
and endotracheal aspirate did not detect SARS-CoV-2 RNA. Pneumocystis jirovecii pneumonia
PCR was negative as was sputum for acid-fast
bacillus smear/c/st. Autoimmune workup including
antinuclear antibody, antineutrophil autoantibodies
and immunoglobulin pattern was negative.
The patient was initially prescribed empirical
meningitis treatment with intravenous ceftriaxone
2 g Q12H and intravenous acyclovir 500 mg Q8H,
and antimicrobials were switched to piperacillin-tazobactam
on 2 October 2022 after cerebrospinal
fluid results excluded meningitis. Her condition
continued to deteriorate while on antibiotics.
The patient was suspected of having multisystem
inflammatory syndrome in adults (MIS-A) and
was started on intravenous methylprednisolone
and intravenous immunoglobulin (IVIG) from 5
October 2022. She was initially given a daily dose
of methylprednisolone 0.5 g for 3 days and IVIG
20 g for 5 days. She gradually improved with decreased
oxygen requirement and ventilatory support and
was extubated on 7 October 2022. There was
significant improvement in inflammatory markers
with C-reactive protein level decreased to 30.8 mg/L,
ferritin level decreased to 4746 pmol/L, and lactate
dehydrogenase level decreased to 526 IU/L at the end
of treatment. Serial chest X-ray showed radiological
improvement with decreased bilateral opacities (Fig d) and near-resolution of chest X-ray upon discharge
(Fig e) 16 days after starting MIS-A treatment. She
completed a 9-week course of steroids with full
recovery. A repeated magnetic resonance imaging
of the brain was scheduled 7 months after discharge
to monitor her progress, which showed resolution
of previously noted oedema in the splenium of the
corpus callosum.
Case 2
A 39-year-old Malawian man presented to Ruttonjee
and Tang Shiu Kin Hospitals on 1 November 2022
with a history of fever since 29 October 2022.
He had had confirmed COVID-19 infection with
nasopharyngeal swab SARS-CoV-2 PCR positive on
7 October 2022 but recovered without the need of
antivirals. He had a history of malaria 21 years ago but
no travel history over the last 2 years. He otherwise
enjoyed good past health apart from obesity (body
weight: 120 kg; body mass index: >30 kg/m2). He had
received two doses of CoronaVac and one dose of
Comirnaty vaccines with the last dose administered
on 2 March 2022. His fever persisted and he was
noted to have bilateral conjunctivitis and petechiae
over the throat. Electrocardiogram later revealed
new atrial fibrillation and serial echocardiograms
showed accumulation of pericardial effusion and
worsening left ventricular ejection fraction of 30%.
He had acute liver failure with elevated parenchymal
enzyme level (alanine transaminase level: 1079 IU/L),
coagulopathy (international normalised ratio:
2.61), hyperammonaemia (serum ammonia level:
108 μmol/L), and hyperlactatemia (lactate
concentration: 6.65 mmol/L). He also developed acute
kidney injury (creatinine level: 256 μmol/L, estimated
glomerular filtration rate: 26 mL/min/1.73 m2) and thrombocytopenia (platelet count: 35×109/L). He
was transferred to the intensive care unit for further
management on 6 November 2022. He was in a
hyperinflammatory state with ferritin level of 131 351
pmol/L, C-reactive protein level of 442 mg/mL,
lactate dehydrogenase level of 7710 IU/L, and
persistently elevated D-dimer level of >8000 ng/mL.
Immunoglobulin G antibody level against SARS-CoV-2 receptor-binding domain on 6 November 2022 was >40 000 AU/mL. He was haemodynamically
stable throughout his admission.
The patient was suspected of having MIS-A and
was commenced on intravenous methylprednisolone
(0.5 g for 6 days) and IVIG (20 g for 5 days) on 8
November 2022. His fever subsided soon after
steroids were given, with resolution of organ failure.
He was discharged 11 days after starting MIS-A
treatment with prednisolone 40 mg twice daily.
Repeated echocardiogram on 16 November 2022
prior to discharge showed significant improvement
with left ventricular ejection fraction of 55% and
decreased pericardial effusion of up to 1.1 cm
in thickness. He had no further episodes of atrial
fibrillation. He was last seen 4 weeks post-discharge
and remained well on a tapering dose of prednisolone.
He remained well and was eventually weaned off
immunosuppressants in early October 2023.
Discussion
The Centers for Disease Control and Prevention case
definition for MIS-A was developed through expert
opinion and states that the patient should be aged
≥21 years, have been hospitalised for at least 1 day
or died as a result, and fulfilled certain clinical and
laboratory criteria with no more likely alternative
diagnosis.1 Case 1 did not fulfil these primary clinical
criteria for MIS-A. Nonetheless she exhibited the
neurological and haematological components of
the secondary clinical criteria and also met the
laboratory criteria with no other cause identified.
Fulminant pulmonary involvement is unusual
since pulmonary involvement has been used to
distinguish MIS-A patients from patients with severe
COVID-19 infection.2 Chronologically the patient
developed fulminant pneumonitis 3 weeks after her
initial COVID-19 infection, within the commonly
described 2- to 5-week interval between onset of
typical COVID-19 symptoms and onset of MIS-A,
that likely represented a post-acute phenomenon
rather than part of the initial infection. A case
series in the United States also reported that MIS-A
patients may have pulmonary involvement and
require mechanical ventilation when compared with
multisystem inflammatory syndrome in paediatric
patients.3 Although 86% to 89% of MIS-A patients
have one or more cardiovascular abnormalities,4
the lack of cardiac involvement in this patient with
otherwise compatible clinical features should not
have excluded the diagnosis of MIS-A.
Case 2 fulfilled the Centers for Disease Control
and Prevention case definition as well as having
markedly deranged liver and renal function. Despite
his impaired left ventricular ejection fraction,
there was no shock or congestion to explain the
deranged liver and renal function. Both liver and
renal function recovered with immunosuppression,
suggesting reversibility with treatment of the
hyperinflammatory state. Such hepatic involvement
has been reported in a case in Croatia5 and renal
involvement has been reported previously, albeit
usually associated with shock.2
Both cases responded rapidly to
methylprednisolone and IVIG. This treatment
regimen was with reference to the guidelines
published by the National Institutes of Health
and extrapolated from multisystem inflammatory
syndrome in children data.6 Case 2 had a longer course of methylprednisolone based on body weight
and higher level of inflammatory markers. Steroid
tapering was initiated afterwards and continued for at least 2 months in both our patients. Further studies would be helpful to guide the management for MIS-A.
Author contributions
All authors contributed to the concept or design of the study,
acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patients were treated in accordance with the Declaration
of Helsinki. Verbal consent for treatments, procedures and for
publication has been obtained from the patients.
References
1. Centers for Disease Control and Prevention, United States
Department of Health and Human Services. CDC case
definition for MIS-A. Updated January 2023. Available
from: https://www.cdc.gov/mis/mis-a/hcp.html. Accessed 15 Jan 2023.
2. Morris SB, Schwartz NG, Patel P, et al. Case series of
multisystem inflammatory syndrome in adults associated
with SARS-CoV-2 infection—United Kingdom and United
States, March–August 2020. MMWR Morb Mortal Wkly Rep 2020;69:1450-6.Crossref
3. Patel P, DeCuir J, Abrams J, Campbell AP, Godfred-Cato S, Belay ED. Clinical characteristics of multisystem inflammatory syndrome in adults: a systematic review.
JAMA Netw Open 2021;4:e2126456. Crossref
4. Lai CC, Hsu CK, Hsueh SC, Yen MY, Ko WC, Hsueh
PR. Multisystem inflammatory syndrome in adults:
characteristics, treatment, and outcomes. J Med Virol
2023;95:e28426. Crossref
5. Vujaklija Brajković A, Zlopaša O, Gubarev Vrdoljak N,
Goran T, Lovrić D, Radonić R. Acute liver and cardiac
failure in multisystem inflammatory syndrome in
adults after COVID-19. Clin Res Hepatol Gastroenterol
2021;45:101678. Crossref
6. National Institutes of Health, United States Government.
Therapeutic management of hospitalized children with
MIS-C, plus a discussion on MIS-A. Updated February 2024.
Available from: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-children/hospitalized-pediatric-patients--therapeutic-management-of-mis-c/. Accessed 8 Apr 2024.

