Hong Kong Med J 2023 Dec;29(6):514–23 | Epub 16 Nov 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Ten-year territory-wide trends in the utilisation and clinical outcomes of extracorporeal membrane oxygenation in Hong Kong
Pauline Y Ng, MB, BS, FHKCP1,2; Vindy WS Chan1; April Ip, MPH1; Lowell Ling, MB, BS, FHKCA3; KM Chan, MB, ChB, FCICM3; Anne KH Leung, MB, ChB, FHKCA4; Kenny KC Chan, MB, ChB, MStat5; Dominic So, MB, BS, HKCA6; HP Shum, MB, BS, MD7; CW Ngai, MB, ChB, FHKCP2; WM Chan, MB, ChB, FHKCP2; WC Sin, MB, ChB, FHKCP2,8
1 Department of Medicine, The University of Hong Kong, Hong Kong SAR, China
2 Department of Adult Intensive Care, Queen Mary Hospital, Hong Kong SAR, China
3 Department of Anaesthesia and Intensive Care, The Chinese University of Hong Kong, Hong Kong SAR, China
4 Department of Intensive Care, Queen Elizabeth Hospital, Hong Kong SAR, China
5 Department of Intensive Care, Tuen Mun Hospital, Hong Kong SAR, China
6 Department of Intensive Care, Princess Margaret Hospital, Hong Kong SAR, China
7 Department of Intensive Care, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
8 Department of Anaesthesiology, The University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr PY Ng (pyeungng@hku.hk)
Abstract
Introduction: The utilisation of extracorporeal
membrane oxygenation (ECMO) has been rapidly
increasing in Hong Kong. This study examined
10-year trends in the utilisation and clinical
outcomes of ECMO in Hong Kong.
Methods: We retrospectively reviewed the records
of all adult patients receiving ECMO who were
admitted to the intensive care units (ICUs) of public
hospitals in Hong Kong between 2010 and 2019.
Temporal trends across years were assessed using
the Mann–Kendall test. Observed hospital mortality
was compared with the Acute Physiology and
Chronic Health Evaluation (APACHE) IV–predicted
mortality.
Results: The annual number of patients receiving
ECMO increased from 18 to 171 over 10 years. In
total, 911 patients received ECMO during the study
period: 297 (32.6%) received veno-arterial ECMO,
450 (49.4%) received veno-venous ECMO, and 164
(18.0%) received extracorporeal cardiopulmonary
resuscitation. The annual number of patients aged
≥65 years increased from 0 to 47 (27.5%) [P for
trend=0.001]. The median (interquartile range)
Charlson Comorbidity Index increased from
1 (0-1) to 2 (1-3) [P for trend<0.001] while the
median (interquartile range) APACHE IV score
increased from 90 (57-112) to 105 (77-137) [P for
trend=0.003]. The overall standardised mortality
ratio comparing hospital mortality with APACHE
IV–predicted mortality was 1.11 (95% confidence
interval=1.01-1.22). Hospital and ICU length of stay
both significantly decreased (P for trend=0.011 and
<0.001, respectively).
Conclusions: As ECMO utilisation increased in
Hong Kong, patients put on ECMO were older,
more critically ill, and had more co-morbidities.
It is important to combine service expansion
with adequate resource allocation and training to
maintain quality of care.
New knowledge added by this study
- During the 10-year study period, there was increasing utilisation of extracorporeal membrane oxygenation (ECMO) in older patients, patients with more co-morbidities, and patients with greater disease severity.
- Patients receiving ECMO require significant resources for out-of-hours services, inter-hospital transfers, and major operations.
- Although the observed hospital mortality was comparable with the Acute Physiology and Chronic Health Evaluation IV–predicted mortality, efforts should be made to systematically collect physiological data for computation of Survival after Veno-Arterial ECMO and Respiratory ECMO Survival Prediction scores in the future.
- Among patients receiving ECMO in Hong Kong, clinical outcomes can be improved by revising patient selection criteria, enhancing therapy for bridge to transplantation and promoting organ transplantation, and consolidating ECMO services in specialised centres.
Introduction
Extracorporeal membrane oxygenation (ECMO)
offers life-sustaining support by supplementing
heart and lung functions in patients with circulatory
or respiratory failure. There is increasing utilisation
of ECMO in intensive care units (ICUs) worldwide;
for example, the Extracorporeal Life Support
Organization (ELSO) registry reported a 10-fold
increase in ECMO runs from 1643 in 1990 to 18 260 in
2020.1 Hong Kong is a Special Administrative Region
of the People’s Republic of China, with a population
of 7.4 million and an independent healthcare
system.2 In Hong Kong, various assessments of
ICU performance have been performed for other
disease entities,3 but there have been few reports
of ECMO-specific data and patient outcomes.4 In
particular, Hong Kong has a higher ECMO centre–to-population ratio compared with international
guidelines.5 6 A retrospective study examined
the risk score–mortality association in patients
receiving ECMO, but it only included data from a
single tertiary ICU and was not fully representative
of territory-wide practices.7 Because ECMO is a
high-cost, labour-intensive ICU treatment modality,
it is important to understand how ECMO is utilised
in Hong Kong, its associated resource implications,
and review patient outcomes for future planning efforts.
In this study, using a territory-wide
administrative registry of all patients receiving
ECMO in the ICUs of public hospitals in Hong Kong,
we examined trends in ECMO utilisation and clinical
outcomes. Our primary objective was to summarise
the status of ECMO services in Hong Kong over the
past decade.
Methods
Study population
This retrospective observational study covered the
period from 1 January 2010 to 31 December 2019.
All adult patients aged ≥18 years with an ECMO
episode and admission to the ICU of a public hospital
under the Hospital Authority were identified using
an administrative ECMO patient registry managed
by a centralised ICU committee. An episode of
ECMO was defined on the basis of the International
Classification of Diseases, Ninth Revision, Clinical
Modification (ICD-9-CM) procedure code for
ECMO.8 The need for ICU admission was determined
using the Acute Physiology and Chronic Health
Evaluation IV (APACHE IV) evaluation form.9 10
Patients with missing ECMO details (eg, ECMO
duration and configuration) and patients managed
in non-mixed disciplinary ICUs were excluded from
the study.
Data collection
Extracorporeal membrane oxygenation data were extracted from the administrative patient registry,
which contained information about ECMO
configuration, time of initiation, and time of
discontinuation that had been entered by qualified
nurses at the corresponding ECMO centre. The
Clinical Data Analysis and Reporting System
(CDARS), a central de-identified data repository
comprising electronic health records from all public
hospitals in Hong Kong, was accessed to collect
patient baseline characteristics and components
of the following disease severity scores: Sequential
Organ Failure Assessment (SOFA),11 Survival
after Veno-Arterial ECMO (SAVE),12 Respiratory
ECMO Survival Prediction (RESP),13 Charlson
Comorbidity Index (CCI), and APACHE IV14 (online supplementary Table 1). For patients with multiple
ICU admissions during a single hospital stay, the
APACHE IV score for the first ICU admission was
used. Clinical outcomes including length of stay
(LOS) and mortality were retrieved from the CDARS.
Study outcomes and definitions
The primary outcomes were trends in ECMO utilisation over 10 years, including number of patients
receiving ECMO, illness severity (as measured by
disease severity scores), and numbers of tertiary and quaternary inter-hospital transfers. Secondary
outcomes were mortality, hospital and ICU LOS,
transplantation procedures, ventricular assistive
device (VAD) implantation, and complications. For
patients who were transferred between hospitals,
hospital mortality was defined as death during the
final hospitalisation. Four common complications of
ECMO, namely haemorrhagic, neurological, renal
and cardiovascular complications, were identified
using ICD-9-CM diagnostic and procedural codes
(online supplementary Table 2).8 Major non-cranial
bleeding was identified as the diagnosis of
gastrointestinal, major internal, and/or postoperative
bleeding; alternatively, it was identified by the need
for haemostatic procedures, transfusion of >2 units
of packed red blood cells over 24 hours, and/or use
of recombinant factor VII. Stroke was subdivided
into haemorrhagic and ischaemic types. Patients
with acute ischaemic limbs were identified by the
diagnosis of acute limb ischaemia or compartment
syndrome or by the performance of limb-saving
procedures (eg, fasciotomy and amputation). Brain
death was identified by the appropriate diagnostic
code or by a procedure code indicating organ
collection from a deceased donor.
For the purposes of subsequent analyses,
ECMO centres referred to designated ICUs
under the governance of the Hospital Authority
Central Organising Committee in ICU Services.
An emergency admission was defined as an
admission in which the patient had emergency room
attendance records within the preceding 12 hours.
Extracorporeal membrane oxygenation initiation
in the emergency room was defined as an ECMO
episode in which the patient had emergency room
attendance records within the preceding 24 hours.
An inter-hospital transfer was defined when ECMO
was started at another institution before patient
transferal with ECMO in situ to one of six ECMO
centres. A transfer to a quaternary cardiothoracic
unit was defined as an instance of intra- or inter-hospital
transfer from a mixed ICU to cardiothoracic
care in one of three centres, either during ECMO
care or within 12 hours after stopping ECMO.
Statistical analysis
Frequencies and percentages were used to describe
categorical variables. The Shapiro–Wilk test was
used to assess data normality; data were expressed
as means with standard deviations or medians with
interquartile ranges, as appropriate. Categorical
variables were compared between groups using
the Chi squared test; continuous variables were
compared by the t test or Mann-Whitney U test,
as appropriate. The Mann–Kendall test was used
to assess temporal trends in patient characteristics
and outcomes across years, in sequential order from
2010 to 2019. Model discrimination and model calibration of risk scores in predicting hospital
mortality were examined using the area under the
receiver operating characteristic (AUROC) curve
and the Hosmer–Lemeshow test. The observed
hospital mortality was compared with that predicted
from risk scores using standardised mortality ratios
(SMRs). Patients with missing APACHE IV scores
were excluded from this analysis.
All statistical analysis and data visualisation
procedures were performed in Stata 16 (StataCorp;
College Station [TX], United States). Tests were
considered statistically significant when two-tailed P
values were <0.05.
Results
Patient characteristics and co-morbidities
From January 2010 to December 2019, among
125 101 ICU admissions overall in Hong Kong,
911 (0.73%) involved patients receiving ECMO as
follows: 297 (32.6%) veno-arterial (V-A) ECMO, 450
(49.4%) veno-venous (V-V) ECMO, and 164 (18.0%)
extracorporeal cardiopulmonary resuscitation
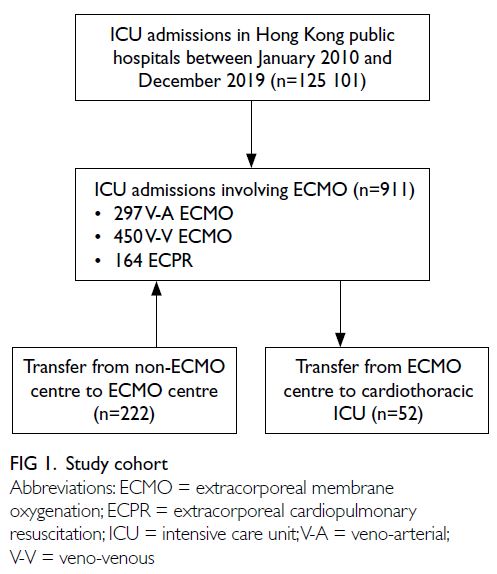
(ECPR) [Fig 1]. There was a steady increase in the
annual number of patients receiving ECMO, with
a 9.5-fold increase from 18 episodes in 2010 to
171 episodes in 2019 (Fig 2). The annual number
of V-A ECMO episodes significantly increased
from 3 (16.7%) to 67 (39.2%) over 10 years (P for
trend=0.001) [Table 1]. The total number of ECMO
patient-days increased from 109 in 2010 to 1565 in
2019 (online supplementary Fig 1).
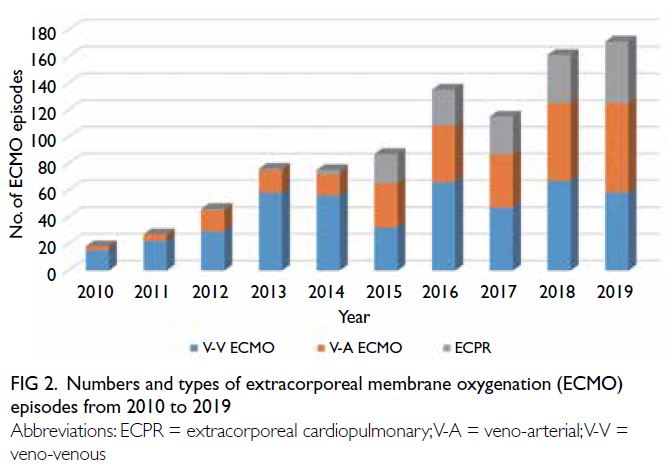
Figure 2. Numbers and types of extracorporeal membrane oxygenation (ECMO) episodes from 2010 to 2019
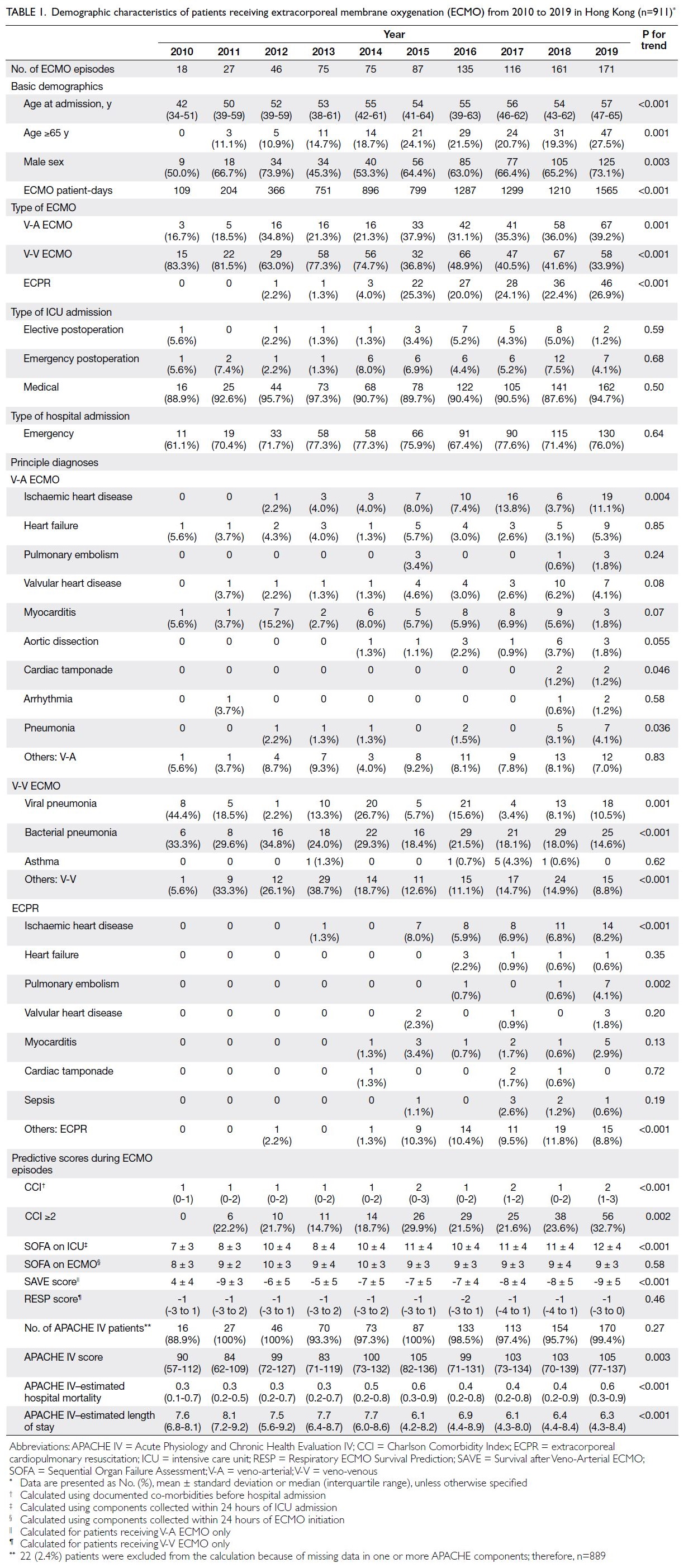
Table 1. Demographic characteristics of patients receiving extracorporeal membrane oxygenation (ECMO) from 2010 to 2019 in Hong Kong (n=911)
A total of 583 (64.0%) patients were male, with
a median age at admission of 54 years (interquartile
range, 42-62), and 185 (20.3%) patients of ≥65 years.
There was increasing utilisation of ECMO among
patients aged ≥65 years (P for trend=0.001). The
median CCI was 1 (0-2), and an increasing number of
patients had a CCI ≥2 (P for trend=0.002) [Table 1].
Among the 889 (97.6%) patients with complete
APACHE IV data, the median APACHE IV score was
100 (73-132), with an increase from 90 (57-112) in
2010 to 105 (77-137) in 2019 (P for trend=0.003); the
median APACHE IV–estimated risk of death was 0.5
(0.2-0.8). Complete demographic details are shown
in Table 1; trends in co-morbidities and disease
severity scores are shown in online supplementary Figure 2.
Extracorporeal membrane oxygenation
resources and inter-hospital transfers
Within the publicly funded hospital system, the
number of ECMO centres under centralised ICU
governance increased from three in 2010 to five in
2015, and then seven in 2019. The total number of
available ECMO consoles paralleled the increase:
from three in 2010 to nine in 2015, and then 11 in
2019 (online supplementary Table 3).
Among the 911 patients receiving ECMO,
469 (51.5%) were initiated outside of the regular 9
am to 5 pm period, including 247 (52.7%) patients
receiving V-V ECMO, 137 (29.2%) patients receiving
V-A ECMO, and 85 (18.1%) patients receiving ECPR.
In total, 710 (77.9%) emergency admissions were
identified. These patients were younger, had fewer
co-morbidities, and were more likely to receive
V-V ECMO [371/710 (52.3%) vs 79/201 (39.3%);
P=0.001]. In total, 370 (40.6%) patients had ECMO
initiated within 24 hours of emergency admission;
these patients were more likely to receive ECPR
[113/370 (30.5%) vs 51/541 (9.4%); P<0.001] and have higher APACHE IV scores [118 (86-146) vs 91
(69-118); P<0.001].
Overall, there were 222 (24.4%) episodes of
inter-hospital transfer from non-ECMO centres
to ECMO centres; the annual number of episodes
increased from one (1/18 [5.6%]) in 2010 to 22
(22/171 [12.9%]) in 2019 (P for trend<0.001) [online supplementary Fig 3]. In total, 173 (77.9%) patients
were transferred from ICUs in other hospitals;
the remaining 49 patients were transferred from
non-ICU settings. Most transferred patients (66.2%)
received V-V ECMO (online supplementary Fig 4a); their principal diagnoses are shown in online supplementary Figure 4b and 4c. Transferred
patients had worse RESP scores [-2 (-4 to 0) vs -1
(-3 to 2); P<0.001], better SAVE scores (-6 ± 5 vs -8
± 5; P=0.012), and lower APACHE IV scores [89 (69-117) vs 104 (75-136); P<0.001]. Among the patients
transferred to ECMO centres, 54 (24.3%) underwent
a major operation within 7 days of transfer, and 32
(59.3%) of these surgeries involved the cardiovascular
system. Other procedural details are shown in online supplementary Figure 4d and 4e.
There were 52 (5.7%) episodes of inter-hospital
transfer to quaternary cardiothoracic ICUs; the
annual number remained relatively consistent
throughout the 10-year study period (P for
trend=0.121) [online supplementary Fig 3]. Patients
in these transfers were younger (P=0.048); they were
more likely to receive V-A ECMO [31/52 (59.6%) vs
266/859 (31.0%); P<0.001] and ECPR [15/52 (28.8%)
vs 149/859 (17.3%); P=0.036] (online supplementary Fig 5a). The primary diagnoses are shown in online supplementary Figure 5b and 5c. Among the
patients involved in quaternary transfers, 22 (42.3%)
underwent a major operation within 28 days of
transfer, and 18 (81.8%) of these surgeries involved
the cardiovascular system. Other procedural details
are shown in online supplementary Figure 5d and 5e.
Patient outcomes
The overall numbers of hospital mortalities and
ICU mortalities were 456 (50.1%) and 382 (41.9%),
respectively. The numbers of hospital mortalities
among patients receiving V-V ECMO, V-A ECMO,
and ECPR were 152 (33.9%), 178 (59.9%), and 126
(76.8%), respectively (online supplementary Table 4). The median hospital LOS was 26.8 (interquartile range, 10.7-55.6) days, and the median ICU LOS
was 10.2 (interquartile range, 4.8-20.1) days [Table 2]. Throughout the 10-year study period, the annual
number of hospital mortalities increased from
one (5.6%) in 2010 to 90 (52.6%) in 2019 (P for
trend<0.001). The hospital LOS decreased from 36.6
(interquartile range, 26.8-57.2) to 25.2 (7.6-50.2)
days [P for trend=0.011], and ICU LOS decreased
from 15.5 (10.8-18.2) days in 2010 to 7.9 (3.9-19.8)
days in 2019 (P for trend<0.001).
After adjustments for age, sex, APACHE
IV score, and type of ECMO, the odds of hospital
mortality were significantly lower in patients with
ECMO initiated within 24 hours of emergency
admission (adjusted odds ratio [OR]=0.56, 95%
confidence interval [CI]=0.40-0.78; P=0.001). There
were no significant associations with hospital
mortality among patients who had emergency admission (adjusted OR=0.78, 95% CI=0.54-
1.12; P=0.17), patients who were transferred to
ECMO centres (adjusted OR=0.74, 95% CI=0.52-
1.05; P=0.09), or patients who were transferred to
quaternary cardiothoracic ICUs (adjusted OR=0.58,
95% CI=0.30-1.13; P=0.11). Patients transferred to
quaternary cardiothoracic ICUs had significantly
lower ICU mortality [4 (7.7%) vs 378 (44.0%); P<0.001] and significantly longer hospital LOS [38.3
(22.1-111.0) vs 25.6 (9.4-53.1) days, P<0.001]. The
unadjusted and adjusted outcomes in various patient
subgroups are presented in online supplementary Table 5.
In total, 41 (4.5%) patients were successfully
bridged to VAD or transplantation. Among 461
patients who were receiving V-A ECMO and ECPR,
31 (6.7%) patients underwent VAD implantation
and eight (1.7%) patients underwent heart transplantation. Among 450 patients who were
receiving V-V ECMO, one (0.2%) patient underwent
lung transplantation.
In terms of complications, there were 466
(51.2%) cases of major bleeding, 28 (3.1%) ischaemic
limb complications, and nine (1.0%) patients who
were declared brain-dead. Among 76 (8.3%) patients
with stroke, 54 (5.9%) had haemorrhagic stroke
(Table 2).
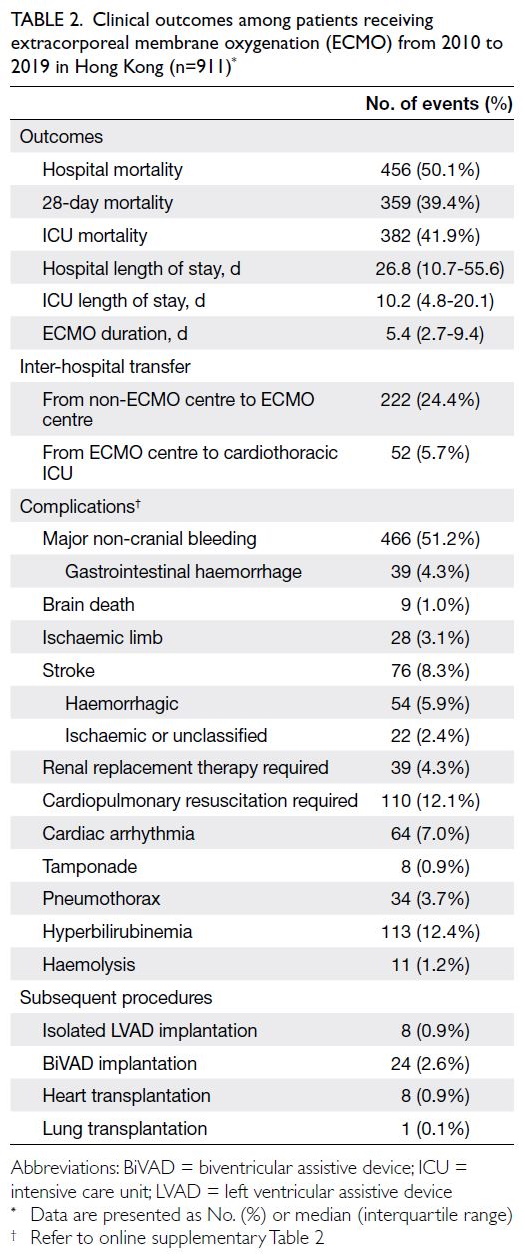
Table 2. Clinical outcomes among patients receiving extracorporeal membrane oxygenation (ECMO) from 2010 to 2019 in Hong Kong (n=911)
Prediction of hospital mortality
The ability of risk scores to predict post-ECMO
hospital mortality was examined. There was a
significant increase in the annual median APACHE
IV score from 90 (57-112) in 2010 to 105 (77-137) in
2019 (P for trend=0.003). The SOFA score on the first
day of ICU admission and the SAVE score in patients
receiving V-A ECMO also showed significant trends
(P for trend<0.001). No significant trends were
observed regarding the SOFA score on the first day
of ECMO (P for trend=0.58) or the RESP score in
patients receiving V-V ECMO (P for trend=0.46)
[Table 1].
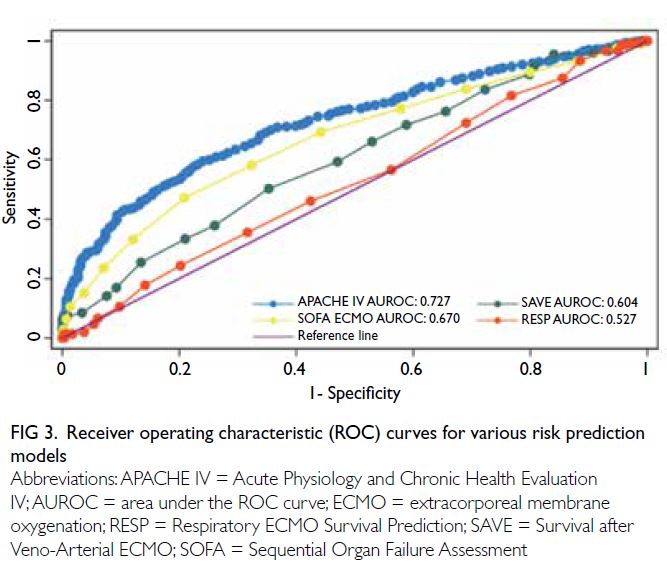
The APACHE IV score showed good
discriminatory power and was well calibrated for
the prediction of hospital mortality (AUROC=0.727;
Hosmer–Lemeshow test P=0.356); as was SOFA
score on the first day of ECMO (AUROC=0.670;
Hosmer–Lemeshow test P=0.322) [Fig 3]. The overall
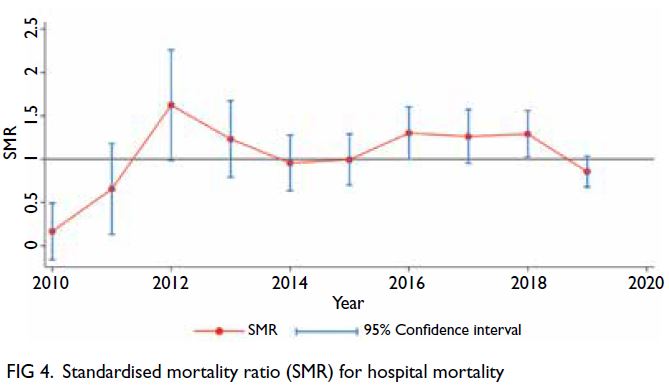
SMR for hospital mortality compared with APACHE
IV–predicted mortality was 1.11 (95% CI=1.01-1.22) and there was no significant trend over the
10-year study period (P for trend=0.135) [Fig 4].
The SAVE and RESP scores, estimated using data
from electronic health records, displayed limited
discriminatory power for the prediction of hospital
mortality in patients receiving V-A and V-V ECMO
(AUROC=0.604 and 0.527, respectively). The ROC
curves for various risk prediction models are shown
in Fig 3.
Discussion
To our knowledge, this is the first 10-year longitudinal
study of the majority of patients receiving ECMO in
Hong Kong; the results showed that the numbers
of patients and complexities of medical conditions
increased throughout the study period. Although
patients receiving ECMO represent a small
proportion of ICU patients overall, they require
significant resource utilisation including out-of-hours
services, inter-hospital transfers, and major
operations. Comparisons with standardised risk
scores suggested satisfactory performance based
on the APACHE IV model, but the lack of complete
and granular patient data precluded meaningful
conclusions with respect to ECMO-specific risk
scores.
Trends in patient characteristics
In addition to the observation of a 9.5-fold increase
in ECMO utilisation in Hong Kong over the 10-year
study period, including greater use of V-A ECMO
after 2012 and rapid uptake of ECPR after 2015, this
study revealed that patients receiving ECMO were
increasingly older, had an increasing co-morbidity
burden, and displayed greater disease severity upon
ICU admission. This is not only attributable to the
overall advances in ECMO,15 but also encouraged
by the multiple studies showing indistinguishable
survival after ECMO in older adult patients
compared with the younger ones.16 17 18 The increased utilisation of ECMO in ECPR is supported by clinical
trials demonstrating the efficacy of this approach. In
the CHEER trial (mechanical CPR, Hypothermia,
ECMO and Early Reperfusion), treatment with
mechanical cardiopulmonary resuscitation,
hypothermia, ECMO, and early reperfusion led to
increased survival among patients with refractory
cardiac arrest.19 The ARREST trial (Advanced
Reperfusion Strategies for Refractory Cardiac
Arrest) showed a similar increase in survival upon
initiation of early ECPR among patients with out-of-hospital cardiac arrest and refractory ventricular
fibrillation.20 The increasing numbers of patients
receiving ECMO have also resulted from greater
utilisation of V-A ECMO to manage conditions
such as acute myocardial infarction complicated
by refractory cardiogenic shock,21 as well as efforts
to transition to therapies including VAD and heart
transplantation.22 The overall growth of ECMO
utilisation in Hong Kong is similar to global patterns
evident in the ELSO registry.1
Patient mortality
The observed overall SMR for post-ECMO hospital
mortality was slightly worse than the predicted
overall SMR, possibly because ECMO services
were in early phases of development at various
centres throughout the study period. The decrease
in SMR in the later portion of the study period,
when ECMO services had matured at most centres,
may be an indication of progress. When the results
were stratified according to the type of ECMO, we
found that the rate of hospital mortality among
patients receiving V-V ECMO was better in Hong
Kong than in the global ELSO registry (33.9% vs
40.8%), whereas the rates of hospital mortality
among patients receiving V-A ECMO and ECPR
were worse (V-A ECMO: 59.8% in Hong Kong vs
55.4% globally; ECPR: 76.8% in Hong Kong vs 69.8%
globally). The sharp increase in ECPR utilisation may
have contributed to an artificially elevated SMR,
considering that ECPR is associated with worse
survival relative to V-V ECMO and V-A ECMO19;
notably, in a pilot cohort of patients receiving ECPR
in Hong Kong, ICU survival was 32.4%.23 The low
rate of ECMO bridging to transplantation in Hong
Kong—nine (1.0%) patients over 10 years—also
reduces overall cohort survival. Among developed
countries/regions, Hong Kong has a very low rate
of registration in the Centralised Organ Donation
Register (3.8%) and limited motivation to participate
in organ donation.24 Nevertheless, it remains
important to actively explore methods to lower
the SMR. One possibility involves consolidating
ECMO services to a few specialised centres, based
on evidence of a volume-outcome relationship
repeatedly identified in other observational cohorts
across various geographical regions and healthcare settings.25 26 Furthermore, a study in the United
States showed that multidisciplinary interventions—including coordination among surgeons,
cardiologists, and ECMO specialists, as well as the
implementation of standardised ECMO admission
and weaning protocols—were associated with lower
mortality in patients receiving ECMO,27 indicating a
need to strengthen interdisciplinary communication
or expand collaborations with allied health services
to maintain standards of care.
Risk prediction
The comparative utility of various risk scores for
outcome prediction in Hong Kong merits attention.
In terms of predicting hospital mortality among
patients receiving ECMO in the present study, the
APACHE IV score performed best, followed by the
SOFA score on the first day of ECMO; the SAVE and
RESP scores had moderate discriminatory power.
The satisfactory performance of the APACHE IV
score in Hong Kong was previously demonstrated
in a large retrospective cohort study of ICU patients
(c-statistic=0.889).28 Importantly, most data were
available for APACHE IV scores in the present study,
and the corresponding accuracy was high. However,
the main limitation of APACHE IV scores is the
lack of definite correlation with the time and patient
condition upon ECMO initiation,29 which likely
leads to a systemic under-representation of disease
severity. The SOFA score, which can be calculated
on a daily basis, has the theoretical advantage of
more closely reflecting disease severity and clinical
progression30; the SOFA score on the date of
ECMO initiation demonstrated good performance
in predicting hospital mortality among patients
in our cohort. We note that the limited predictive
performances of ECMO-specific SAVE and RESP
scores are mainly related to the difficulty of retrieving
accurate physiological data from the CDARS; various
components of the scores were determined by a
combination of diagnostic codes, procedural codes,
and laboratory parameters. Although these scores
have been validated in international cohorts,12 13
their systematic adoption as benchmarks for ECMO
service performance in Hong Kong is hindered by
the lack of available patient data. Among the six
ECMO centres included in the present study, only
four routinely collect patient and ECMO data for
submission to the international ELSO registry; none
of the centres compute SAVE and RESP scores.
Within the community of ECMO providers in Hong
Kong, we strongly encourage collaborative efforts to
routinely document ECMO-specific severity scores
and improve coding practices within electronic
health records and the CDARS; these approaches
will facilitate outcome monitoring and resource
allocation. Moreover, validation of these scores in
Hong Kong will be informative because Asians were substantially underrepresented in the original scoredevelopment
cohorts established using the ELSO
international registry.12 13
Limitations
There were some limitations in this study. First, the
retrospective observational design utilised data that
were not recorded in a manner intended for research
purposes; systematic biases in missing data may
be present. Inaccurate diagnoses and procedural
coding practices may have led to insufficient
collection of relevant clinical data and information
regarding ECMO circuit complications. However,
the clinical outcomes of hospital mortality and
LOS were captured from administrative data with a
low risk of error. Second, the presence of between-centre
heterogeneity related to non-uniform
clinical practices may have contributed to outcome
differences that were not reflected in the overall
cohort. Third, patients receiving ECMO in non-mixed
disciplinary ICUs or coronary care units were
excluded from the study; outcomes and resource
utilisation may have been considerably different
among these patients. Finally, the collected data did
not allow examination of ECMO cost-effectiveness,
an important metric for service and resource
planning.
Conclusion
In this territory-wide study, we observed increasing
trends in ECMO utilisation in Hong Kong that were
similar to global patterns. The overall observed
mortality was reasonably close to the APACHE IV–predicted mortality. Systematic documentation of
ECMO-specific risk scores is needed to ensure high-quality
data for ECMO service benchmarking and
development efforts.
Author contributions
Concept or design: PY Ng, A Ip.
Acquisition of data: VWS Chan, A Ip.
Analysis or interpretation of data: PY Ng, VWS Chan.
Drafting of the manuscript: PY Ng, VWS Chan.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: VWS Chan, A Ip.
Analysis or interpretation of data: PY Ng, VWS Chan.
Drafting of the manuscript: PY Ng, VWS Chan.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research was supported by an unrestricted philanthropic
donation from Mr and Mrs Laurence Tse. The funder had no
role in study design, data collection, analysis and interpretation
of the data, or manuscript preparation.
Ethics approval
This research was approved by the Institutional Review Board of The University of Hong Kong/Hospital Authority Hong Kong West Cluster (Ref No.: UW 20-573). The research was
conducted in accordance with the Declaration of Helsinki.
The requirement for informed consent was waived by the
Board due to the retrospective nature of the research.
References
1. Extracorporeal Life Support Organization. ECLS Registry
Report. International Summary. October 2021. Report
data through 2020. Available from: https://www.elso.org/Portals/0/Files/Reports/2021_October/International%20Report%20October_page1.pdf. Accessed 20 Dec 2021.
2. Department of Health, Hong Kong SAR Government. Health Facts of Hong Kong. 2021 Edition. Available from:
https://www.dh.gov.hk/english/statistics/statistics_hs/files/2021.pdf. Accessed 20 Dec 2021
3. Lam KW, Lai KY. Evaluation of outcome and performance
of an intensive care unit in Hong Kong by APACHE IV
model: 2007-2014. J Emerg Crit Care Med 2017;1:16. Crossref
4. Sin SW, Young K. Development of extracorporeal
membrane oxygenation in Hong Kong: current challenges
and future development. Hong Kong Med J 2017;23:216-7. Crossref
5. Combes A, Brodie D, Bartlett R, et al. Position paper for
the organization of extracorporeal membrane oxygenation
programs for acute respiratory failure in adult patients. Am
J Respir Crit Care Med 2014;190:488-96. Crossref
6. Ng PY, Ip A, Fang S, et al. Effect of hospital case volume
on clinical outcomes of patients requiring extracorporeal
membrane oxygenation: a territory-wide longitudinal
observational study. J Thorac Dis 2022;14:1802-14. Crossref
7. Ng WT, Ling L, Joynt GM, Chan KM. An audit of mortality
by using ECMO specific scores and APACHE II scoring
system in patients receiving extracorporeal membrane
oxygenation in a tertiary intensive care unit in Hong Kong.
J Thorac Dis 2019;11:445-55. Crossref
8. National Center for Health Statistics, Centers for Disease
Control and Prevention. International Classification of
Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Available from: https://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed 10 Oct 2023.
9. Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute
Physiology and Chronic Health Evaluation (APACHE)
IV: hospital mortality assessment for today's critically ill
patients. Crit Care Med 2006;34:1297-310. Crossref
10. Zimmerman JE, Kramer AA, McNair DS, Malila FM,
Shaffer VL. Intensive care unit length of stay: benchmarking
based on Acute Physiology and Chronic Health Evaluation
(APACHE) IV. Crit Care Med 2006;34:2517-29. Crossref
11. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related
Organ Failure Assessment) score to describe organ
dysfunction/failure. On behalf of the Working Group
on Sepsis-Related Problems of the European Society of
Intensive Care Medicine. Intensive Care Med 1996;22:707-10. Crossref
12. Schmidt M, Burrell A, Roberts L, et al. Predicting survival
after ECMO for refractory cardiogenic shock: the survival
after veno-arterial-ECMO (SAVE)–score. Eur Heart J
2015;36:2246-56. Crossref
13. Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival
after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal
Membrane Oxygenation Survival Prediction (RESP) score.
Am J Respir Crit Care Med 2014;189:1374-82. Crossref
14. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A
new method of classifying prognostic comorbidity in
longitudinal studies: development and validation. J Chronic
Dis 1987;40:373-83. Crossref
15. Mosier JM, Kelsey M, Raz Y, et al. Extracorporeal
membrane oxygenation (ECMO) for critically ill adults in
the emergency department: history, current applications,
and future directions. Crit Care 2015;19:431. Crossref
16. Lee SN, Jo MS, Yoo KD. Impact of age on extracorporeal
membrane oxygenation survival of patients with cardiac
failure. Clin Interv Aging 2017;12:1347-53. Crossref
17. Narotsky DL, Mosca MS, Mochari-Greenberger H, et al.
Short-term and longer-term survival after veno-arterial
extracorporeal membrane oxygenation in an adult patient
population: does older age matter? Perfusion 2016;31:366-75. Crossref
18. Saito S, Nakatani T, Kobayashi J, et al. Is extracorporeal life
support contraindicated in elderly patients? Ann Thorac
Surg 2007;83:140-5. Crossref
19. Stub D, Bernard S, Pellegrino V, et al. Refractory cardiac
arrest treated with mechanical CPR, hypothermia, ECMO
and early reperfusion (the CHEER trial). Resuscitation
2015;86:88-94. Crossref
20. Yannopoulos D, Bartos J, Raveendran G, et al. Advanced
reperfusion strategies for patients with out-of-hospital
cardiac arrest and refractory ventricular fibrillation
(ARREST): a phase 2, single centre, open-label, randomised
controlled trial. Lancet 2020;396:1807-16. Crossref
21. Tsao NW, Shih CM, Yeh JS, et al. Extracorporeal
membrane oxygenation–assisted primary percutaneous
coronary intervention may improve survival of patients
with acute myocardial infarction complicated by profound
cardiogenic shock. J Crit Care 2012;27:530.e1-11. Crossref
22. Brugts JJ, Caliskan K. Short-term mechanical circulatory
support by veno-arterial extracorporeal membrane
oxygenation in the management of cardiogenic shock
and end-stage heart failure. Expert Rev Cardiovasc Ther
2014;12:145-53. Crossref
23. Ng PY, Li AC, Fang S, et al. Predictors of favorable
neurologic outcomes in a territory-first extracorporeal
cardiopulmonary resuscitation program. ASAIO J
2022;68:1158-64. Crossref
24. Tsai NW, Leung YM, Ng PY, et al. Attitudes of visitors at
adult intensive care unit toward organ donation and organ
support. Chin Med J (Engl) 2019;132:373-6. Crossref
25. Muguruma K, Kunisawa S, Fushimi K, Imanaka Y.
Epidemiology and volume-outcome relationship of
extracorporeal membrane oxygenation for respiratory
failure in Japan: a retrospective observational study using
a national administrative database. Acute Med Surg
2020;7:e486. Crossref
26. Barbaro RP, Odetola FO, Kidwell KM, et al. Association
of hospital-level volume of extracorporeal membrane
oxygenation cases and mortality. Analysis of the
extracorporeal life support organization registry. Am J
Respir Crit Care Med 2015;191:894-901. Crossref
27. Ratnani I, Tuazon D, Zainab A, Uddin F. The role and
impact of extracorporeal membrane oxygenation in critical
care. Methodist Debakey Cardiovasc J 2018;14:110-9. Crossref
28. Ling L, Ho CM, Ng PY, et al. Characteristics and outcomes of patients admitted to adult intensive care units in Hong
Kong: a population retrospective cohort study from 2008
to 2018. J Intensive Care 2021;9:2. Crossref
29. Ko M, Shim M, Lee SM, Kim Y, Yoon S. Performance
of APACHE IV in medical intensive care unit patients: comparisons with APACHE II, SAPS 3, and MPM0 III.
Acute Crit Care 2018;33:216-21. Crossref
30. Lambden S, Laterre PF, Levy MM, Francois B. The SOFA
score–development, utility and challenges of accurate
assessment in clinical trials. Crit Care 2019;23:374. Crossref