Hong Kong Med J 2023 Dec;29(6):542–4 | Epub 1 Dec 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PERSPECTIVE
Can a microbiota-derived health supplement mitigate adverse events after COVID-19 vaccination in children?
CM Chow, FHKCPaed, FHKAM (Paediatrics)1; PK Cheong, MPH2; J Hu, PhD2; Jessica YL Ching, MPH, PhD2
1 Department of Paediatrics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
2 GenieBiome Limited, Hong Kong SAR, China
Corresponding author: Dr Jessica YL Ching (jessicaching@g-niib.com)
Introduction
Coronavirus disease 2019 (COVID-19), caused by
severe acute respiratory syndrome coronavirus 2,
has caused a global pandemic with high rates of
morbidity and mortality. Vaccination is effective in
reducing the risk and severity of COVID-19; recent
evidence suggests that gut microbiota have important
effects on the immune response to vaccination.1
Furthermore, there is increasing concern about
adverse events associated with COVID-19 vaccines,
which range from local inflammatory responses to
anaphylactic reactions and thromboembolic events.
Members of the phylum Bacteroidota are suspected
to stimulate macrophages and monocytes to secrete
a complex array of pro-inflammatory cytokines
(eg, interferon-γ, tumour necrosis factor-α, and
neurotoxins), which could trigger an aberrant
immune response and contribute to a cytokine
storm–induced abnormal inflammatory reaction.2
Thus, the correction of gut dysbiosis through
prebiotic/probiotic supplementation might offer a
solution for the management of COVID-19 vaccine–related adverse reactions.
Exploration of a microbiota-derived health supplement
During the fifth wave of COVID-19 in Hong Kong,
the Hong Kong SAR Government promoted the
vaccination of children aged 6 months to 17 years.
Because there is no prior information regarding
the efficacy or safety of microbiota-derived health
supplements in children undergoing COVID-19
vaccination, we performed a pilot study to evaluate
the use of a health supplement available in Hong
Kong, the G-NiiB Immunity formula (SIM01,
containing 10 billion colony-forming units per
sachet; developed by The Chinese University of Hong
Kong), in alleviating adverse events after COVID-19
vaccination in children aged 5 to 17 years. Our
primary objective was to investigate the safety of
SIM01 in children after COVID-19 vaccination
(first or second dose), when SIM01 use was initiated
prior to vaccination and continued for 7 days after vaccination. We also evaluated the effects of SIM01
on rates of adverse events in vaccinated children,
compared with historical data published by the
vaccine manufacturers. We excluded children with
a known history of COVID-19; a known chronic
illness requiring long-term medication (ie, three
standard doses per week); a known history of allergy
to probiotics or prebiotics; a known history of lactose
intolerance; a known history of (or active) infective
endocarditis; and any use of other antibiotics,
probiotics, or prebiotics during the study period.
Each child’s parents provided written informed
consent to participate in the study.
The children received SIM01 (one sachet twice
daily), beginning 1 week before the first dose of
vaccine and continuing until 1 week after completion
of the second dose of vaccine, or beginning 3 weeks
before the second dose of vaccine and continuing
until 1 week after vaccination; the supplementation
protocol was determined by the vaccine dose that
the participants received during the study period.
Adverse events were recorded using a semi-structured
adverse event assessment form that
included 17 known paediatric adverse events after
COVID-19 vaccination.3 4 Additional symptoms
and adverse event–related medical consultations
were also recorded. The adverse events were
assessed using the following four categories: none,
minimal, tolerable, and distressing. Trained research
personnel conducted phone interviews 1 week after
vaccination to assess the effect of SIM01 on adverse
event severity after COVID-19 vaccination, as well
as adherence to the supplementation protocol.
Participants received medical care for adverse
events as needed. The demographic and medical
characteristics of the participants were recorded,
including the date of COVID-19 vaccination and
type of vaccine received. Logistic regression was
used to adjust for the effects of age, vaccine type, and
vaccine dose on adverse events.
Adverse events with a microbiota-derived
health supplement
Between April 2022 and August 2022, 102 children aged 5 to 17 years were enrolled in the study. Seven
children were excluded from analysis because
they did not undergo vaccination or use SIM01.
The remaining 95 children (mean age ± standard
deviation [SD]=9.03 ± 3.123 years; 48.4% boys and
51.6% girls) used SIM01 supplementation while
receiving at least one vaccine dose; they underwent
assessments of adverse events. The supplementation
protocol adherence rate was 97% across 124
vaccine doses. Notably, three participants reported
temporary abdominal distension, loose stool, or
mouth sores.
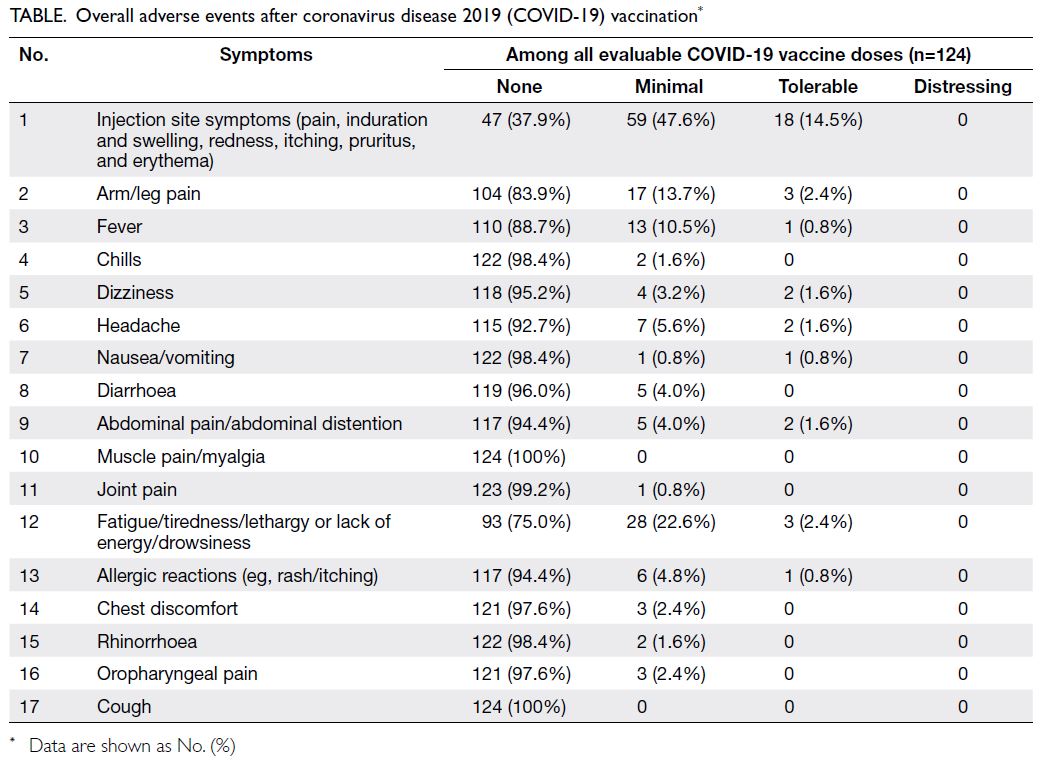
In terms of overall adverse events, the most
common event was injection site symptoms (pain,
induration and swelling, redness, itching, pruritus,
and erythema) [Table]. The second most common
event was fatigue/tiredness/lethargy or lack of
energy/drowsiness. Other common adverse events
were arm/leg pain and fever. BioNTech/Pfizer
vaccine BNT162b2 has a higher rate of reported
adverse events among children, compared with
CoronaVac (Sinovac Biotech). The odds ratio (OR)
for injection site symptoms was 2.59 (95% confidence
interval [CI]=1.14-5.95; P=0.02) with BNT162b2.
The ORs for arm/leg pain and fever were 6.5 (95%
CI=1.44-60.89; P=0.08) and 13.78 (95% CI=2.22 to
infinity; P=0.02), respectively. Other adverse event
ORs were not statistically significant.
Thirty-eight children received the first dose
of vaccine, whereas 86 received the second dose.
Adverse events were similar between the two doses,
although some effects were more common after the
second dose: arm/leg pain (second dose: 18.6%, first
dose: 10.5%), fever (12.8% vs 7.9%), chills (2.3% vs
0%), dizziness (5.8% vs 2.6%), headache (10.5% vs 0%),
diarrhoea (4.7% vs 2.6%), fatigue (including fatigue/tiredness/lethargy or lack of energy/drowsiness;
26.7% vs 21.1%), chest discomfort (3.5% vs 0%),
rhinorrhoea (2.3% vs 0%), and oropharyngeal pain
(3.5% vs 0%). Most of these between-dose differences
were not statistically significant; headache tended to
be more common after the second dose (P=0.056).
Among the 95 evaluable participants, 81 were
aged 5 to 12 years (mean ± SD=8.09 ± 2.24 years)
and 14 were aged 13 to 17 years (mean ± SD=14.5 ±
1.40 years). Although the incidences of adverse
events generally did not differ according to age,
older children more frequently reported dizziness
(OR=8.59, 95% CI=1.14-99.69; P=0.03) and headache
(OR=9.05, 95% CI=1.80-61.98; P=0.005).
Adverse events without a microbiota-derived health supplement
The BNT162b2 Product Monograph showed that the common adverse events among children aged 5
to 15 years are injection site pain or local reaction,
tiredness, headache, chills, and myalgia.3 Adverse
events are more common in older children (aged 12
to 15 years). Children receiving SIM01 in our study
had fewer adverse events when receiving BNT162b2,
particularly regarding injection pain or local reaction
(71% vs 84.3%-90.5% in Product Monograph data),
tiredness (29% vs 51%-77%), headache (10% vs 38.2%-78.5%), chills (3% vs 12.4%-49.2%), and myalgia (0%
vs 17.5%-42.2%). However, a direct comparison
between our data and Product Monograph data
could not be performed because there were
considerable differences in study conditions. There
was no obvious decrease in adverse events among
children receiving CoronaVac, possibly because this
vaccine has fewer adverse events.5 A larger sample
size may be necessary to identify any effect of SIM01
on adverse events after receipt of CoronaVac.
Implications and future work
Recent research indicates that baseline gut microbiota
composition can predict immune responses to
COVID-19 vaccines and vaccine-related adverse
events in adults.6 7 However, corresponding data
for children have been limited. Our study showed
that the microbiota-derived SIM01 formula is well
tolerated in children aged 5 to 17 years. Among the 95
children in this study, only two discontinued SIM01
supplementation because of abdominal distension
and loose stool or mouth sores. Most adverse events
were mild and transient; none were considered
distressing. Future studies should include a control
group to validate the findings; they should also focus
on mechanistic analyses. Our key findings were that
SIM01 supplementation was safe for children before
COVID-19 vaccination, and the rates of adverse
events after vaccination appeared to be lower in
children undergoing SIM01 supplementation than
in a historical control group who did not use SIM01.
These findings offer insights to support randomised
controlled trials in the future, as well as information
that may reduce vaccine hesitancy among parents
and children.
Author contributions
Concept or design: CM Chow.
Acquisition of data: JYL Ching, PK Cheong.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: CM Chow, JYL Ching.
Critical revision of the manuscript for important intellectual content: J Hu, JYL Ching.
Acquisition of data: JYL Ching, PK Cheong.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: CM Chow, JYL Ching.
Critical revision of the manuscript for important intellectual content: J Hu, JYL Ching.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
CM Chow has disclosed no conflicts of interest. PK Cheong is an employee of GenieBiome Limited. J Hu is a scientist at GenieBiome Limited. JYL Ching is a clinical research consultant for GenieBiome Limited.
Funding/support
All funding was provided by GenieBiome Limited (Ref No.: GB-IRB 0001CT/2022). The funder had no role in study design, data collection/analysis/interpretation, or manuscript
preparation.
Ethics approval
This study was approved by the GenieBiome Independent
Review Board (Ref No.: GB-IRB 0001CT/2022) and was
conducted in compliance with the Declaration of Helsinki.
Written informed consent form was signed by the parent of
all participants prior to study recruitment.
References
1. de Jong SE, Olin A, Pulendran B. The impact of the
microbiome on immunity to vaccination in humans. Cell
Host Microbe 2020;28:169-79. Crossref
2. Zimmermann P, Curtis N. The influence of the intestinal microbiome on vaccine responses. Vaccine 2018;36:4433-9. Crossref
3. Comirnaty® (COVID-19 vaccine, mRNA) product
monograph. 18 February 2022, Pfizer Canada. Available
from: https://covid-vaccine.canada.ca/info/pdf/pfizer-biontech-covid-19-vaccine-pm1-en.pdf. Accessed 19 May 2022.
4. Department of Health, Hong Kong SAR Government.
Vaccination fact sheet for CoronaVac. 3 April 2023.
Available from: https://www.chp.gov.hk/files/pdf/covid19vaccinationfactsheet_coronavac_eng.pdf. Accessed 9 Nov 2023.
5. Han B, Song Y, Li C, et al. Safety, tolerability, and
immunogenicity of an inactivated SARS-CoV-2 vaccine
(CoronaVac) in healthy children and adolescents: a double-blind,
randomised, controlled, phase 1/2 clinical trial.
Lancet Infect Dis 2021;21:1645-53. Crossref
6. Ng SC, Peng Y, Zhang L, et al. Gut microbiota composition
is associated with SARS-CoV-2 vaccine immunogenicity
and adverse events. Gut 2022;71:1106-16. Crossref
7. Zhang L, Xu Z, Mak JW, et al. Gut microbiota-derived
synbiotic formula (SIM01) as a novel adjuvant therapy
for COVID-19: an open-label pilot study. J Gastroenterol
Hepatol 2022;37:823-31. Crossref