Hong Kong Med J 2023 Aug;29(4):337–41 | Epub 16 Aug 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PERSPECTIVE
Continuous subcutaneous insulin infusion: a local perspective
Tiffany TL Yau, MRCP, FHKAM (Medicine)1; Alicia J Jenkins, MD, FRCP2,3,4; Ronald CW Ma, FHKAM (Medicine), FRCP5,6
1 Department of Medicine and Therapeutics, Prince of Wales Hospital, Hong Kong SAR, China
2 NHMRC Clinical Trials Centre, The University of Sydney, Camperdown, Australia
3 Department of Endocrinology and Diabetes, St Vincent’s Hospital, Melbourne, Australia
4 The Baker Heart and Diabetes Institute, Melbourne, Australia
5 Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong SAR, China
6 Hong Kong Institute of Diabetes and Obesity, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Prof Ronald CW Ma (rcwma@cuhk.edu.hk)
Introduction
Intensive treatment of diabetes mellitus (DM)
improves short-term well-being while mitigating
micro- and macrovascular complications,1 2 but
it can be associated with a three-fold increase
in the risk of severe hypoglycaemia (61.2 vs 18.7
cases per 100 patient-years) as well as weight gain.1
Intensive treatment of type 1 diabetes mellitus
(T1DM) involves multiple daily injection (MDI)
insulin therapy or continuous subcutaneous insulin
infusion (CSII) therapy. During the T1DM Diabetes
Control and Complications Trial (DCCT), over a
mean intervention period of 6.5 years, intensive
treatment with MDI insulin therapy or CSII therapy
(using less sophisticated pumps that lacked modern
continuous glucose monitoring [CGM] technology
to measure glucose levels in interstitial fluid) yielded
a mean glycated haemoglobin (HbA1c) level of 7.2%
(vs 9.1% for the conventional therapy group with
one or two daily insulin injections) and reductions
of 26% to 76% in microvascular complications.1 The
observational Epidemiology of Diabetes Interventions
and Complications study continued to follow DCCT
participants, with all participants being recommended
intensive diabetes management and returned to their
usual healthcare team. In both DCCT treatment
arms, HbA1c levels converged at around 8.1%;
sustained reductions in micro- and macrovascular
complications were observed in the prior intensive
treatment group over 18 years of follow-up.2 3
Severe hypoglycaemia risk is strongly
associated with HbA1c level, with a 13% to 15%
increase in such risk for every 10% decrease in HbA1c
level.3 Newer forms of insulin, modern pumps, and
CGM technology have led to substantial decreases in
hypoglycaemia, including severe hypoglycaemia.4 For
instance, CSII technology has considerably advanced
over the past two decades, which allows similar
HbA1c levels to be achieved without an increased
risk of hypoglycaemia; hybrid closed loop (HCL)
pumps have also become commercially available.
This article provides an overview of insulin
pump therapy, evidence concerning its clinical
efficacy, its limitations, and local challenges in Hong
Kong.
History and evolution of insulin pumps
The first pump prototype, developed in the 1960s,
was a heavy machine worn as a backpack. Early
pumps had suboptimal characteristics (eg, quality
control, battery power, and dosing flexibility), along
with technical failures and rigid blockage-prone
infusion sets. A modern pump is a battery-powered
device that is worn externally and continuously with
an internal reservoir for rapid-acting insulin (100 IU
in strength), which is subcutaneously delivered
through an infusion set. The reservoir and infusion
set are changed by the user or caregivers every few
days and batteries are changed every 2 weeks.
Basal insulin is continuously delivered at
modifiable infusion rates, or by a control algorithm
in advanced CGM-linked HCL pumps; insulin
boluses for meals and instances of hyperglycaemia
require user input. Continuous subcutaneous
insulin infusion therapy offers greater flexibility over
MDI insulin therapy; it allows variations in basal
infusion rate and the use of temporary basal rates
(eg, higher for sedentary periods or illnesses and
lower for aerobic exercise), as well as precise insulin
delivery, various bolus patterns (for some devices),
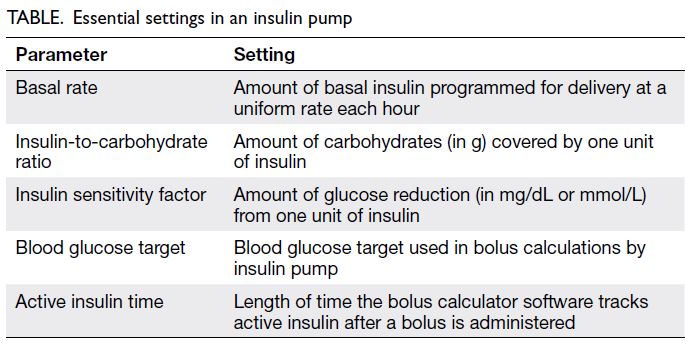
and lower MDI burden. Essential pump settings are
shown in the Table; on HCL pumps in automated
mode (see below), adjustable settings are the insulin-to-carbohydrate ratio and active insulin time.
Standalone (non-HCL) pumps solely deliver
insulin; users measure blood and/or interstitial fluid
glucose level (by CGM systems) to help determine
appropriate insulin doses. Continuous glucose
monitoring tracks interstitial fluid glucose level
for 7 to 14 days; it provides optional alarms for
hyperglycaemia and hypoglycaemia. The United States Food and Drug Administration–approved
CGM systems available in Hong Kong include
Abbott’s FreeStyle Libre, Dexcom, and Medtronic’s
MiniMed systems.
The most advanced commercial pumps are
HCL pumps. The first HCL pump, the Medtronic
MiniMed 670G, was approved by the United States
Food and Drug Administration in 2016 for people
with T1DM aged ≥7 years5; it became available in Hong Kong in 2019. Hybrid closed loop pumps
use a built-in computer algorithm with learning
capabilities to modify basal insulin delivery in
response to CGM measurements. Algorithm-based
automatic adjustments are made at 5-minute
intervals according to the current sensor glucose
value, the extent and duration of deviation from
the glucose target, the speed of changes in glucose
level, and the amount of insulin already delivered.
The pumps can function in two modes: manual
and automated mode. In automated mode, the
Medtronic SmartGuard algorithm in MiniMed
670G adjusts basal insulin to a glucose target of
6.7 mmol/L, or to a user- or caregiver-initiated higher
temporary target (eg, 8.3 mmol/L for exercise). The
pumps function in manual mode if CGM data are
unavailable or if glucose reading or insulin delivery
rate is persistently high.
Pump data can be uploaded to a cloud-based
programme, which can be accessed (with patient
permission) by the diabetes care team. Uploaded
report analytics include device usage duration;
glucose levels, trends, and variability; comparisons
of pre- and postprandial glucose levels; estimated
HbA1c level; pump settings; and suggestions to
improve glycaemic control.
Newer commercial HCL pumps include the
Medtronic MiniMed 770G and 780G as well as
the t:slim X2/Dexcom G6 CGM system/Control-IQ algorithm. Advances include optional lower
glucose targets (eg, 5.7, 6.1 or 6.7 mmol/L), better
algorithms, Bluetooth functionality, a smartphone
application for pump control, and wireless data uploads that enable others to remotely monitor the wearer’s glucose levels.
Clinical efficacy
Continuous subcutaneous insulin infusion
therapy lowers HbA1c level, mitigates hyper- and
hypoglycaemia, improves quality of life, reduces
chronic complications, and—particularly when
using sensor-augmented pumps—increases time
in range (TIR) and decreases glucose variability. A
meta-analysis of trials from 2008 to 2015 showed
that CSII therapy reduced HbA1c level by 0.37%
(95% confidence interval [CI]=0.24-0.51) compared
with MDI insulin therapy; it also reduced the
incidence of nocturnal hypoglycaemia.6 However,
the included trials had a moderate to high risk of
bias related to funding sources, considerable loss
to follow-up, and lack of or unclear descriptions of
concealment and masking. In most studies, a higher
HbA1c level before pump initiation was associated
with greater glycaemic improvement.7 8 Sensor-augmented
pumps reduced severe hypoglycaemia,
frequent hypoglycaemic episodes, and nocturnal
hypoglycaemia in adults and children with T1DM,
with no change or worsening of HbA1c level.9
A Swedish National Diabetes Register–based
observational study of people with T1DM (n=18 168,
including 2441 CSII users; mean follow-up interval,
6.8 years) demonstrated lower cardiovascular
mortality in CSII users than in MDI insulin therapy
users, despite similar mean HbA1c levels.9 Adjusted
hazard ratios for CSII therapy were significantly
lower: 0.55 (95% CI=0.36-0.83) for fatal coronary
heart disease, 0.58 (95% CI=0.40-0.85) for fatal
cardiovascular disease (coronary heart disease or
stroke), and 0.73 (95% CI=0.58-0.92) for all-cause
mortality.
For decades, HbA1c has been regarded
as the main indicator of glycaemic control in
clinical and research settings.10 11 An important
limitation of HbA1c is its poor responsiveness
to hypoglycaemia. Glycaemic variability is a risk
factor for hypoglycaemia, hyperglycaemia, and
chronic complications; it can also be used as an
indicator during treatment optimisation. Regardless
of mean HbA1c level, higher glycaemic variability
is associated with an increased risk of adverse
DM outcomes,12 including chronic complications
and mortality. In clinical management of people
with T1DM, CSII therapy is associated with lower
glycaemic variability, compared with MDI insulin
therapy.13 14
The Medtronic MiniMed 670G is safe and
effective in the treatment of T1DM; it increases TIR,
lowers HbA1c level, and mitigates hyperglycaemia/hypoglycaemia without increasing the rates of severe
hypoglycaemia or diabetic ketoacidosis.15 In a pivotal
trial, the use of an HCL system significantly reduced HbA1c level compared with a sensor-augmented
pump (HCL: 8.3% to 7.4% vs sensor-augmented
pump: 8.2% to 7.7%), with longer TIR and shorter
hypoglycaemia duration.16 The first randomised
trial of the Medtronic MiniMed 670G HCL
pump in adults was completed by our Australian
colleagues.17 Participants were randomised to 6
months of HCL pump use (n=61) or the control
group that consisted of ongoing MDI insulin therapy
or standard pump use (without CGM) with access
to a glucose meter and insulin bolus calculator
(n=59). The primary outcome was a TIR of 70 to 180
mg/dL by masked CGM during the final 3 weeks.
Hybrid closed loop pump use was associated with
significant improvements in all glucose metrics,
leading to 3.6 additional hours of TIR per day. Such
use also improved diabetes-specific well-being;
no participants exhibited worsened sleep quality,
diabetes-related distress or cognition. Various studies
have consistently demonstrated a 6% to 11% increase
in TIR during HCL pump use, compared with MDI
insulin therapy.15 16 18 19 20 Subsequent real-world data
have been similar to clinical trial results.21 22
Limitations
Continuous subcutaneous insulin infusion
therapy increases flexibility regarding mealtimes,
carbohydrate intake, and physical activity; however,
it increases costs, time, and educational burden for
clinicians and users.
Patient selection for CSII therapy involves
consideration of HbA1c level, DM complications,
vision, numeracy, problem-solving skills,
psychological status, hypoglycaemia awareness,
prior adherence to diabetes self-care and follow-up,
expectations, and ability to afford pump therapy.
A supportive multidisciplinary team is needed:
an endocrinologist familiar with CSII therapy, a
diabetes educator, a dietitian, and a representative
from the device company. Patient responsibilities
include regular self-monitoring of glucose, possible
CGM calibration, reliable carbohydrate counting and
bolusing, uploading pump data (unless automated),
and responding to pump alerts.
Potential skin and infusion set issues include
set dislodgement, occlusion, pump malfunction,
infusion site infection, site scarring, lipohypertrophy,
and lipoatrophy. The pump delivers rapid-acting
insulin and does not provide background long-acting
insulin (eg, in MDI insulin therapy). Thus,
no insulin is delivered if the pump is disconnected
or malfunctions; if alerts are ignored, diabetic
ketoacidosis can occur. Accordingly, relevant
education, an alternative insulin delivery method
(eg, syringe or pen), and ketone-testing supplies
(preferably for blood samples) are key considerations.
Cost, privacy, and constant hardware attachment
may impact patient preferences.
Glycaemic control is a key driver of patient
preference for CSII therapy.23 Less glycaemic
variability, shorter hypoglycaemia duration, and
fewer chronic complications are moderately
important to users; these factors had similar ratings
relative to components of treatment burden,
including device size and appearance, cost, ease of
use, and embarrassment related to public use. Hybrid
closed loop pumps provide reassurance to some
patients, along with reduced anxiety, improved sleep,
improved confidence, and ‘time off’ from diabetes
demands.24 Pump discontinuation is uncommon;
temporary pump holidays may occur (eg, during a
beach vacation). In a longitudinal study (n=8935),
pump discontinuation rates were 3% (all ages), 4%
(adolescents), and 1% (older adults). Participants
who discontinued pump use had a higher baseline
HbA1c level. Reasons for discontinuation included
problems with wearability (57%), pump-related
discomfort or anxiety (44%), and problems with
glycaemic control (30%).25
Insulin pumps in Hong Kong
In Hong Kong, between 2002 and 2015, T1DM
incidence increased from 3.5 (95% CI=2.2-4.9) to
5.3 (95% CI=3.4-7.1) cases per 100 000 person-years
in boys and from 4.3 (95% CI=2.7-5.8) to 6.4
(95% CI=4.3-8.4) cases per 100 000 person-years
in girls. Among people aged ≥20 years, T1DM
incidence remained stable.26
The public healthcare system in Hong Kong
provides support for approximately 2500 patients
with T1DM,26 including around 100 CSII users. In
contrast, 10% to 50% of adults with T1DM and >50%
of children with T1DM receive CSII therapy in many
Western countries.27 28 29 30 31 32 Factors affecting CSII therapy
uptake include treatment cost and reimbursement
schemes, availability of standardised criteria or
clinical recommendations concerning CSII therapy,
availability of a multidisciplinary team and trained
staff, resources and workload, and patient awareness
of CSII therapy benefits and willingness to use the
technology.
Additionally, many patients are not educated
about pumps, nor are they prepared to invest the
necessary time and effort to use a pump. Clinicians
may also lack CSII experience (eg, troubleshooting
and report interpretation) and have no time to gain
appropriate experience.33 Only several Hong Kong
endocrinologists have undergone extensive training
in the use of pumps and CGM at leading centres
in Australia and other countries. Cost remains an
issue in Hong Kong—pumps, CGM, and associated
expenses are not subsidised by the government or
most health insurance schemes. A Swedish National
Diabetes Register–based study showed that the mean
annual cost was approximately US$4000 higher for
CSII therapy than for MDI insulin therapy.32
Future directions
Hybrid closed loop pumps represent a step towards
the ‘artificial pancreas’, although this term is
suboptimal because current systems only deliver
insulin (ie, no other hormones or pancreatic
exocrine functions). Closed-loop research is rapidly
advancing. An ‘ideal’ pump would automatically and
accurately deliver insulin with a very rapid onset and
offset (with or without hormones such as glucagon34)
to maintain normal blood glucose level in various
situations. It would also include a compact pump with
minimal attached hardware, reliable calibration-free
CGM (now available) and movement monitoring (to
adjust for exercise), and other analytes (eg, ketones
and lactate). Finally, it would be user-friendly,
efficient, cost-effective, and affordable for both
healthcare systems and individuals.
In patients with T1DM, glucagon secretion is
also impaired. A bihormonal pump combining insulin
and glucagon infusions is feasible for hypoglycaemia
management; research has shown improvements
in glycaemic control compared with insulin pump
use alone.34 35 However, device complexity remains a
limitation because of the short duration of glucagon
stability and enhancement of insulin resistance
during chronic glucagon administration.
Hybrid closed loop and emerging closed-loop
pumps are important technological advances in life-saving
and life-easing insulin treatment. Greater
availability and access to this technology can improve
glycaemic control and quality of life for people
with T1DM in Hong Kong. These improvements
will require reimbursement from the government
and health insurance schemes, along with medical
expertise, structured care during pump therapy, and
better awareness of CSII therapy and its benefits.
Author contributions
Concept or design: TTL Yau, AJ Jenkins.
Acquisition of data: TTL Yau.
Analysis or interpretation of data: TTL Yau.
Drafting of the manuscript: TTL Yau.
Critical revision of the manuscript for important intellectual content: AJ Jenkins, RCW Ma.
Acquisition of data: TTL Yau.
Analysis or interpretation of data: TTL Yau.
Drafting of the manuscript: TTL Yau.
Critical revision of the manuscript for important intellectual content: AJ Jenkins, RCW Ma.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
TTL Yau has disclosed no conflicts of interest. AJ Jenkins has
been on advisory boards for Medtronic, Sanofi, and Abbott,
and has received pump- and sensor-related research grant
support from Medtronic, Sanofi, Abbott, Juvenile Diabetes
Research Foundation, and The Helmsley Charitable Trust.
RCW Ma has received research support from Bayer, Novo
Nordisk, Roche Diagnostics (Hong Kong) Limited, Sanofi, as
well as consultancy or speaker fees from Bayer, Merck, and Medtronic, all of which has been donated to support diabetes research at The Chinese University of Hong Kong.
Acknowledgement
TTL Yau thanks the training and experience provided by the diabetes unit clinicians of Royal North Shore Hospital
(Sydney), Royal Prince Alfred Hospital (Sydney), St Vincent’s
Hospital (Melbourne), and Ms Kerryn Roem, a practising
dietitian in Melbourne, during her period of training in
Australia. RCW Ma acknowledges financial support from a
Croucher Foundation Senior Medical Research Fellowship.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, et al. The effect of intensive
treatment of diabetes on the development and progression
of long-term complications in insulin-dependent diabetes
mellitus. N Engl J Med 1993;329:977-86. Crossref
2. Lachin JM, Orchard TJ, Nathan DM; DCCT/EDIC Research Group. Update on cardiovascular outcomes at
30 years of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications
Study. Diabetes Care 2014;37:39-43. Crossref
3. Nathan DM; DCCT/EDIC Research Group. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 years: overview. Diabetes Care 2014;37:9-16. Crossref
4. Anderson SM, Buckingham BA, Breton MD, et al. Hybrid closed-loop control is safe and effective for people with
type 1 diabetes who are at moderate to high risk for
hypoglycemia. Diabetes Technol Ther 2019;21:356-63. Crossref
5. The United States Food and Drug Administration. Devices@
FDA. MiniMed 670G system. Available from: https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm?db=pma&id=388375. Accessed 7 Aug 2023.
6. Benkhadra K, Alahdab F, Tamhane SU, McCoy RG, Prokop LJ, Murad MH. Continuous subcutaneous insulin
infusion versus multiple daily injections in individuals with
type 1 diabetes: a systematic review and meta-analysis.
Endocrine 2017;55:77-84. Crossref
7. Orr CJ, Hopman W, Yen JL, Houlden RL. Long-term efficacy of insulin pump therapy on glycemic control in
adults with type 1 diabetes mellitus. Diabetes Technol Ther
2015;17:49-54. Crossref
8. Calandro DA, Januszewski AS, Cuper KK, et al. Substantial and sustained HbA1c reductions in Australian insulin
pump services for adults with type 1 diabetes. Benefit also
evident for older and high HbA1c subjects. Madridge J
Diabetes 2016;1:23-8. Crossref
9. Steineck I, Cederholm J, Eliasson B, et al. Insulin pump therapy, multiple daily injections, and cardiovascular
mortality in 18,168 people with type 1 diabetes:
observational study. BMJ 2015;350:h3234. Crossref
10. Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J 2015;39:273-82. Crossref
11. Kovatchev B. Glycemic variability: risk factors, assessment, and control. J Diabetes Sci Technol 2019;13:627-35. Crossref
12. Gorst C, Kwok CS, Aslam S, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic
review and meta-analysis. Diabetes Care 2015;38:2354-69. Crossref
13. Scott ES, McGrath RT, Januszewski AS, et al. HbA1c variability in adults with type 1 diabetes on continuous
subcutaneous insulin infusion (CSII) therapy compared
to multiple daily injection (MDI) treatment. BMJ Open
2019;9:e033059. Crossref
14. Abraham MB, de Bock M, Smith GJ, et al. Effect of a hybrid closed-loop system on glycemic and psychosocial
outcomes in children and adolescents with type 1 diabetes:
a randomized clinical trial. JAMA Pediatr 2021;175:1227-35. Crossref
15. Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop
insulin delivery system in adolescents and adults with type
1 diabetes. Diabetes Technol Ther 2017;19:155-63. Crossref
16. Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018;392:1321-9. Crossref
17. McAuley SA, Lee MH, Paldus B, et al. Six months of hybrid closed-loop versus manual insulin delivery with fingerprick blood glucose monitoring in adults with type 1 diabetes: a
randomized, controlled trial. Diabetes Care 2020;43:3024-33. Crossref
18. Stone MP, Agrawal P, Chen X, et al. Retrospective analysis of 3-month real-world glucose data after the MiniMed 670G system commercial launch. Diabetes Technol Ther 2018;20:689-92. Crossref
19. Bally L, Thabit H, Kojzar H, et al. Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol 2017;5:261-70. Crossref
20. Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373:2129-40. Crossref
21. Arunachalum S, Velado K, Vigersky RA, Cordero TL. Glycemic outcomes during real-world hybrid closedloop system use by individuals with type 1 diabetes in the United States. J Diabetes Sci Technol 2023;17:951-8. Crossref
22. Da Silva J, Bosi E, Jendle J, et al. Real-world performance of the MiniMedTM 670G system in Europe. Diabetes Obes Metab 2021;23:1942-9. Crossref
23. Muñoz-Velandia O, Guyatt G, Devji T, et al. Patient values and preferences regarding continuous subcutaneous insulin infusion and artificial pancreas in adults with type 1
diabetes: a systematic review of quantitative and qualitative
data. Diabetes Technol Ther 2019;21:183-200. Crossref
24. Farrington C. Psychosocial impacts of hybrid closed-loop systems in the management of diabetes: a review. Diabet
Med 2018;35:436-49. Crossref
25. Wong JC, Boyle C, DiMeglio LA, et al. Evaluation of pump
discontinuation and associated factors in the T1D exchange
clinic registry. J Diabetes Sci Technol 2017;11:224-32. Crossref
26. Luk AO, Ke C, Lau ES, et al. Secular trends in incidence of
type 1 and type 2 diabetes in Hong Kong: a retrospective
cohort study. PLoS Med 2020;17:e1003052. Crossref
27. van den Boom L, Karges B, Auzanneau M, et al. Temporal
trends and contemporary use of insulin pump therapy
and glucose monitoring among children, adolescents,
and adults with type 1 diabetes between 1995 and 2017.
Diabetes Care 2019;42:2050-6. Crossref
28. Wheeler BJ, Braund R, Galland B, et al. District health board
of residence, ethnicity and socioeconomic status all impact
publicly funded insulin pump uptake in New Zealand
patients with type 1 diabetes. N Z Med J 2019;132:78-89.
29. Gajewska KA, Biesma R, Bennett K, Sreenan S. Barriers and facilitators to accessing insulin pump therapy by adults with type 1 diabetes mellitus: a qualitative study. Acta
Diabetol 2021;58:93-105. Crossref
30. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971-8. Crossref
31. Kampmann U, Madsen LR, Bjerg L, et al. Prevalence and geographical distribution of insulin pump therapy in the
Central Denmark Region and its association with metabolic
parameters. Diabetes Res Clin Pract 2018;141:148-55. Crossref
32. Toresson Grip E, Svensson AM, Miftaraj M, et al. Real-world costs of continuous insulin pump therapy and multiple daily injections for type 1 diabetes: a population-based
and propensity-matched cohort from the Swedish
National Diabetes Register. Diabetes Care 2019;42:545-52. Crossref
33. White HD, Goenka N, Furlong NJ, et al. The U.K. service level audit of insulin pump therapy in adults. Diabet Med
2014;31:412-8. Crossref
34. El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy
in adults with type 1 diabetes: a multicentre randomised
crossover trial. Lancet 2017;389:369-80. Crossref
35. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes.
N Engl J Med 2014;371:313-25. Crossref