Hong Kong Med J 2023 Aug;29(4):349–50 | Epub 4 Aug 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Sevelamer crystal–associated peritonitis in a patient on continuous ambulatory peritoneal dialysis: a case report
YH Wong, MB, ChB, FHKAM (Medicine)1; SM Li, B Pharm, MSc2; Will WL Pak, MB, BS, FHKAM (Medicine)1; KL Chan, MB, BS, FHKAM (Medicine)1; Z Chan, MB, BS, FHKAM (Medicine)1; WP Law, MB, ChB, FHKAM (Medicine)1; CK Lam, MB, ChB, FHKAM (Medicine)1; Sunny SH Wong, MB, BS, FHKAM (Medicine)1
1 Department of Medicine and Geriatrics, United Christian Hospital, Hong Kong SAR, China
2 Pharmacy Department, United Christian Hospital, Hong Kong SAR, China
Corresponding author: Dr YH Wong (wyh114@ha.org.hk)
Case report
A 60-year-old Chinese lady was admitted in August
2019 with fever, abdominal pain, and turbid peritoneal
dialysate effluent. She had a history of end-stage
renal disease due to immunoglobulin A nephropathy
and had been on continuous ambulatory peritoneal
dialysis for 3 years. Her usual medication included
aspirin, calcitriol, cetirizine, ferrous sulphate,
metoprolol, mirtazapine, pantoprazole, pregabalin,
and sevelamer carbonate (1600 mg three times a
day). Peritoneal dialysate fluid grew Escherichia coli,
and intra-peritoneal gentamicin and ceftazidime
were started. Her peritoneal dialysis catheter was
removed 1 week later due to refractory peritonitis,
but her abdominal pain persisted with development
of paralytic ileus. A contrast computed tomography
scan of abdomen performed 2 days following catheter
removal revealed gross pneumoperitoneum and
mural thickening over the small bowel. Laparotomy
was performed and a proximal descending colonic
ulcer with 2-mm perforation was identified. The
patient underwent a left hemicolectomy but later
succumbed in the intensive care unit due to hospital-acquired
pneumonia.
An analysis of the colonic specimen
demonstrated full thickness necrosis of the colonic
wall with associated acute suppurative inflammation
and peripheral ulceration. Incidentally there were
abundant polygonal, non-refractile crystals with
a brown, fish-scale configuration in the necrotic
debris (Fig 1). The crystals appeared violet on
periodic acid–Schiff stain staining. On haematoxylin
and eosin staining, they had a two-toned colour
imparted by pink linear accentuations (Fig 2).
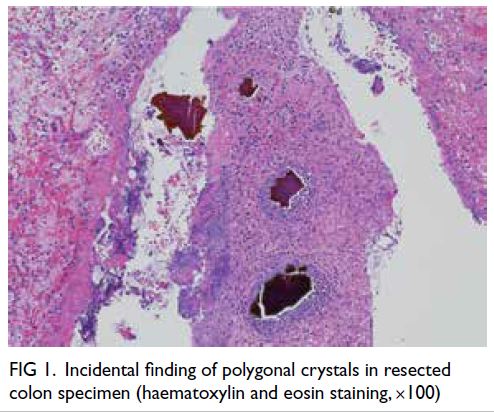
Figure 1. Incidental finding of polygonal crystals in resected colon specimen (haematoxylin and eosin staining, ×100)
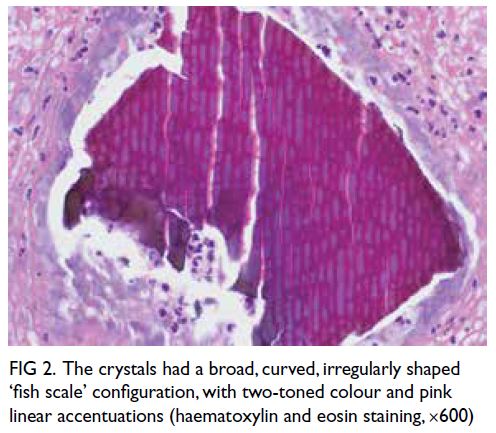
Figure 2. The crystals had a broad, curved, irregularly shaped ‘fish scale’ configuration, with two-toned colour and pink linear accentuations (haematoxylin and eosin staining, ×600)
Discussion
Sevelamer is a calcium-free anion-exchange resin
prescribed as a phosphate binder in patients with
chronic kidney disease. It is composed of a non-absorbable
hydrogel with ammonia on a carbon
backbone. Stomach acid releases sevelamer polymer that binds phosphate in the intestine and forms
a crystalline aggregate.1 Initially approved by the
United States Food and Drug Administration
in October 1998 as sevelamer hydrochloride,
sevelamer has been largely replaced since 2007 by sevelamer carbonate.1 Sevelamer is commonly
associated with gastrointestinal (GI) tract side-effects
such as dyspepsia, abdominal pain, flatulence,
and constipation.2 Sevelamer crystals (SCs) are
non-polarised, have a broad curved and irregularly
spaced fish-scale pattern, appear violet on periodic
acid–Schiff staining, and have a two-tone yellowish/brownish colour on haematoxylin and eosin
staining.1 About 19 cases of sevelamer-associated
GI ulcers have been reported in the literature.3
The lesion can be found in all segments of the GI
tract although the colon is the most common site.
Sevelamer crystals are usually found inside the GI
tract mucosa.2 Endoscopic findings include erosions
and ulcerations, pseudo-inflammatory polyps and
bezoar. Although a dose-dependent association had
been reported, a recent review could not confirm the
association.2 In one case report, a colonic mucosal
ulcer developed while taking sevelamer carbonate
800 mg three times a day (duration not known).4 In
another case report, a recto-sigmoid ulcer developed
after taking sevelamer carbonate 1600 mg three
times a day for 2 months.5 Diabetic patients appeared
to be more prone to SC-associated GI lesions.2 They
developed SC-associated GI lesions with a smaller
dose compared with non-diabetics. Most reported
cases required discontinuation of sevelamer,3 6 7
although improvement in clinical condition and
cessation of rectal bleeding following dose reduction
were reported in one case.2
In our patient, there were two possible
explanations for her clinical course. She may
have developed severe continuous ambulatory
peritoneal dialysis peritonitis as a primary disease
with secondary paralytic ileus, predisposing to SC
deposition in the GI tract mucosa. Alternatively, she
may have sustained GI tract injury by SC leading to
colonic perforation and secondary peritonitis.
To the best of our knowledge, SC has not been
previously reported to present with continuous
ambulatory peritoneal dialysis peritonitis. The
treating physician must be vigilant for potential
complications of sevelamer prescribed in patients
on peritoneal dialysis, especially when they have
paralytic ileus.
Author contributions
Concept or design: YH Wong, SSH Wong.
Acquisition of data: YH Wong, SM Li.
Analysis or interpretation of data: YH Wong, SM Li.
Drafting of the manuscript: YH Wong, SM Li.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: YH Wong, SM Li.
Analysis or interpretation of data: YH Wong, SM Li.
Drafting of the manuscript: YH Wong, SM Li.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Acknowledgement
We thank Dr Ching-ki Fung and Dr Ngai-sheung Fung,
pathologists of United Christian Hospital, for the detailed
analysis of the colonic specimen and illustrative pathology
images.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. The patient’s next-of-kin has granted permission for submission and publication of this case report.
References
1. Swanson BJ, Limketkai BN, Liu TC, et al. Sevelamer crystals in the gastrointestinal tract (GIT): a new entity associated with mucosal injury. Am J Surg Pathol 2013;37:1686-93. Crossref
2. Yuste C, Mérida E, Hernández E, et al. Gastrointestinal complications induced by sevelamer crystals. Clin Kidney J 2017;10:539-44. Crossref
3. Uy PP, Vinsard DG, Hafeez S. Sevelamer-associated rectosigmoid ulcers in an end-stage renal disease patient.
ACG Case Rep J 2018;5:e83. Crossref
4. Nambiar S, Pillai UK, Devasahayam J, Oliver T, Karippot A. Colonic mucosal ulceration and gastrointestinal bleeding associated with sevelamer crystal deposition in a
patient with end stage renal disease. Case Rep Nephrol
2018;2018:4708068. Crossref
5. Tieu C, Moreira RK, Song LMWK, Majumder S, Papadakis KA, Hogan MC. A case report of sevelamer-associated
recto-sigmoid ulcers. BMC Gastroenterol
2016;16:20. Crossref
6. Magee J, Robles M, Dunaway P. Sevelamer-induced gastrointestinal injury presenting as gastroenteritis. Case
Rep Gastroenterol 2018;12:41-5. Crossref
7. Lai T, Frugoli A, Barrows B, Salehpour M. Sevelamer carbonate crystal–induced colitis. Case Rep Gastrointest
Med 2020;2020:4646732. Crossref

