© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Chest computed tomography analysis of lung sparing morphology: differentiation of COVID-19 pneumonia from influenza pneumonia and bacterial pneumonia using the arched bridge and vacuole signs
Tiffany Y So, FRANZCR1; Simon CH Yu, FRCR1; WT Wong, FRCR2; Jeffrey KT Wong, FRCR1; Heather Lee, FRCR3; YX Wang, MMed, PhD1
1 Department of Imaging and Interventional Radiology, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong
2 Department of Anaesthesia and Intensive Care, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong
3 Department of Diagnostic Radiology, Princess Margaret Hospital, Hong Kong
Corresponding author: Prof YX Wang (yixiang_wang@cuhk.edu.hk)
Abstract
Introduction: This study evaluated the arched bridge
and vacuole signs, which constitute morphological
patterns of lung sparing in coronavirus disease 2019
(COVID-19), then examined whether these signs
could be used to differentiate COVID-19 pneumonia
from influenza pneumonia or bacterial pneumonia.
Methods: In total, 187 patients were included:
66 patients with COVID-19 pneumonia, 50 patients
with influenza pneumonia and positive computed
tomography findings, and 71 patients with bacterial
pneumonia and positive computed tomography
findings. Images were independently reviewed by
two radiologists. The incidences of the arched bridge
sign and/or vacuole sign were compared among the
COVID-19 pneumonia, influenza pneumonia, and
bacterial pneumonia groups.
Results: The arched bridge sign was much
more common among patients with COVID-19
pneumonia (42/66, 63.6%) than among patients
with influenza pneumonia (4/50, 8.0%; P<0.001)
or bacterial pneumonia (4/71, 5.6%; P<0.001). The
vacuole sign was also much more common among
patients with COVID-19 pneumonia (14/66, 21.2%)
than among patients with influenza pneumonia
(1/50, 2.0%; P=0.005) or bacterial pneumonia (1/71,
1.4%; P<0.001). The signs occurred together in 11 (16.7%) patients with COVID-19 pneumonia,
but they did not occur together in patients with
influenza pneumonia or bacterial pneumonia.
The arched bridge and vacuole signs predicted
COVID-19 pneumonia with respective specificities
of 93.4% and 98.4%.
Conclusion: The arched bridge and vacuole signs
are much more common in patients with COVID-19
pneumonia and can help differentiate COVID-19
pneumonia from influenza and bacterial pneumonia.
New knowledge added by this study
- On computed tomography, the arched bridge sign is characterised by ground-glass opacities or consolidation with an arched margin outlining unaffected lung parenchyma. The vacuole sign refers to a focal oval or round lucent area (typically <5 mm) that is present within ground-glass opacities or sites of consolidation.
- These signs were commonly observed in patients with coronavirus disease 2019 (COVID-19) in Hong Kong, consistent with data from other populations.
- Patients with COVID-19 pneumonia are much more likely to exhibit the arched bridge sign and/or the vacuole sign, compared with patients who have influenza pneumonia or bacterial pneumonia.
- The presence of the arched bridge sign and/or the vacuole sign on computed tomography may support a diagnosis of COVID-19 pneumonia and assist in differentiation from other types of pneumonia.
- The duration of total hospitalisation did not differ between patients with COVID-19 pneumonia who had and did not have these two signs, suggesting that they do not indicate a better or worse prognosis if appropriate treatments are administered.
Introduction
A diagnosis of coronavirus disease 2019 (COVID-19)
is made on the basis of epidemiological and clinical
history, as well as the results of severe acute
respiratory syndrome coronavirus 2 real-time reverse
transcriptase polymerase chain reaction (RT-PCR)
testing. Chest computed tomography (CT) has been
proposed as a useful alternative investigation method
for COVID-19 diagnosis or triage, particularly in
healthcare settings with restricted access to RT-PCR
testing and in the context of lower RT-PCR sensitivity
during early stages of the disease; it may also be useful
for imaging-mediated evaluation of disease severity
and progression.1 2 The most common CT findings
in early-stage COVID-19 pneumonia (illness days
0-5) are pure ground-glass opacities (GGOs); the
second most common finding is consolidation.3 4 In
the later stages (illness days 6-17), findings usually
evolve to a combination of GGOs, consolidation,
and reticular opacities with architectural distortion.4
These imaging features are not specific to
COVID-19 pneumonia; they can overlap with other
types of viral or bacterial pneumonia, particularly
influenza pneumonia, as well as other non-infectious
inflammatory lung diseases.5 6 Influenza, one of
the most common causes of viral pneumonia,7 and
bacterial pneumonia, historically the most common
type of community-acquired pneumonia worldwide,8 maintained high incidences during the early
COVID-19 pandemic when this study was
conducted; thus, they had the potential to
substantially contribute to hospitalisations in this
period. However, COVID-19 pneumonia and other
types of viral or bacterial pneumonia distinctly
differ in terms of their disease course, temporal
progression, and available therapeutics9 10 11; thus,
there is a need for early and accurate differentiation
among these entities.
Studies in 2020 revealed several CT imaging
features that can aid in differential diagnosis.
Compared with influenza pneumonia, patients with
COVID-19 pneumonia are more likely to exhibit a
peripheral distribution,12 13 14 patchy combination of
GGOs and consolidation,15 fine reticular opacities,16
and vascular thickening or enlargement16 17;
patients with influenza pneumonia are more likely
to exhibit nodules,18 tree-in-bud sign,18 bronchial
wall thickening,15 lymphadenopathy,16 and pleural
effusions.12 In the past, diffuse airspace consolidation,
centrilobular nodules, bronchial wall thickening,
and mucous impaction19 have been identified as
typical signs of bacterial pneumonia. Nevertheless,
CT assessment of COVID-19 generally remains
challenging, with reported accuracies for radiologists
ranging from 60 to 83%16 in terms of differentiating
patients with COVID-19 pneumonia from patients
with influenza pneumonia; considering these rates,
further studies of relevant imaging findings are
needed.
A report by Wu et al20 highlighted the arched
bridge sign, which may be a distinct CT feature
of COVID-19 pneumonia. In their analysis of
11 patients with COVID-19 pneumonia, the sign
was present in 72.7%.20 The arched bridge sign refers
to a specific pattern of GGOs or consolidation,
commonly in a subpleural location, which forms
an arched contour with a smooth concave margin
towards the pleural side. The arched margin outlines
the spared parenchyma between the GGOs or
consolidation and the pleural surface. Another
reported sign, regarded as the vacuole sign,21 22 23 24 is
presumably based on the morphological pattern of
parenchymal sparing in areas of affected lung. The
vacuole sign refers to a focal oval or round lucent
area (typically <5 mm) that is observed within
GGOs or sites of consolidation. In clinical practice,
we often observed these two novel signs on CT
scans of patients with COVID-19 pneumonia. We
hypothesised that these two signs are common in
patients with COVID-19 pneumonia and thus could
be used to differentiate such pneumonia from other
types of infection-related pneumonia. However,
considering the limited prior evidence (solely
from small retrospective studies20 21 22 23 24) regarding
the prevalence of the vacuole sign in COVID-19
pneumonia, and because the arched bridge sign has—to our knowledge—only been reported in a
single previous publication,20 additional assessments
of these signs are needed. The utilities of the arched
bridge and vacuole signs in COVID-19 pneumonia
have not been directly assessed in prior reports,
nor have they been compared between COVID-19
pneumonia and other types of infection-related
pneumonia. In this study, we evaluated the arched
bridge and vacuole signs in patients with COVID-19
pneumonia, then examined whether these signs
could be used to differentiate such pneumonia from
influenza pneumonia or bacterial pneumonia.
Methods
Patients
This retrospective study included consecutive
patients who were admitted to two hospitals in Hong
Kong (Prince of Wales Hospital and Princess Margaret
Hospital) with RT-PCR–confirmed COVID-19,
along with positive CT findings, from 24 January
2020 to 16 April 2020. These patients represent
most patients with COVID-19 in Hong Kong during
the study period, when all patients with confirmed
COVID-19 were hospitalised regardless of clinical
status; moreover, Princess Margaret Hospital also
served as a centralised treatment centre for patients
with COVID-19. The study recruitment period
reflects the early days of the COVID-19 pandemic
in Hong Kong, during which CT examinations were
commonly performed during the diagnosis and
treatment of patients with COVID-19. All patients
with COVID-19 underwent complete PCR-based
assessment of multiple respiratory pathogens on
admission; patients with COVID-19 were excluded
from the present study if they exhibited evidence
of other concomitant viral or bacterial respiratory
infections.
The influenza pneumonia and bacterial
pneumonia comparison groups comprised
consecutive patients who were admitted to Prince of
Wales Hospital in Hong Kong, with pure influenza
pneumonia or pure bacterial pneumonia and positive
CT findings from 20 February 2018 to 13 January
2020. The diagnosis of pure influenza pneumonia
was determined by RT-PCR–mediated detection
of influenza A or B viral RNA, in the absence of
evidence (eg, respiratory or blood cultures, PCR
tests, or serological tests) suggesting concomitant
infection with other viral or bacterial pathogens.
The diagnosis of pure bacterial pneumonia was
determined by positive bacterial culture on sputum
or bronchoalveolar lavage, in the absence of evidence
suggesting concomitant infection with other viral or
bacterial pathogens. Patients with pre-existing lung
parenchymal disease (eg, interstitial lung disease)
or known lung malignancy were excluded from the
study.
Image acquisition
Computed tomography scans were performed using
64-section multidetector scanners (LightSpeed
VCT or LightSpeed Pro 32, GE Medical Systems,
Milwaukee [WI], United States). The following scan
parameters were used: voltage, 120 kV; tube current,
50-502 mA; and slice thickness, 0.625 mm or
1.25 mm. Scans were performed with the patient in
the supine position during end-inspiration.
Image evaluation
All CT images were reviewed in random order
by two trained radiologists (TY So and YX Wang)
with 7 and 5 years of experience in diagnostic chest
imaging, respectively, using a dedicated picture
archiving and communication system workstation.
Each radiologist was blinded to demographic and
clinical information for all patients. The images were
independently reviewed by each radiologist, and
the consensus findings for any discrepancies from
discussion are reported.
Each CT image was initially subjected to broad
assessment of abnormalities. Subsequently, the
arched bridge and vacuole signs were specifically
assessed; the presence or absence of each sign was
recorded. The arched bridge sign was defined as the
presence of GGOs or consolidation with an arched
concave margin outlining a region of spared lung;
the vacuole sign was defined as the presence of a
vacuole-like region of normal lung (<5 mm) within
GGOs or sites of consolidation.21
For patients with COVID-19 pneumonia and
patients with influenza pneumonia, CT findings of
GGOs (hazy areas of parenchymal opacities that
did not conceal underlying vessels), consolidation
(parenchymal opacities that concealed underlying
vessels), reticular opacities (coarse linear or
curvilinear opacities, interlobular septal thickening,
or subpleural reticulation), and crazy paving pattern
(GGOs with interlobular and intralobular septal
thickening) were recorded. Other signs such as air
bronchograms (air-filled bronchi on a background
of opaque lung), nodules (small rounded focal
opacities <3 cm), cavitation (gas-filled spaces within
sites of pulmonary consolidation), bronchiectasis,
pleural retraction or thickening, pleural effusion,
pericardial effusion, pneumothorax, and mediastinal
lymphadenopathy (lymph nodes >1 cm in short-axis
diameter) were also recorded. The distributions
of pulmonary abnormalities were classified as
unilateral or bilateral, and peripheral (involving
mainly the peripheral one-third of the lung), central
(involving mainly the central two-thirds of the lung),
or diffuse (involving both peripheral and central
regions). Lobar involvement was also recorded
(right upper lobe, right middle lobe, right lower
lobe, left upper lobe, and/or left lower lobe). For patients with bacterial pneumonia, only the
arched bridge and vacuole signs were recorded.
Other CT changes and their distributions were
not individually recorded. This component of the
analysis was determined based on reports that it is
easier to differentiate COVID-19 pneumonia from
bacterial pneumonia, whereas it is more difficult
to differentiate COVID-19 pneumonia from other
types of viral pneumonia.6 25 26 This manuscript was
written in accordance with the Strengthening the
Reporting of Observational Studies in Epidemiology
(STROBE) guidelines for reporting observational
studies.
Statistical analysis
Imaging findings were compared using the Chi
squared test or Fisher’s exact test, as appropriate,
followed by Bonferroni correction. Comparisons of
disease stage, severity, and clinical course among
patients with COVID-19 who had the arched bridge
and/or vacuole signs were performed using the
non-parametric Mann-Whitney U test. P values
<0.05 were considered indicative of statistical
significance. For the arched bridge and vacuole
signs, the sensitivity, specificity, positive predictive
value, and negative predictive value were calculated,
along with the respective 95% confidence intervals.
All analyses were conducted using SPSS software
(Windows version 25.0; IBM Corp, Armonk [NY],
United States).
Results
Patients
Among 76 patients with bacterial pneumonia
who were admitted for treatment during the
study period, five patients with pre-existing lung
parenchymal disease were excluded from the
analysis: organising pneumonia (n=2), non-specific
interstitial pneumonia (n=1), and idiopathic
interstitial pneumonia of uncertain subtype (n=2).
No patients with COVID-19 required exclusion
because of concomitant viral or bacterial infections.
The final study population comprised 187 patients:
66 patients with COVID-19 pneumonia, 50 patients
with influenza pneumonia, and 71 patients with
bacterial pneumonia. The following organisms were
detected in patients with bacterial pneumonia:
Streptococcus pneumoniae, Staphylococcus aureus,
Haemophilus influenzae, Enterococcus spp.,
Klebsiella pneumoniae, Pseudomonas aeruginosa,
Escherichia coli, Stenotrophomonas spp., Serratia
spp., Acinetobacter spp., and Moraxella catarrhalis.
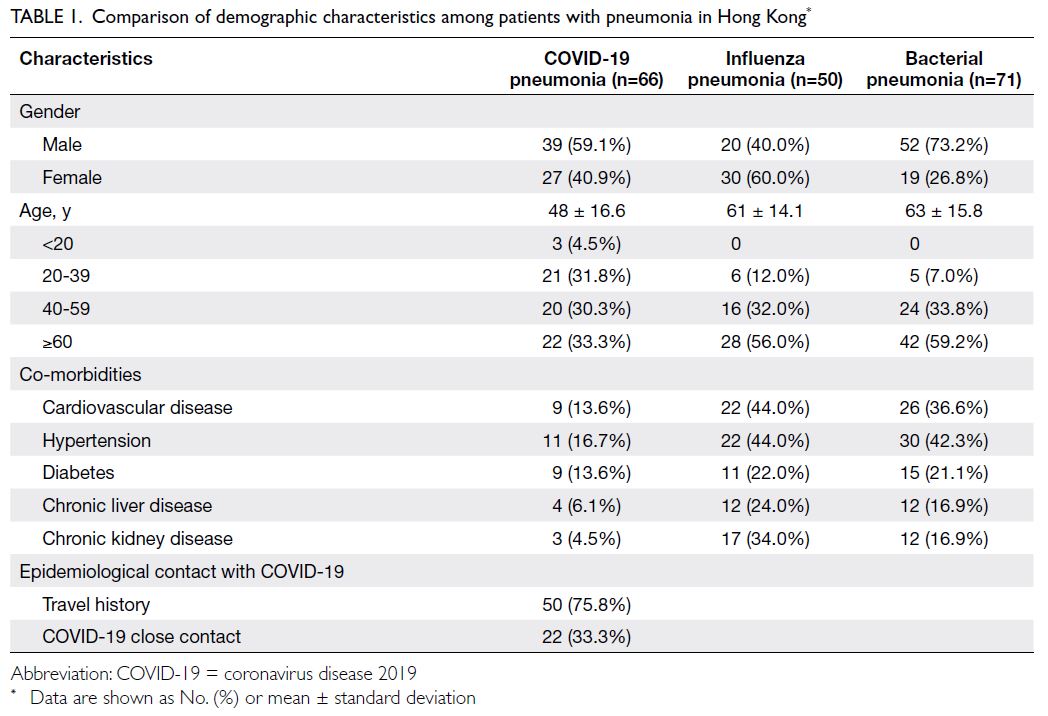
Demographic and clinical characteristics of the study
population are shown in Table 1. Compared with
patients in the influenza pneumonia and bacterial
pneumonia groups, patients with COVID-19
pneumonia tended to be younger and healthier.
Arched bridge and vacuole signs
The arched bridge and vacuole signs were present in 42 (63.6%) and 14 (21.2%) of 66 patients with
COVID-19 pneumonia, respectively (Table 2). The
arched bridge sign was commonly in a subpleural
location, and there was a smooth arched margin
outlining the underside of the GGO or consolidation
in all cases (Fig a and b). The vacuole sign was
present with GGOs or sites of consolidation in
various locations (Fig c and d). The arched bridge
sign was much more common in patients with
COVID-19 pneumonia than in patients with
influenza pneumonia (63.6% vs 8.0%) or bacterial
pneumonia (63.6% vs 5.6%, P<0.001). Similarly, the
vacuole sign was much more common in patients
with COVID-19 pneumonia than in patients with
influenza pneumonia (21.2% vs 2.0%, P=0.005) or
bacterial pneumonia (21.2% vs 1.4%, P<0.001).
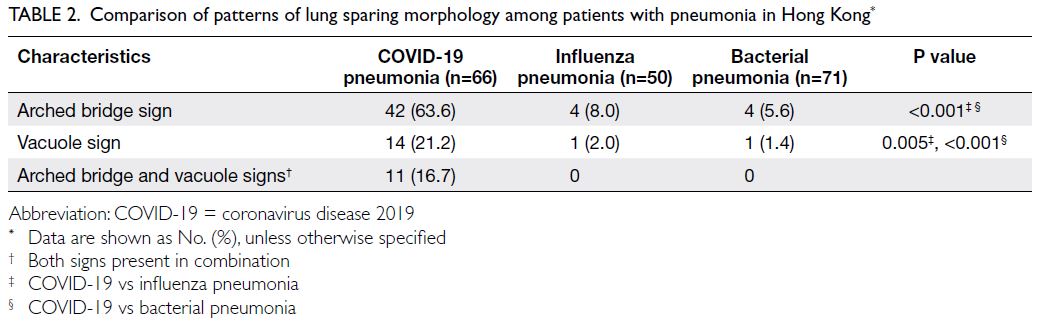
Table 2. Comparison of patterns of lung sparing morphology among patients with pneumonia in Hong Kong
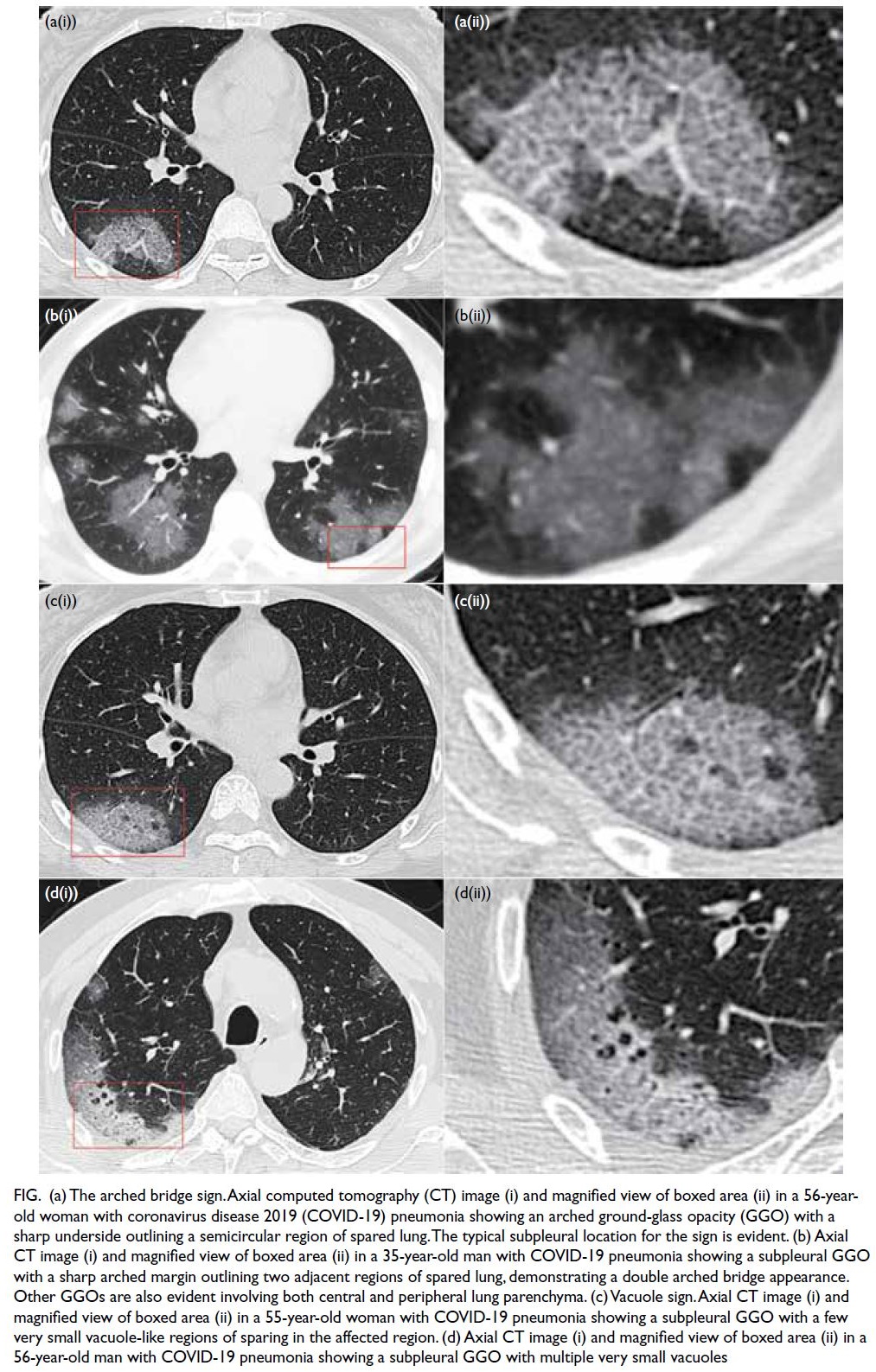
Figure. (a) The arched bridge sign. Axial computed tomography (CT) image (i) and magnified view of boxed area (ii) in a 56-year-old woman with coronavirus disease 2019 (COVID-19) pneumonia showing an arched ground-glass opacity (GGO) with a sharp underside outlining a semicircular region of spared lung. The typical subpleural location for the sign is evident. (b) Axial CT image (i) and magnified view of boxed area (ii) in a 35-year-old man with COVID-19 pneumonia showing a subpleural GGO with a sharp arched margin outlining two adjacent regions of spared lung, demonstrating a double arched bridge appearance. Other GGOs are also evident involving both central and peripheral lung parenchyma. (c) Vacuole sign. Axial CT image (i) and magnified view of boxed area (ii) in a 55-year-old woman with COVID-19 pneumonia showing a subpleural GGO with a few very small vacuole-like regions of sparing in the affected region. (d) Axial CT image (i) and magnified view of boxed area (ii) in a 56-year-old man with COVID-19 pneumonia showing a subpleural GGO with multiple very small vacuoles
The arched bridge and vacuole signs occurred
together in 11 (16.7%) of 66 patients with COVID-19
pneumonia, but they did not occur together in any
patients with influenza pneumonia or bacterial
pneumonia. Additionally, a review of the five
excluded patients with bacterial pneumonia and
concurrent pre-existing lung parenchymal disease
revealed that none of those patients exhibited the
arched bridge sign or the vacuole sign.
In this study, the arched bridge and vacuole
signs exhibited high specificities (93.4% and 98.4%,
respectively) in terms of identifying COVID-19
pneumonia (Table 3), with moderate or low
sensitivities (63.6% and 21.2%, respectively). They
also exhibited high positive predictive values (84.0%
and 87.5%, respectively) and high or moderate
negative predictive values (82.5% and 69.6%,
respectively).
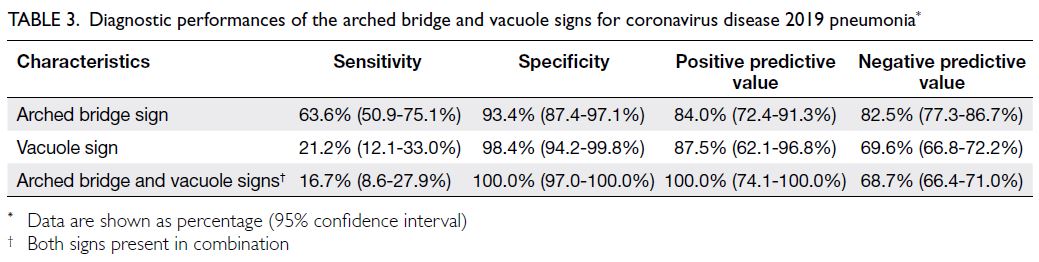
Table 3. Diagnostic performances of the arched bridge and vacuole signs for coronavirus disease 2019 pneumonia
The relationships of the arched bridge and
vacuole signs with disease course are shown in Table 4. Computed tomography was mainly performed
during admission, at a mean of 5.3 days after
admission, suggesting these two signs generally
appeared at an early stage. Comparisons of patients
with COVID-19 pneumonia who had and did
not have these two signs revealed that the arched
bridge sign was associated with more extensive lung
involvement (diseased lobes: 4.0 [present] vs 2.4 [absent], P<0.001). This trend was not evident for
the vacuole sign (diseased lobes: 3.8 [present] vs 3.3
[absent]). There was no significant difference in the
duration of total hospitalisation between patients
with COVID-19 who had and did not have these
two signs, suggesting they were not associated with
a better or worse prognosis if appropriate treatment
was administered.
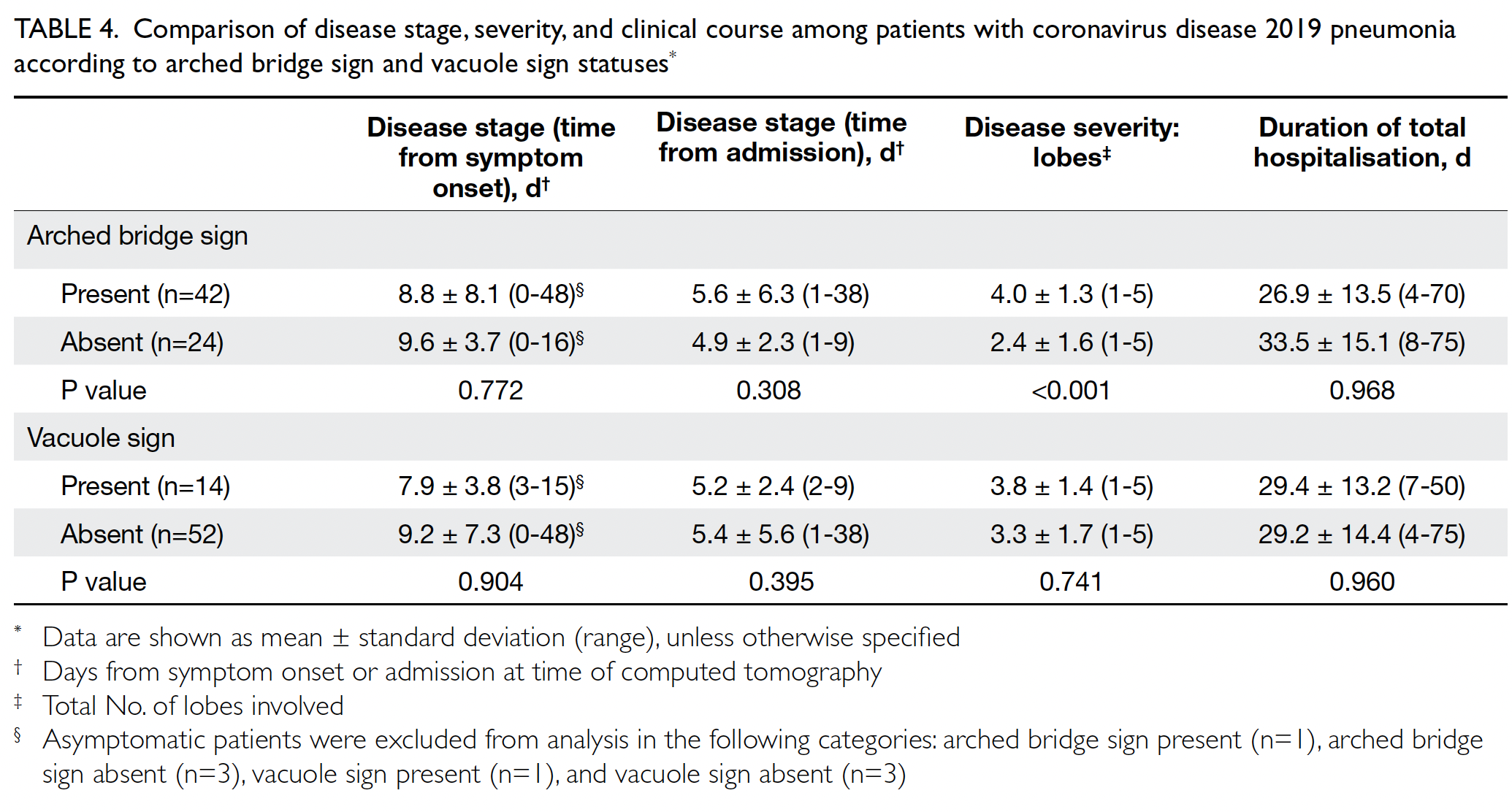
Table 4. Comparison of disease stage, severity, and clinical course among patients with coronavirus disease 2019 pneumonia according to arched bridge sign and vacuole sign statuses
Other computed tomography findings
Table 5 shows the comparison of other CT findings
between COVID-19 pneumonia and influenza
pneumonia. No significant differences were
observed in the incidences of GGOs, consolidation,
reticular opacities, or crazy paving between patients
with COVID-19 and patients with influenza
pneumonia (all P>0.05). Air bronchograms
(P=0.003), nodules (P=0.009), cavitation (P=0.004),
bronchiectasis (P<0.001), pleural effusion (P<0.001),
pericardial effusion (P=0.032), and mediastinal
lymphadenopathy (P<0.001) were significantly more
common in patients with influenza pneumonia.
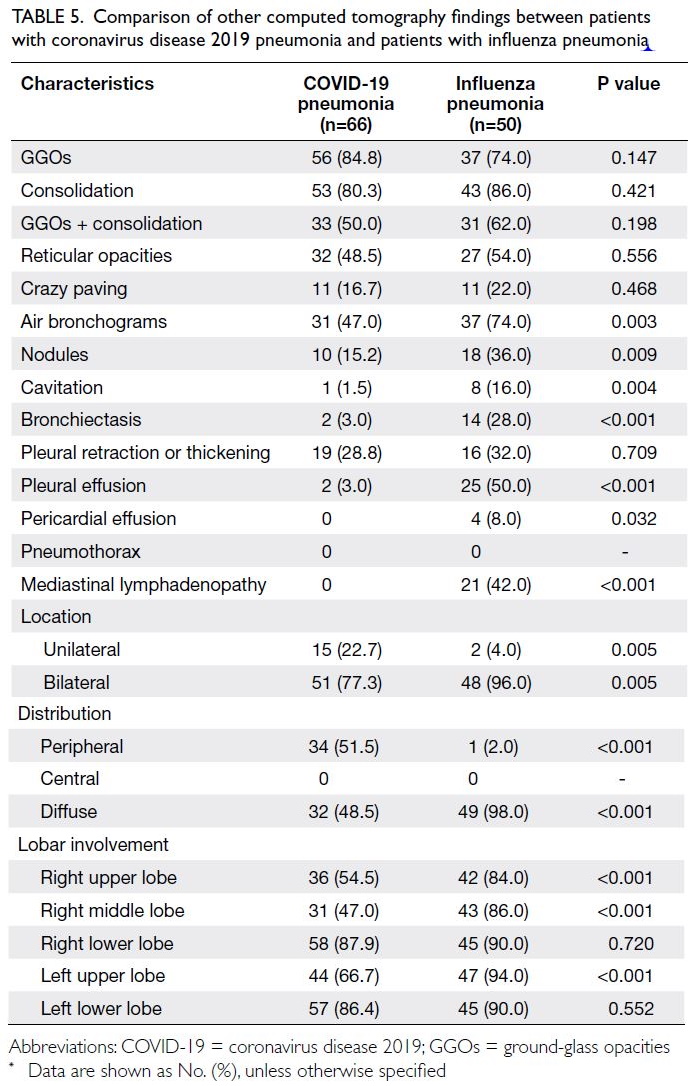
Table 5. Comparison of other computed tomography findings between patients with coronavirus disease 2019 pneumonia and patients with influenza pneumonia
Abnormalities were more commonly bilateral
in patients with COVID-19 pneumonia (77.3%)
and patients with influenza pneumonia (96%).
The distribution was more likely to be peripheral
in patients with COVID-19 pneumonia (51.5% vs
2.0%, P<0.001), and was more likely to be diffuse
in patients with influenza pneumonia (98% vs
48.5%, P<0.001). The right upper lobe (P<0.001),
right middle lobe (P<0.001), and left upper lobe
(P<0.001) were less commonly involved in patients
with COVID-19 pneumonia than in patients with
influenza pneumonia.
Discussion
Arched bridge and vacuole signs
This study evaluated the incidences and diagnostic
values of the arched bridge and vacuole signs among
patients with COVID-19 pneumonia in a Hong Kong
Chinese population. Since the initial description of
Wu et al20 in a series of 11 patients with COVID-19, our study is the first to validate the arched bridge sign in patients with COVID-19. To our knowledge,
this is also the first study to evaluate the vacuole sign
in non–COVID-19–related pneumonia. The arched
bridge sign was significantly more common in
COVID-19 pneumonia than in influenza pneumonia
or bacterial pneumonia. Additionally, the incidences
of the vacuole sign and both signs observed in
combination were higher (or tended to be higher) in
patients with COVID-19 pneumonia than in patients
with influenza pneumonia or bacterial pneumonia.
Our results imply that these two signs generally
appeared at an early stage; the arched bridge sign
is more likely to be observed in patients with more
severe lung pathology. These results suggest that the
arched bridge and vacuole signs can be used in CT-based
identification of COVID-19 pneumonia, as
well as efforts to differentiate COVID-19 pneumonia
from other types of infection-related pneumonia.
Currently, chest CT is not recommended for the
screening or diagnosis of COVID-19 pneumonia
when RT-PCR tests are available. In selected
cases, CT can be used to monitor clinical progress and identify complications of the disease. In
some scenarios, CT can be a useful alternative
investigation method for COVID-19 diagnosis or
triage, such as healthcare settings with restricted
access to RT-PCR tests.27 28 When these two signs
are observed on CTs performed for COVID-19
pneumonia or other indications during the
COVID-19 pandemic, physicians should carefully
consider a diagnosis of COVID-19 pneumonia.
However, our findings indicated there was no
significant difference in the duration of total
hospitalisation between patients with COVID-19
pneumonia who had and did not have these two
signs, suggesting that they are not indicative of a
better or worse prognosis if appropriate treatments
are administered.
The underlying pathophysiological
mechanisms behind these signs remain unclear.
However, the morphological appearances of the
arched bridge and vacuole signs may indicate
different pathophysiological mechanisms of
lung sparing that occur during infection-related
pneumonia. Histopathological examinations of lung biopsy tissues from patients with COVID-19
pneumonia have provided evidence of variations
in diffuse alveolar damage.29 30 The curved concave
margin in the arched bridge sign may be the result
of secondary pulmonary lobule sparing, with the
interlobular septum of the secondary pulmonary
nodule forming some resistance to the spread of
infection among lobules.20 In contrast, the vacuole
sign (ie, a very small focal lucent area) may reflect a
spared alveolar cluster or dilated alveolar sac within
an area of otherwise involved lung.21 23 Zhang et al23
reported that the vacuole sign was often present
in patients with advanced COVID-19 pneumonia,
where alveolar sac dilation could result from damage
to the alveolar wall.
The incidence of the arched bridge sign in
patients with COVID-19 (63.6%) was similar to
the incidence reported by Wu et al20 (72.7%). The
incidence of the vacuole sign (21.2%) in patients
with COVID-19 pneumonia is also within the
range reported in prior studies describing this sign
(17-66%).21 22 23 24 Notably, three additional case series
have revealed spared airspaces in patients with
COVID-19 pneumonia, comprising ‘round cystic
changes’31, ‘cystic air spaces’32 and ‘cavity signs’,33
with prevalences of 10 to 30%; these phenomena
may include the vacuole sign. However, these case
series did not include formal definitions of the
findings. The differences in definitions of the vacuole
sign (and phenomena that include the sign) may also
explain the disparate prevalences (17%-66%, as noted
above) reported in the literature.
The arched bridge and vacuole signs
differentiated COVID-19 pneumonia from influenza
pneumonia and bacterial pneumonia with high
specificities and high positive predictive values,
suggesting that these signs can help to provide a
specific imaging diagnosis of COVID-19 pneumonia.
When encountering inconclusive CT features of
COVID-19 pneumonia, these signs can be identified
with minimal additional effort; their presence may
be sufficient to increase suspicion or add to the
evidence confirming a diagnosis of COVID-19
pneumonia. The respective sensitivities of the arched
bridge and vacuole signs were moderate (63.6%) and
low (21.2%); the arched bridge sign may be more
useful in this context. Our findings suggest that the
combined presence of the arched bridge and vacuole
signs strongly supports a diagnosis of COVID-19
pneumonia.
Consistent with previous studies, the presence
of nodules, cavitations, bronchiectasis, pleural
effusion, pericardial effusion, and/or mediastinal
lymphadenopathy was uncommon in patients
with COVID-19 pneumonia; these features
were more common in patients with influenza
pneumonia.12 16 17 18 34 35 Our results indicated that
COVID-19-related abnormalities on CT were
generally bilateral and peripheral, compatible with
the findings in prior studies.12 13 14
Limitations
This study had several limitations. First, it used a retrospective design, and patients were imaged in
a cross-sectional manner at various time intervals
after symptom onset. Computed tomography
was not regularly performed, which hindered the
monitoring or analysis of imaging signs over time.
Second, CT was not routinely performed for patients
with influenza pneumonia or bacterial pneumonia;
it was performed based on clinical judgement,
generally because of patient deterioration or poor
response to treatment. We did not assess differences in the clinical features of patients with influenza
pneumonia and patients with bacterial pneumonia
between patients who did and did not undergo CT.
Third, we attempted to implement diversity in our
analysis of COVID-19 pneumonia by comparisons
with influenza pneumonia and bacterial pneumonia,
whereas prior studies have generally been limited
to comparisons of COVID-19 pneumonia with
influenza pneumonia. However, we did not examine
other types of viral pneumonia; we also did not
conduct subgroup analysis according to influenza
subtype. Additionally, we did not systematically
compare the prognoses of patients with non–COVID-19 pneumonia who had and did not have
the arched bridge or vacuole signs. This comparison
was hindered by the sample size, because these
two signs were very uncommon in patients with
non–COVID-19 pneumonia. However, additional
analysis did not reveal a clear pattern whereby these
two signs would be predictive for clinical prognosis
in patients with non–COVID-19 pneumonia.
Finally, the sample size in this study was moderate.
Although the prevalences of the arched bridge
and vacuole signs in our patients with COVID-19
pneumonia were consistent with findings in the
literature, their diagnostic specificities should be
validated in other types of pneumonia. Despite these
limitations, the high diagnostic specificities of these
CT signs provide insights that will be useful in future
studies. Additional work is needed regarding the
relationships of these CT signs with clinical status,
and our findings require validation in larger and
more diverse patient populations.
Conclusion
In conclusion, two morphological patterns of lung sparing, namely the arched bridge and vacuole signs,
are much more common in patients with COVID-19
pneumonia; they have the potential to differentiate
COVID-19 pneumonia from influenza pneumonia
and bacterial pneumonia. In this study, these signs
had high specificities and positive predictive values
for COVID-19 pneumonia. The identification of these
signs in clinical practice may be useful for increasing
suspicion or providing confirmatory evidence to
support a diagnosis of COVID-19 pneumonia.
Author contributions
Concept and design: TY So, SCH Yu, JYX Wang.
Acquisition of data: All authors.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: TY So, S Yu, JYX Wang.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: TY So, S Yu, JYX Wang.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Acknowledgement
The authors thank Ms Apurva Sawhney, Department of Imaging and Interventional Radiology, The Chinese University
of Hong Kong, for assistance with data collection.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research
Ethics Committee (REC Ref. No.: 2020.232), which waived the
requirement for informed consent due to the retrospective
nature of the study. The study was conducted in compliance
with the established ethical standards and principles of the
Declaration of Helsinki.
References
1. Kim H. Outbreak of novel coronavirus (COVID-19): what is the role of radiologists? Eur Radiol 2020;30:3266-7. Crossref
2. Yang W, Sirajuddin A, Zhang X, et al. The role of imaging in 2019 novel coronavirus pneumonia (COVID-19). Eur
Radiol 2020;30:4874-82. Crossref
3. Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 2020;295:715-21. Crossref
4. Wang Y, Dong C, Hu Y, et al. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a
longitudinal study. Radiology. 2020;296:E55-64. Crossref
5. Koo HJ, Lim S, Choe J, Choi SH, Sung H, Do KH. Radiographic and CT features of viral pneumonia.
Radiographics 2018;38:719-39. Crossref
6. Sun Z, Zhang N, Li Y, Xu X. A systematic review of chest
imaging findings in COVID-19. Quant Imaging Med Surg
2020;10:1058-79. Crossref
7. Marcos MA, Esperatti M, Torres A. Viral pneumonia. Curr Opin Infect Dis 2009;2:143-7. Crossref
8. Apisarnthanarak A, Mundy LM. Etiology of community-acquired pneumonia. Clin Chest Med 2005;26:47-55. Crossref
9. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019
(COVID-19): a review. JAMA 2020;323:1824-36. Crossref
10. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis,
and treatment of coronavirus disease 2019 (COVID-19): a
review. JAMA 2020;324:782-93. Crossref
11. Zayet S, Kadiane-Oussou NJ, Lepiller Q, et al. Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte cluster. Microbes Infect
2020;22:481-8. Crossref
12. Lin L, Fu G, Chen S, et al. CT manifestations of coronavirus disease (COVID-19) pneumonia and influenza virus pneumonia: a comparative study. AJR Am J Roentgenol 2021;216:71-9. Crossref
13. Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology
2020;295:202-7. Crossref
14. Song F, Shi N, Shan F, et al. Emerging 2019 novel coronavirus
(2019-nCoV) pneumonia. Radiology 2020;295:210-7. Crossref
15. Wang H, Wei R, Rao G, Zhu J, Song B. Characteristic CT
findings distinguishing 2019 novel coronavirus disease
(COVID-19) from influenza pneumonia. Eur Radiol
2020;30:4910-7. Crossref
16. Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists
in differentiating COVID-19 from non–COVID-19 viral
pneumonia on chest CT. Radiology 2020;296:E45-54. Crossref
17. Yin Z, Kang Z, Yang D, Ding S, Luo H, Xiao E. A
comparison of clinical and chest CT findings in patients
with influenza A (H1N1) virus infection and coronavirus
disease (COVID-19). AJR Am J Roentgenol 2020;215:1065-71. Crossref
18. Liu M, Zeng W, Wen Y, Zheng Y, Lv F, Xiao K. COVID-19
pneumonia: CT findings of 122 patients and differentiation
from influenza pneumonia. Eur Radiol 2020;30:5463-9. Crossref
19. Tanaka N, Matsumoto T, Kuramitsu T, et al. High resolution
CT findings in community-acquired pneumonia. J Comput
Assist Tomogr 1996;20:600-8. Crossref
20. Wu R, Guan W, Gao Z, et al. The arch bridge sign: a
newly described CT feature of the coronavirus disease-
19 (COVID-19) pneumonia. Quant Imaging Med Surg
2020;10:1551-8. Crossref
21. Zhou S, Wang Y, Zhu T, Xia L. CT features of coronavirus
disease 2019 (COVID-19) pneumonia in 62 patients in
Wuhan, China. AJR Am J Roentgenol 2020;214:1287-94. Crossref
22. Sabri YY, Nassef AA, Ibrahim IM, Abd El Mageed MR,
Khairy MA. CT chest for COVID-19, a multicenter study—
experience with 220 Egyptian patients. Egypt J Radiol Nucl
Med 2020;51:144. Crossref
23. Zhang L, Kong X, Li X, et al. CT imaging features of
34 patients infected with COVID-19. Clin Imaging
2020;68:226-31. Crossref
24. Zhou S, Zhu T, Wang Y, Xia L. Imaging features and
evolution on CT in 100 COVID-19 pneumonia patients in
Wuhan, China. Eur Radiol 2020;30:5446-54. Crossref
25. Elmokadem AH, Bayoumi D, Abo-Hedibah SA, El-Morsy A.
Diagnostic performance of chest CT in differentiating COVID-19 from other causes of ground-glass opacities. Egypt J Radiol Nucl Med 2021;52:12. Crossref
26. Zheng F, Li L, Zhang X, et al. Accurately discriminating
COVID-19 from viral and bacterial pneumonia according
to CT images via deep learning. Interdiscip Sci 2021;13:273-85. Crossref
27. Simpson S, Kay FU, Abbara S, et al. Radiological Society of
North America Expert consensus statement on reporting
chest CT findings related to COVID-19. Endorsed by the
Society of Thoracic Radiology, the American College of
Radiology, and RSNA - Secondary Publication. J Thorac
Imaging 2020;35:219-27. Crossref
28. Dennie C, Hague C, Lim RS, et al. Canadian Society of
Thoracic Radiology/Canadian Association of Radiologists
consensus statement regarding chest imaging in suspected
and confirmed COVID-19. Can Assoc Radiol J 2020;71:470-81. Crossref
29. Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 2020;396:320-32. Crossref
30. Zhang H, Zhou P, Wei Y, et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med 2020;172:629-32. Crossref
31. Shi H, Han X, Jiang N, et al. Radiological findings from 81
patients with COVID-19 pneumonia in Wuhan, China: a
descriptive study. Lancet Infect Dis 2020;20:425-34. Crossref
32. Rodrigues RS, Barreto MM, Werberich GM, Marchiori E. Cystic airspaces associated with COVID-19 pneumonia.
Lung India 2020;37:551-3. Crossref
33. Kong W, Agarwal PP. Chest imaging appearance of COVID-19 infection. Radiol Cardiothorac Imaging
2020;2:e200028. Crossref
34. Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A.
Coronavirus disease 2019 (COVID-19): a systematic review
of imaging findings in 919 patients. AJR Am J Roentgenol
2020;215:87-93. Crossref
35. Xu Z, Pan A, Zhou H. Rare CT feature in a COVID-19
patient: cavitation. Diagn Interv Radiol 2020;26:380-1. Crossref