Hong Kong Med J 2024 Feb;30(1):44–55 | Epub 8 Feb 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
The Omicron variant of COVID-19 and its association with croup in children: a single-centre study in Hong Kong
Michelle CY Lam, MB, ChB, MRCPCH; David SY Lam, MB, BS, FHKAM (Paediatrics)
Department of Paediatrics and Adolescent Medicine, Tuen Mun Hospital, Hong Kong SAR, China
Corresponding author: Dr Michelle CY Lam (lcy766@ha.org.hk)
Abstract
Introduction: The fifth wave of the coronavirus
disease 2019 (COVID-19) pandemic in Hong Kong
was dominated by the Omicron variant, which may
cause more upper airway involvement in children.
This study was performed to identify any associations
between the Omicron variant of COVID-19 and
croup in children.
Methods: This retrospective study reviewed the
electronic medical records of patients admitted to
Tuen Mun Hospital in Hong Kong from 1 January
2018 to 31 March 2022 under the diagnostic code
for croup (J05.0 in the International Classification
of Diseases 10th Edition). Patients were categorised
into three groups according to their admission
periods, namely, non–COVID-19, COVID-19–pre-Omicron, and COVID-19–Omicron groups.
Disease associations and severity were compared
according to incidence, Westley Croup Score, length
of hospital stay, medication use, respiratory support,
and intensive care unit admissions.
Results: The COVID-19 incidence among patients
with croup was significantly higher in the COVID-19–Omicron group than in the COVID-19–pre-Omicron group (90.0% vs 2.0%; P<0.001). Compared with patients in the COVID-19–pre-Omicron and non–COVID-19 groups, patients in the COVID-19–Omicron group also had a higher Westley score (moderate and severe disease in the COVID-19–Omicron group: 56.7%; COVID-19–pre-Omicron
group: 22.0%, P=0.004; non–COVID-19 group:
24.8%, P<0.001), longer median hospital stay
(COVID-19–Omicron group: 3.00 days; COVID-19–pre-Omicron group: 2.00 days, P<0.001; non–COVID-19 group: 2.00 days, P=0.034), and higher mean dexamethasone requirement (COVID-19–Omicron group: 0.78 mg/kg; COVID-19–pre-Omicron group: 0.49 mg/kg, P<0.001; non–COVID-19 group: 0.58 mg/kg, P=0.001).
Conclusion: The Omicron variant of COVID-19 is
associated with croup and can cause more severe
disease in Hong Kong children.
New knowledge added by this study
- The Omicron variant is associated with higher risk of croup than previously circulating variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
- The presence of croup in a patient infected with the Omicron variant of SARS-CoV-2 could lead to a more prolonged and severe disease course.
- Omicron-associated croup may require more doses and a larger total amount of dexamethasone, as well as a longer hospital stay.
- Paediatricians should be aware of the potential for prolonged courses of croup during the Omicron era of the coronavirus disease 2019 (COVID-19) pandemic.
- More healthcare resources may be needed for paediatric patients with croup in the Omicron era of the COVID-19 pandemic.
- Further research and policies promoting COVID-19 vaccination may be warranted to prevent COVID-19 and associated complications in children.
Introduction
Coronavirus disease 2019 (COVID-19) was first
detected in Wuhan, China on 31 December
2019.1 Since then, COVID-19 has affected adults
and children worldwide. On 31 December 2021,
the Centre for Health Protection of Hong Kong announced that the fifth wave of the pandemic, also
known as the ‘Omicron surge’, had begun.2 There was
evidence that the Omicron variant of severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2)
replicated more rapidly and effectively than other
strains in bronchial and nasal epithelial cells, resulting in higher infectivity and transmissibility,
along with more severe upper respiratory tract
manifestations.3 4
Croup, or laryngotracheitis, is an upper
airway disease that primarily affects children aged
6 months to 3 years. Causative viruses infect the
nasopharyngeal epithelium and spread along the
respiratory tract up to the laryngotracheal region,
leading to upper airway narrowing, inspiratory
stridor, barking cough, and hoarseness.5 6 Thus far,
parainfluenza viruses have been the most common
causative agents of croup.7
Compared with other SARS-CoV-2 variants
and other respiratory viruses, the new Omicron
variant of SARS-CoV-2 may have a stronger
association with croup.3 4 Case reports and case
series have been published regarding COVID-19–associated croup8 9 10 11 12; however, few studies in Hong
Kong or other countries have focused on possible
causative relationships between the Omicron variant
and croup.8 12 Analyses of epidemiological data from
Hong Kong are needed to guide further management
of croup in children during the COVID-19 pandemic.
By exploring the incidence, clinical
characteristics, treatment options, and outcomes of
croup before and after the emergence of COVID-19, as well as after the emergence of the Omicron
variant, this study aimed to identify differences
among these three groups of patients and provide
insights concerning COVID-19‐associated croup in
Hong Kong.
Methods
Study design
This retrospective observational study was
conducted in the Department of Paediatrics and
Adolescent Medicine at Tuen Mun Hospital, a large
public hospital serving a population of >1.1 million
(15% of the total Hong Kong population),13 among
which >15% are children.14 15 Clinical data and
medical records were retrieved from the Clinical
Data Analysis and Reporting System of the Hospital
Authority.
Inclusion and grouping criteria
All hospital admissions with a diagnostic code of
‘Croup’ (J05.0 in the International Classification of
Diseases 10th Edition) from 1 January 2018 to 31
March 2022 were included in this study. Patients
were grouped into the following three admission
periods: (1) non‐COVID-19 (1 January 2018 to
31 December 2019); (2) COVID-19‐pre-Omicron
(1 January 2020 to 31 December 2021); and (3)
COVID-19‐Omicron (1 January 2022 to 31 March
2022). This grouping approach coincided with the
World Health Organization’s announcement of the
discovery of a novel coronavirus in Wuhan, China
on 31 December 20191 and the Centre for Health
Protection’s announcement that the fifth wave of the
pandemic (also known as the ‘Omicron surge’) had
begun in Hong Kong on 31 December 2021.2 The
2-year cohort from 2018 to 2019 (before the World
Health Organization’s announcement) was included
for comparisons of characteristics before and after
the emergence of SARS-CoV-2.
Exclusion criteria
The study population was limited to inpatients at
Tuen Mun Hospital, excluding individuals solely
managed in the Emergency Department. The
study also excluded patients with a final diagnosis
(eg, foreign body inhalation) that could mimic the
clinical presentation of croup.
Clinical data and outcome measurements
Baseline clinical characteristics including age, sex,
ethnicity, and significant medical history were
retrieved from the medical records of the included
patients. Diagnoses of COVID-19 were made by
laboratory confirmation of viral infection through
real-time polymerase chain reaction (RT-PCR) assays
of nasopharyngeal specimens. Diagnoses of specific
respiratory viruses were also confirmed by RT-PCR assays of patients’ nasopharyngeal specimens. The
incidences of all viruses were analysed.
The total numbers of admitted patients
with confirmed COVID-19 in the COVID-19–pre-Omicron and COVID-19–Omicron groups
were retrieved from the Clinical Data Analysis
and Reporting System. Among these patients,
individuals with a diagnosis of croup were identified
to determine the incidence rate of croup in each
group.
The Westley Croup Score was calculated
on the basis of physical findings documented in
the retrieved medical records. It evaluates croup
severity using five clinical parameters16: (1) level
of consciousness (normal=0, disoriented=5); (2)
cyanosis (none=0, with agitation=4, at rest=5); (3)
stridor (none=0, with agitation=1, at rest=2); (4) air
entry (normal=0, mildly decreased=1, substantially
decreased=2); and (5) retraction (none=0, mild=1,
moderate=2, severe=3). The raw score ranges from
0 to 17; croup can be categorised as mild (score
0-2), moderate (score 3-5), severe (score 6-11), or
impending respiratory failure (score ≥12).
The following outcome measurements were also assessed:
- Length of hospital stay (days);
- Dexamethasone use (number of doses and total amount used);
- Use of nebulised adrenaline;
- Respiratory support (oxygen therapy and high-flow nasal cannula oxygen therapy);
- Paediatric intensive care unit admission;
- Other associated medical co-morbidities during the same admission (febrile convulsion, wheezing attacks/acute bronchiolitis, gastrointestinal symptoms, pneumonia, poor feeding/dehydration requiring intravenous fluid therapy, or readmission/abnormal blood test results).
Multivariate analysis was performed to
examine a range of risk factors. Age, sex, ethnicity,
history of croup, history of respiratory diseases,
and timing of croup diagnosis were included as
possible factors affecting croup severity. The Westley
score and number of doses of dexamethasone used
were selected as outcome measurements for croup
severity.
History of croup and history of respiratory
diseases were included in multivariate analyses
because they are known risk factors for severe or
recurrent croup.17 18 Patients in the COVID-19–Omicron group were younger; thus, we regarded
age as a possible confounding factor. Considering
that croup had a male predominance in previous
studies, sex was included as a potential risk factor.
Ethnicity was included to determine whether the
predominately Chinese population in Hong Kong
would influence the outcomes compared with findings in previous studies primarily involving Caucasians or Asians.
Statistical analysis
The statistical significance of categorical variables
was determined using the Pearson Chi squared test
or Fisher’s exact test. The Mann-Whitney U test and
Kruskal–Wallis test were utilised to identify any
statistically significant differences among groups
regarding continuous variables (eg, age and length
of stay). Multivariate analysis was performed by
logistic regression. SPSS software (Windows version
28.0; IBM Corp, Armonk [NY], United States) was
used for statistical analysis.
The STROBE (Strengthening the Reporting of
Observational Studies in Epidemiology) checklist
was followed when preparing this article.
Results
In total, 423 inpatients were diagnosed with croup during the study periods: 343 were diagnosed in
the non–COVID-19 period, 50 were diagnosed in
the COVID-19–pre-Omicron period, and 30 were
diagnosed in the COVID-19–Omicron period.
Baseline characteristics
The baseline characteristics for patients in each
time period are shown in Table 1. There were no
significant differences (P>0.05) across the three
groups in terms of sex ratio, ethnicity, history
of prematurity, or significant medical history
(including histories of croup and/or respiratory,
neurodevelopmental, and cardiac diseases). Male
sex predominance was observed across all groups
(male-to-female ratio in the non–COVID-19
group=1.77; COVID-19–pre-Omicron group=2.33;
COVID-19–Omicron group=5; P=0.079). Most
patients were Chinese (non–COVID-19 group:
92.4%, COVID-19–pre-Omicron group: 92.0%,
COVID-19–Omicron group: 93.3%; P=0.725), born
at term (non–COVID-19 group: 90.1%, COVID-19–pre-Omicron group: 94.4%, COVID-19–Omicron
group: 100.0%; P=0.223), and had previous good
health (non–COVID-19 group: 66.5%, COVID-19–pre-Omicron group: 72.0%, COVID-19–Omicron group: 70.0%; P=0.143).
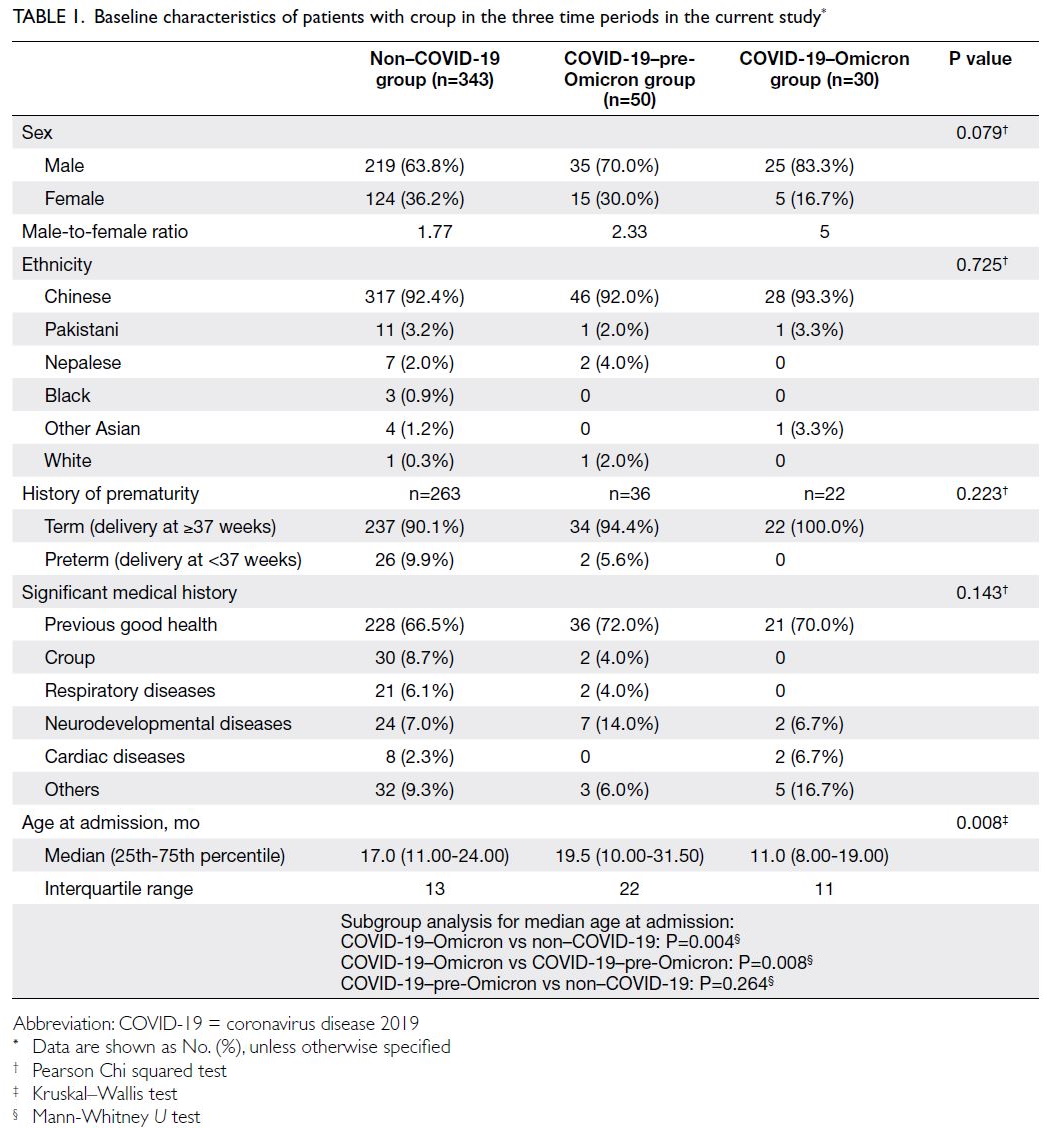
Table 1. Baseline characteristics of patients with croup in the three time periods in the current study
Patients in the COVID-19–Omicron group
had a median age of 11.0 months (interquartile
range [IQR]=11), which was significantly younger
than the median ages of patients in the COVID-19–pre-Omicron group (19.5 months, IQR=22) and the non–COVID-19 group (17.0 months, IQR=13) [P=0.008].
Incidence
Among patients diagnosed with croup, one (infection rate=2.6%) and 27 (infection rate=90.0%) were SARS-CoV-2–positive in the COVID-19–pre-Omicron and COVID-19–Omicron groups, respectively (Table 2). Patients diagnosed with croup in the COVID-19–Omicron group were more likely
to be SARS-CoV-2–positive than patients with such
a diagnosis in the COVID-19–pre-Omicron group
(P<0.001) [Table 2].
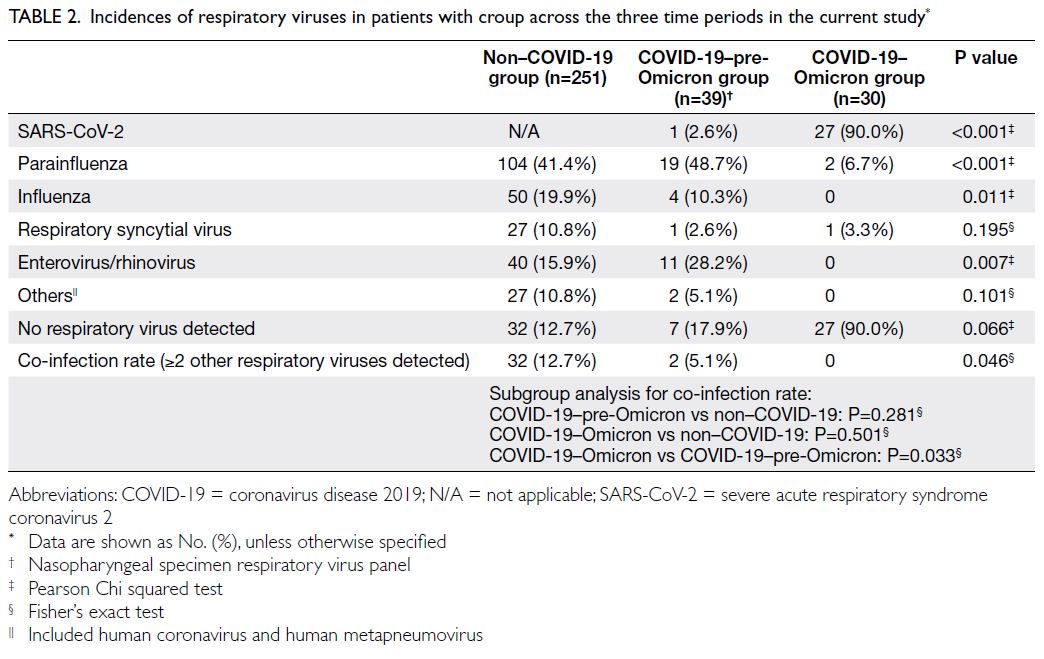
Table 2. Incidences of respiratory viruses in patients with croup across the three time periods in the current study
Additionally, 386 and 170 paediatric patients
(aged 0-18 years) admitted to Tuen Mun Hospital
were SARS-CoV-2–positive in the COVID-19–Omicron and COVID-19–pre-Omicron groups,
respectively. Among these patients, 27 were
diagnosed with croup in the COVID-19–Omicron
group and one was diagnosed with croup in the COVID-19–pre-Omicron group; these values
indicated that the incidence of croup among patients
with COVID-19 was much higher in the COVID-19–Omicron group (rate=7.0%, 95% confidence
interval [CI]=4.61%-10.17%; P=0.0019) than in
the COVID-19–pre-Omicron group (rate=0.59%,
95% CI=0.015%-3.28%; P=0.0019). Compared with
other SARS-CoV-2 variants, the Omicron variant
may be more strongly associated with croup.
Respiratory virus infection
Before the emergence of Omicron, among patients
with croup, there were no differences in the rates
of infection by respiratory viruses such as influenza (non–COVID-19 group: n=50, 19.9% vs COVID-19–pre-Omicron group: n=4, 10.3%; P=0.149),
respiratory syncytial virus (non–COVID-19 group:
n=27, 10.8% vs COVID-19–pre-Omicron group:
n=1, 2.6%; P=0.146), and enterovirus/rhinovirus
(non–COVID-19 group: n=40, 15.9% vs COVID-19–pre-Omicron group: n=11, 28.2%; P=0.061).
Parainfluenza virus was the main respiratory virus
detected in both groups (non–COVID-19 group:
n=104, 41.4% vs COVID-19–pre-Omicron group:
n=19, 48.7%; P=0.392). There was also no difference
in the co-infection rate in the two groups (≥2 other
respiratory viruses detected) [non–COVID-19
group: n=32, 12.7% vs COVID-19–pre-Omicron
group: n=2, 5.1%; P=0.281] (Table 2).
However, after the emergence of Omicron,
the SARS-CoV-2 Omicron variant became the main
respiratory virus among patients with croup (co-infection
in the COVID-19–Omicron group: n=0, vs
non–COVID-19 group: n=32, rate=12.7%; P=0.033).
Because the respiratory viruses infecting
patients with croup were similar between the
COVID-19–pre-Omicron and non–COVID-19
groups, a pooled analysis was performed by
grouping patients with croup in the two groups and
compared with patients in the COVID-19–Omicron
group. The results revealed that patients with croup
in the COVID-19–Omicron group had significantly
lower rates of infection with parainfluenza (COVID-19–Omicron group: n=2, 6.7% vs pre-Omicron
group [non–COVID-19 group and COVID-19–pre-Omicron group]: n=123, 42.4%; P<0.001), influenza
(COVID-19–Omicron group: n=0 vs pre-Omicron
group: n=54, 18.6%; P=0.011), and enterovirus/rhinovirus (COVID-19–Omicron group: n=0
vs pre-Omicron group: n=51, 17.6%; P=0.007).
There was no difference in the rate of infection
with respiratory syncytial virus (COVID-19–Omicron group: n=1, 3.3% vs pre-Omicron group:
n=28, 9.7%; P=0.499) between the time before
and after the emergence of Omicron. The rates
of infection with individual viruses are shown in
Table 2.
Westley Croup Score
In the COVID-19–Omicron group, significantly
more patients with croup had moderate disease
(50.0%) or severe disease (6.7%) according to the
Westley score, compared with the non–COVID-19
(moderate disease: 23.9%; severe disease: 0.9%;
P<0.001) and COVID-19–pre-Omicron groups
(moderate disease: 22.0%; severe disease: 0%;
P=0.004). The distribution of severity, according to
the Westley score, was similar between the non–COVID-19 and COVID-19–pre-Omicron groups
(P=0.780) [Table 3].
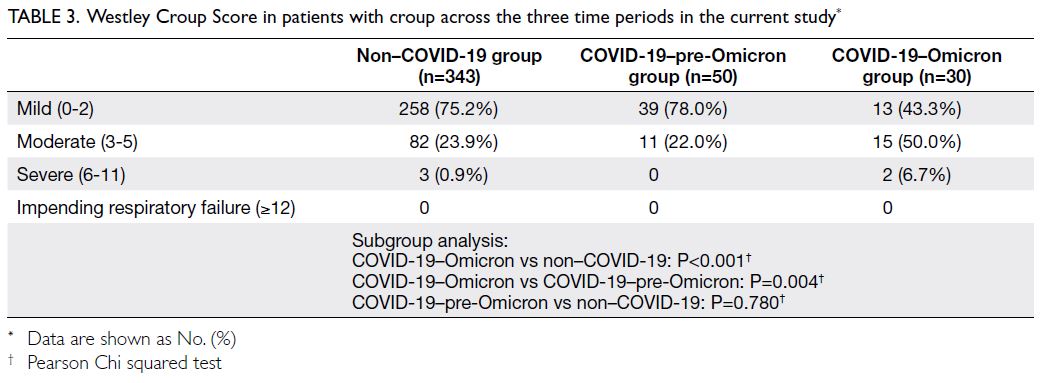
Table 3. Westley Croup Score in patients with croup across the three time periods in the current study
Length of hospital stay
Because causative agents were similar between the non–COVID-19 and COVID-19–pre-Omicron
groups, they were grouped together for analysis again and compared with the COVID-19–Omicron group.
Patients with croup had a significantly longer hospital
stay in the COVID-19–Omicron group (mean=3.63
days, median=3.00, IQR=2) than the pre-Omicron
group (mean=2.67 days, median=2.00, IQR=3;
P=0.016). This finding indicated that patients with
croup who were infected with the Omicron variant
of SARS-CoV-2 required longer hospitalisation,
implying that such patients had more severe disease
than patients infected with other viruses in the pre-Omicron period.
Management strategies and outcomes
Table 4 illustrates treatments and management outcomes during the study periods.
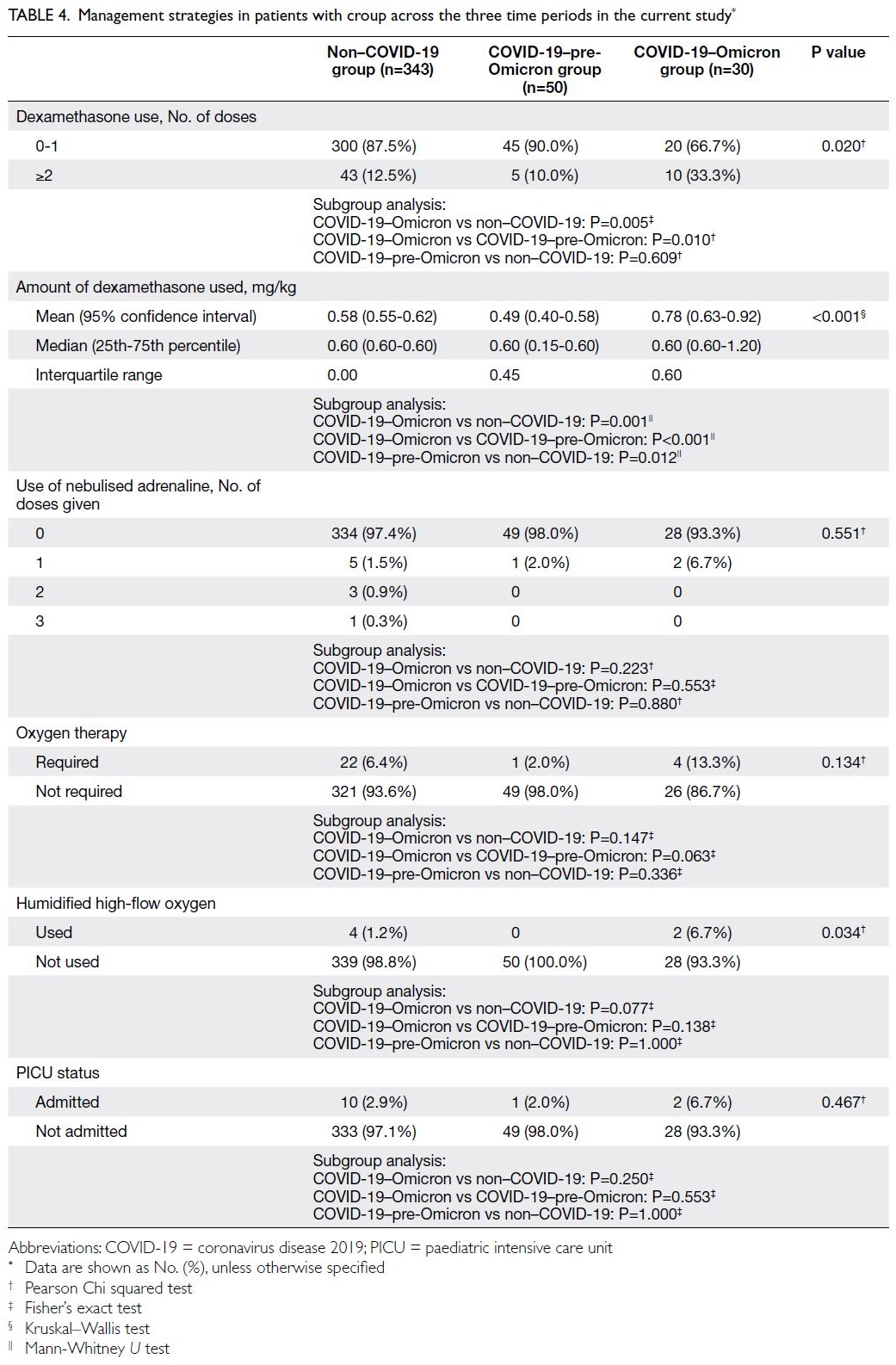
Table 4. Management strategies in patients with croup across the three time periods in the current study
Dexamethasone use
Most patients required zero to one dose of dexamethasone (COVID-19–Omicron group:
66.7%; non–COVID-19 group: 87.5%; COVID-19–pre-Omicron group: 90.0%; P=0.020). Significantly
more patients required ≥2 doses in the COVID-19–Omicron group than in the non–COVID-19 (33.3% vs 12.5%; P=0.005) and COVID-19–pre-Omicron groups (33.3% vs 10.0%; P=0.010). A need for repeated
doses of dexamethasone indicated more severe
disease, considering that guidelines recommend
≥2 doses of dexamethasone for patients with croup
who display suboptimal clinical improvement.5 6 19 20
The difference remained statistically significant
when the total amount of dexamethasone given
was normalised according to the body weight of the
patient; patients in the COVID-19–Omicron group
required a larger total amount of dexamethasone
(mean=0.78 mg/kg) compared with patients in the
other two groups (mean of the non–COVID-19
group=0.58 mg/kg, P=0.001; mean of the COVID-19–pre-Omicron group: 0.49 mg/kg, P<0.001).
Nebulised adrenaline use
Nebulised adrenaline is often administered to patients with severe croup.5 6 19 20 Most patients in the
study did not require nebulised adrenaline. During
the non–COVID-19 period, 1.5% of patients (n=5)
were given one dose, 0.9% (n=3) were given two
doses, and 0.3% (n=1) were given three doses; in the
COVID-19–Omicron and COVID-19–pre-Omicron
groups, only 6.7% (n=2) and 2.0% (n=1) of the
patients, respectively, were given a single dose. No
patients in the COVID-19–Omicron and COVID-19–pre-Omicron groups required more than one
dose. Overall, there was no significant difference in
the need for nebulised adrenaline (P=0.551).
Respiratory support
Overall, 6.4% (n=22) of patients admitted in the non–COVID-19 period required oxygen therapy, whereas
2.0% (n=1) required oxygen in the COVID-19–pre-Omicron period and 13.3% (n=4) required oxygen
in the COVID-19–Omicron period. Although the
oxygen requirement tended to be higher in the
COVID-19–Omicron group than in the other two
groups, this difference was not statistically significant
(P=0.134).
Respiratory support also included the use of
humidified high-flow oxygen. No patients required
intubation or other forms of mechanical ventilation.
Humidified high-flow oxygen was required by 1.2%
(n=4) of patients in the non–COVID-19 period, 6.7%
(n=2) in the COVID-19–Omicron period, and 0% in
the COVID-19–pre-Omicron period. There were no
differences among the groups concerning humidified
high-flow oxygen use (Table 4).
Paediatric intensive care unit admissions
In total, 2.9% (n=10), 2.0% (n=1), and 6.7% (n=2)
of patients required paediatric intensive care
unit admission while hospitalised among the
non–COVID-19, COVID-19–pre-Omicron, and
COVID-19–Omicron groups, respectively; there
was no significant difference across the three groups
(P=0.467) [Table 4].
Other co-morbidities
Patients with croup had a higher overall incidence
of co-morbidities in the COVID-19–Omicron
group (46.7%, n=14) than in the non–COVID-19
(25.4%, n=87) and COVID-19–pre-Omicron groups
(30.0%, n=15) [Table 5]. Patients with croup had a
significantly higher incidence of new co-morbidities
in the COVID-19–Omicron group than in the non–COVID-19 group, with an odds ratio (OR) of 2.575
(95% CI=1.207-5.491; P=0.012); this incidence did
not differ between the COVID-19–pre-Omicron and
non–COVID-19 groups (OR=1.427, 95% CI=0.749-2.718; P=0.278).
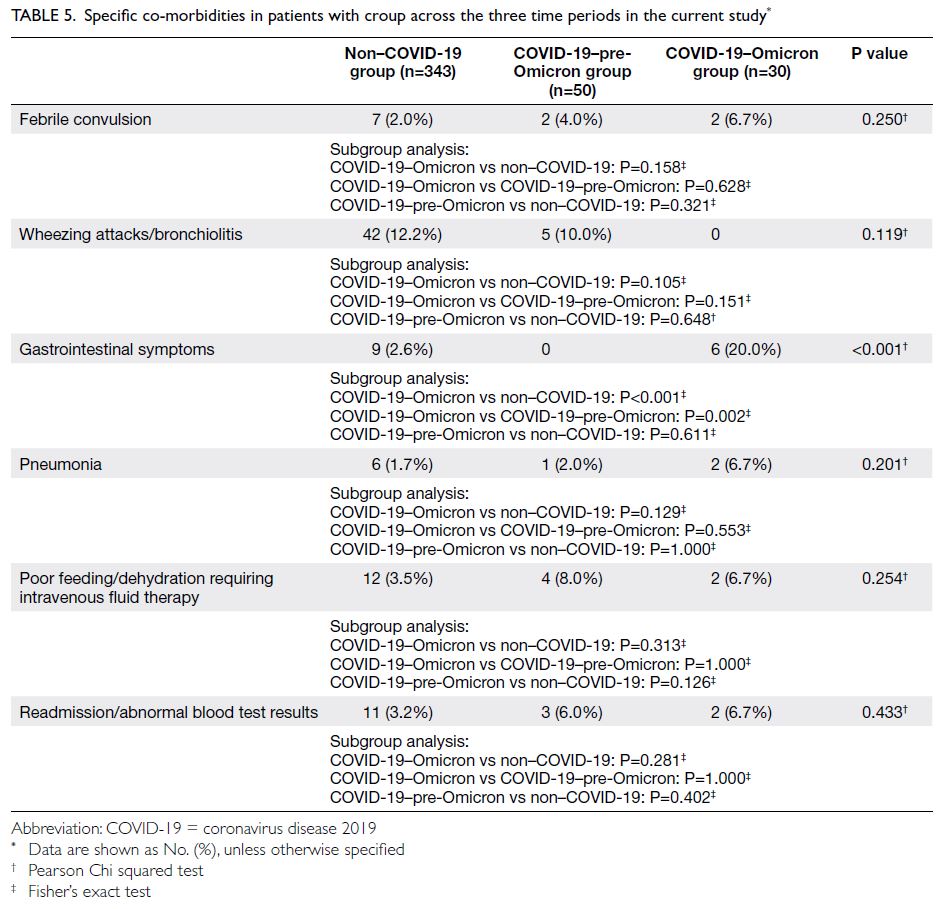
Table 5. Specific co-morbidities in patients with croup across the three time periods in the current study
With respect to specific co-morbidities (Table 5), there were no significant differences in the rates
of febrile convulsion, pneumonia, intravenous fluid
therapy requirement, readmission, or abnormal
blood test results. However, significantly more
patients in the COVID-19–Omicron group had gastrointestinal symptoms compared with patients
in the other groups. Thus, the Omicron variant was
associated with more concomitant gastrointestinal
manifestations among patients with croup
compared with such patients in the non–COVID-19
(OR=9.250, 95% CI=3.039-28.151; P<0.001) and
COVID-19–pre-Omicron groups (OR=3.086,
95% CI=2.217-4.292; P=0.002).
Importantly, no patients with croup in the
COVID-19–Omicron group had concomitant
wheezing attacks or bronchiolitis (n=0), compared
with a rate of approximately 1 in 10 during the
other two groups (non–COVID-19: n=42, 12.2%;
COVID-19–pre-Omicron: n=5, 10.0%). However,
the overall difference was not statistically significant
(P=0.119) [Table 5].
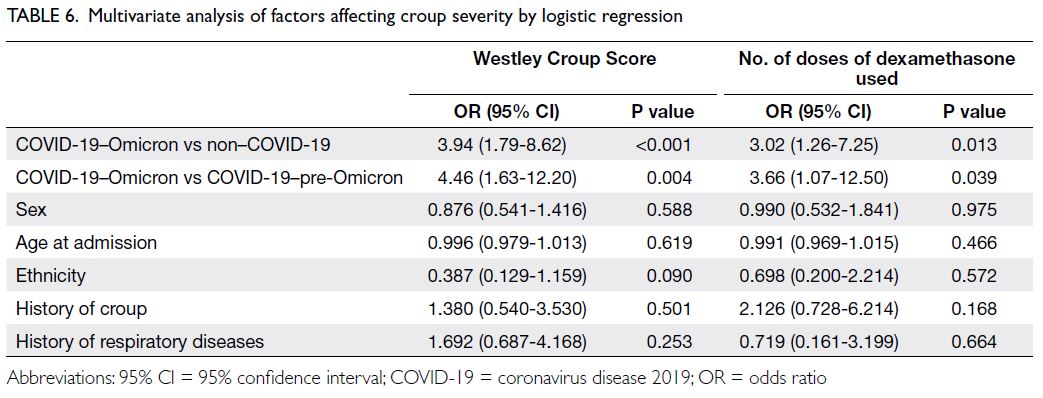
Risk factors
The results in Table 6 indicate that differences in age (P=0.619), sex (P=0.588), ethnicity (P=0.090),
history of croup (P=0.501), and history of respiratory
diseases (P=0.253) did not affect the risk of greater
croup severity. The timing of croup diagnosis was a
significant risk factor for greater croup severity. After
adjustment for the other factors, the OR for greater
croup severity in the COVID-19–Omicron group
was 3.94 (95% CI=1.79-8.62; P<0.001) compared
with the non–COVID-19 group. Comparison of the
COVID-19–Omicron and COVID-19–pre-Omicron
groups revealed an OR of 4.46 (95% CI=1.63-12.20;
P=0.004) [Table 5].
The results were consistent when the number
of doses of dexamethasone was regarded as the
analysis outcome. Patients diagnosed with croup in
the COVID-19–Omicron group had an increased
risk of greater croup severity. The OR for requiring
≥2 doses of dexamethasone in the COVID-19–Omicron group, compared with the non-COVID
group, was 3.02 (95% CI=1.26-7.25; P=0.013).
Comparison of the COVID-19–Omicron and
COVID-19–pre-Omicron groups showed an OR of
3.66 (95% CI=1.07-12.50; P=0.039) [Table 5].
Discussion
Link between the Omicron variant and croup
Our results showed that SARS-CoV-2 became the
predominant virus in patients with croup after
emergence of the Omicron variant, surpassing
parainfluenza virus, which was previously
considered the most common viral cause of croup.7
This contrasts with the COVID-19–pre-Omicron
group, during which there were no differences in
the rates of detected respiratory viruses compared
with the non–COVID-19 group. Thus, the Omicron
variant was associated with a higher risk of croup,
compared with other SARS-CoV-2 variants.
Additionally, among patients admitted for
treatment of COVID-19, the incidence of croup
was significantly higher in the COVID-19–Omicron group than in the COVID-19–pre-Omicron group,
indicating that the Omicron variant was associated
with a higher risk of croup, compared with other
SARS-CoV-2 variants. This finding is consistent
with previous reports that the Omicron variant
preferentially replicates in the upper respiratory
tract,3 4 which differs from observations concerning
other variants.
The lower co-infection rate during the
COVID-19–Omicron period (0%), compared
with the non–COVID-19 period (12.7%), could
be attributed to the greater replication capacity
and infectivity of the Omicron variant of SARS-CoV-2. Another possible explanation for the lower
co-infection rate and the shift in predominant
respiratory virus from parainfluenza to the
Omicron variant of SARS-CoV-2 could have been
the implementation of social distancing policies
outlined in the Prevention and Control of Disease
Ordinance [Cap 599 (F, G, I) of the Laws of Hong
Kong]21 22 23 and school suspension24 25 26 27 28 29 in Hong Kong,
which may have effectively reduced the transmission
of upper respiratory tract infections. These effects
were revealed through the reduction in the total
number of patients with croup admitted during the
2-year interval since the emergence of COVID-19 in
2020. In the COVID-19–pre-Omicron period, only
50 patients were admitted for croup, compared with
343 during the non–COVID-19 period.
Increased croup severity in patients with the
Omicron variant
The present study revealed the Omicron variant
is causing greater croup severity compared
with other variants and respiratory viruses, in
terms of a significantly higher Westley score,
longer hospitalisation, greater requirement
for dexamethasone, and more concomitant
gastrointestinal manifestations. Multivariate
analysis also showed that patients in the COVID-19–Omicron group, when the Omicron variant of SARS-CoV-2 was the predominant virus, were more likely to develop severe disease.
The decrease in the number of patients with
concomitant wheezing attacks or bronchiolitis
could be attributed to a lower viral load in the lower
respiratory tract (relative to the upper respiratory
tract), as observed in hamsters,19 30 along with the
greater infectivity of the Omicron variant in nasal
epithelial cells.3 4 Considering that wheezing attacks
and bronchiolitis mainly affect small airways in the
lower tract, these findings may explain the lower
risks of such co-morbidities in patients with croup
who exhibit the Omicron variant of COVID-19.
Regarding the length of stay, confounding
factors such as quarantine policies, parental anxiety
about hospitalisation, and various discharge criteria
based on physician preferences could affect the
observed correlation with disease severity.
During the ‘Omicron surge’, hospital discharge
criteria were revised to allow early discharge for
clinically stable patients without repeated RT-PCR
testing; conversely, in the COVID-19–pre-Omicron
group, negative RT-PCR results (or RT-PCR
results with certain cycle threshold values) were
required prior to discharge.31 32 33 A longer length of
stay in patients with croup during the COVID-19–Omicron period despite these relaxed discharge
criteria indicates that croup severity was greater in
the COVID-19–Omicron period, although other
co-morbidities in patients with COVID-19 may also
have contributed to the increased length of hospital stay.
The potentially greater severity of croup in
patients with the Omicron variant of COVID-19
and the diverse range of co-morbidities in such
patients had considerable impacts on patient health,
caregiver stress, and the public health burden. More
healthcare resources, such as in-hospital backup
nebulising facilities, may be required during the
Omicron-dominant era. The results of the present
study will enable paediatricians to be more vigilant
and predict a possibly longer disease course,
along with the need for repeated dexamethasone
administration or enhanced treatment, in patients
with COVID-19‐Omicron–associated croup.
Limitations
There were several limitations in this study. First,
it was a single-centre study, limiting its ability to
represent the overall population; thus, a more
extensive study should be performed in the future.
Second, there was no unified treatment
protocol for croup in our hospital. Exact medication
dosing and timing (eg, concerning addition or
repetition) were largely based on clinical decisions
by multiple physicians, which may have considerably
varied although all administered oral dexamethasone as first-line medication; repeated doses were given
as needed, and nebulised adrenaline was reserved
for patients with more severe disease.5 6 19 20 These
factors could have affected the assessments of
severity across study periods by modifying the doses
of medication administered.
Third, measurement of the Westley score could
have been under- or overestimated because it was
based on clinical records, where data may have been
omitted by attending physicians. These missing data
could affect measurements of croup severity across
study periods.
Finally, patients with croup admitted during
the Omicron period (median age=11.0 months)
were younger than such patients in previous periods
(COVID-19–pre-Omicron group: 19.5 months;
non–COVID-19 group: 17.0 months). Possible
explanations include the greater transmissibility
of the Omicron variant in younger populations
compared with other variants34 and the lack of
eligibility for SARS-CoV-2 vaccination among
patients aged <3 years.35 Thus, overall protection
could be compromised in the younger age-group.
Other possible confounding factors, such as family
history of croup and parental smoking habits, could
not be assessed in this study because the data were
not available in clinical records.
Future directions
This study focusing on croup and its associations
with COVID-19 among Hong Kong children
provides important insights that can help guide
management of the Omicron variant. However,
additional population-based studies involving
patients from various centres in Hong Kong are
needed to achieve a sample size that can facilitate the
development of management protocols specifically
targeting Omicron-associated croup. In the future,
prospective studies could be performed to analyse
the long-term outcomes of such patients, thereby
facilitating the planning and allocation of healthcare
resources in Hong Kong.
Conclusion
This retrospective study demonstrated that the Omicron variant of COVID-19 is associated with
croup in children; on admission, croup severity
was greater compared with past observations of
disease.
Author contributions
Concept or design: MCY Lam.
Acquisition of data: MCY Lam.
Analysis or interpretation of data: Both authors.
Drafting of the manuscript: MCY Lam.
Critical revision of the manuscript for important intellectual content: DSY Lam.
Acquisition of data: MCY Lam.
Analysis or interpretation of data: Both authors.
Drafting of the manuscript: MCY Lam.
Critical revision of the manuscript for important intellectual content: DSY Lam.
Both authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
Both authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank Mr Jaden Lam, Statistical Officer, Quality and Safety Division, New Territories West Cluster for statistical analysis support.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This research was approved by the New Territories West
Cluster Research Ethics Committee of Hospital Authority,
Hong Kong (Ref No.: NTWC/REC/22030). Informed patient
consent waiver was granted by the Committee due to the
retrospective nature of the study.
References
1. World Health Organization. Pneumonia of unknown
cause—China. 2020 Jan 5. Available form: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON229. Accessed 29 Jun 2022.
2. The Government of the Hong Kong Special Administrative
Region. Archive of statistics on 5th wave of COVID-19. Available from: https://www.coronavirus.gov.hk/eng/5th-wave-statistics.html. Accessed 2 Feb 2024.
3. Hui KP, Ho JC, Cheung MC, et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 2022;603:715-20. Crossref
4. Peacock TP, Brown JC, Zhou J, et al. The SARS-CoV-2
variant, Omicron, shows rapid replication in human
primary nasal epithelial cultures and efficiently uses
the endosomal route of entry. bioRxiv [pre-print]
2021.12.31.474653. Available from: https://www.biorxiv.org/content/10.1101/2021.12.31.474653v1. Accessed 29 Jun 2022.
5. Cherry JD. Clinical practice. Croup. N Engl J Med 2008;358:384-91. Crossref
6. Sizar O, Carr B. Croup. StatPearls [Internet]. 2023 July
Available from: https://www.ncbi.nlm.nih.gov/books/NBK431070/. Accessed 24 July 2023.
7. Rihkanen H, Rönkkö E, Nieminen T, et al. Respiratory viruses in laryngeal croup of young children. J Pediatr 2008;152:661-5. Crossref
8. Murata Y, Tomari K, Matsuoka T. Children with croup
and SARS-CoV-2 infection during the large outbreak of
Omicron. Pediatr Infect Dis J 2022;41:e249. Crossref
9. Brewster RC, Parsons C, Laird-Gion J, et al. COVID-19–associated croup in children. Pediatrics 2022;149:e2022056492. Crossref
10. Tsoi K, Chan KC, Chan L, Mok G, Li AM, Lam HS. A child with SARS-CoV2–induced croup. Pediatr Pulmonol 2021;56:2377-8. Crossref
11. Venn AM, Schmidt JM, Mullan PC. Pediatric croup with
COVID-19. Am J Emerg Med 2021;43:287.e1-3. Crossref
12. Tunç EM, Koid Jia Shin C, Usoro E, et al. Croup during the
coronavirus disease 2019 Omicron variant surge. J Pediatr
2022;247:147-9. Crossref
13. Census and Statistics Department, Hong Kong SAR
Government. Population and household statistics analysed
by District Council District. 2020. Available from: https://www.statistics.gov.hk/pub/B11303012020AN20B0100.pdf. Accessed 29 Jun 2022.
14. Social Welfare Department, Hong Kong SAR Government.
Population profile of Tuen Mun District. Available from:
https://www.swd.gov.hk/en/pubsvc/district/tuenmun/districtpr/tmpp/ Accessed 18 Jan 2024.
15. Social Welfare Department, Hong Kong SAR Government.
Population profile of Yuen Long District. Available from
https://www.swd.gov.hk/en/pubsvc/district/yuenlong/districtpr/ylpp/. Accessed 18 Jan 2024.
16. Westley CR, Cotton EK, Brooks JG. Nebulized racemic
epinephrine by IPPB for the treatment of croup: a double-blind
study. Am J Dis Child 1978;132:484-7. Crossref
17. Bensoussan N, Nguyen L, Oosenbrug M, He H, Duval M.
Characterization and risk factor identification in children
with severe croup. Paediatr Child Health 2018;23(suppl
1):e52-3. Crossref
18. Pruikkonen H, Dunder T, Renko M, Pokka T, Uhari M. Risk
factors for croup in children with recurrent respiratory
infections: a case-control study. Pediatr Perinat Epidemiol
2009;23:153-9. Crossref
19. Smith DK, McDermott AJ, Sullivan JF. Croup: diagnosis and management. Am Fam Physician 2018;97:575-80.
20. Bjornson CL, Johnson DW. Croup in children. CMAJ 2013;185:1317-23. Crossref
21. Hong Kong SAR Government. Prevention and Control
of Disease (Requirements and Directions) (Business and
Premises) Regulation (Cap 599 sub. leg. F). Available from:
https://www.elegislation.gov.hk/hk/cap599F. Accessed 29 Jun 2022.
22. Hong Kong SAR Government. Prevention and Control of
Disease (Prohibition on Gathering) Regulation (Cap 599 sub. leg. G). Available from: https://www.elegislation.gov.hk/hk/cap599G. Accessed 29 Jun 2022.
23. Hong Kong SAR Government. Prevention and Control of
Disease (Wearing of Mask) Regulation (Cap 599 sub. leg.
I). Available from: https://www.elegislation.gov.hk/hk/cap599I. Accessed 29 Jun 2022.
24. Education Bureau, Hong Kong SAR Government.
Arrangements on deferral of class resumption for all
schools. 2020 Jan 31. Available from: https://www.edb.gov.hk/attachment/en/sch-admin/admin/about-sch/diseases-prevention/edb_20200131_eng.pdf. Accessed 29 Jun 2022.
25. Education Bureau, Hong Kong SAR Government.
Arrangements of early commencement of summer holiday
for all schools. 2020 Jul 10. Available from: https://www.edb.gov.hk/attachment/en/sch-admin/admin/about-sch/diseases-prevention/edb_20200710_eng.pdf. Accessed 29
Jun 2022.
26. Education Bureau, Hong Kong SAR Government.
Continuation of suspension of face-to-face classes for
schools in Hong Kong: the arrangements. 2021 Jan 4. Available from: https://www.edb.gov.hk/attachment/en/sch-admin/admin/about-sch/diseases-prevention/edb_20210104_eng.pdf. Accessed 29 Jun 2022.
27. Education Bureau, Hong Kong SAR Government. Face-to-face class arrangements for schools in Hong Kong in the 2021/22 school year. 2021 Aug 2. Available from: https://www.edb.gov.hk/attachment/en/sch-admin/admin/about-sch/diseases-prevention/edb_20210802_eng.pdf. Accessed 29 Jun 2022.
28. Education Bureau, Hong Kong SAR Government.
Suspension of face-to-face classes of primary schools,
kindergartens and kindergarten-cum-child care centres
until Chinese New Year. 2022 Jan 11. Available from: https://www.edb.gov.hk/attachment/en/sch-admin/admin/about-sch/diseases-prevention/edb_20220111_eng.pdf. Accessed 29 Jun 2022.
29. Education Bureau, Hong Kong SAR Government.
Arrangement of special vacation in 2021/22 school year.
2022 Feb 28. Available from: https://www.edb.gov.hk/attachment/en/sch-admin/admin/about-sch/diseases-prevention/edb_20220228_eng.pdf. Accessed 29 Jun 2022
30. McMahan K, Giffin V, Tostanoski LH, et al. Reduced
pathogenicity of the SARS-CoV-2 Omicron variant in
hamsters. Med 2022;3:262-8.e4. Crossref
31. Hong Kong SAR Government. Government announces
latest criteria for discharge from isolation and home
quarantine. Available from: https://www.info.gov.hk/gia/general/202202/26/P2022022600750.htm. Accessed 26 Feb 2022.
32. Centre for Health Protection, Hong Kong SAR Government.
Updated consensus recommendations on criteria for
releasing confirmed COVID-19 patients from isolation (as
of 4 August 2021). 2021. Available from: https://www.chp.gov.hk/files/pdf/updated_consensus_recommendations_on_criteria_for_releasing_confirmed_covid19_patients_from_isolation_4_august2021r.pdf . Accessed 4 Aug 2021.
33. Centre for Health Protection, Hong Kong SAR Government.
Updated consensus recommendations on criteria for
releasing confirmed COVID-19 patients from isolation
(July 29, 2020). 2020. Available from: https://www.chp.gov.hk/files/pdf/updated_consensus_recommendations_on_criteria_for_releasing_confirmed_covid19_patients_from_isolation29july2020.pdf . Accessed 29 Jul 2020.
34. Wang L, Berger NA, Kaelber DC, Davis PB, Volkow ND,
Xu R. Incidence rates and clinical outcomes of SARS-CoV-2 infection with the Omicron and Delta variants in
children younger than 5 years in the US. JAMA Pediatr
2022;176:811-3. Crossref
35. Centre for Health Protection, Hong Kong SAR
Government. COVID-19 Vaccine Programme. Available
from: https://www.chp.gov.hk/en/features/106934.html. Accessed 18 Jan 2024.