Hong Kong Med J 2022;28(6):475–81 | Epub 11 Jul 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE CME
Recommendations for the management of advanced and metastatic renal cell carcinoma: joint consensus statements from the Hong Kong Urological Association and the Hong Kong Society of Uro-Oncology
Darren MC Poon, FRCR, FHKAM (Radiology)1; CK Chan, MB, ChB, FHKAM (Surgery)2; Kuen Chan, MB, BS, FHKAM (Radiology)1; WH Chu, FRCSEd (Urol), FHKAM (Surgery)2; Philip WK Kwong, FRCR, FHKAM (Radiology)1; Wayne Lam, MRCS (Eng), FRCSEd (Urol)2; KS Law, MB, BS, FHKAM (Radiology)1; Eric KC Lee, MB, ChB, FHKAM (Radiology)1; PL Liu, MB, BS, FHKAM (Surgery)2; Henry CK Sze, FRCR, FHKAM (Radiology)1; Joseph HM Wong, MB, BS, FHKAM (Surgery)2; Eddie SY Chan, MD, FHKAM (Surgery)2
1 Hong Kong Society of Uro-Oncology, Hong Kong
2 Hong Kong Urological Association, Hong Kong
Corresponding author: Dr Darren MC Poon (darren.mc.poon@hksh.com)
Abstract
Introduction: Kidney cancer, primarily renal cell
carcinoma (RCC), ranks among the top 10 most
common malignancies in the male population of
Hong Kong. In 2019, members of two medical
societies in Hong Kong formed an expert panel
to establish a set of consensus statements for the
management of metastatic RCC. On 22 June 2021,
the same panel met to review recent evidence and
reassess their positions regarding the management
of advanced and metastatic RCC, with the aim of
providing recommendations for physicians in Hong
Kong.
Participants: The panel included 12 experts (6 clinical oncologists and 6 urologists) who had extensive experience managing patients with RCC in Hong Kong.
Evidence: The panel reviewed randomised
controlled trials, observational studies, systematic
reviews/meta-analyses, and international clinical
guidelines to address key clinical questions that were
identified before the meeting.
Consensus Process: In total, 15 key clinical
questions were identified before the meeting,
covering the surgical and systemic treatment of
advanced or metastatic clear cell, sarcomatoid, and
non-clear cell RCCs. At the meeting, the panellists
voted on these questions, then discussed relevant
evidence and practical considerations.
Conclusions: The treatment landscape for
advanced and metastatic RCC continues to evolve.
More immune checkpoint inhibitor (ICI)–based
combination regimens will be indicated for the
treatment of metastatic clear cell RCC. There is
increasing evidence concerning the benefit of
adjuvant ICI treatment for resected advanced RCC.
This article summarises recent evidence and expert
insights regarding a series of key clinical questions
about the management of advanced and metastatic RCC.
Introduction
In 2018, kidney cancer was the ninth most common malignancy in the male population of Hong Kong,
with a relative frequency of 3%.1 The predominant
type of kidney cancer is renal cell carcinoma (RCC),
which mainly comprises the clear cell subtype; non-clear
cell RCC can be subdivided into papillary,
chromophobe, and other rarer forms (eg, collecting
duct).2 Many RCCs are found incidentally without
symptoms suggestive of malignancy; approximately
30% of patients have metastatic disease at the time of diagnosis.2
The management of advanced and metastatic
RCC has been transformed by the development of
vascular endothelial growth factor receptor tyrosine
kinase inhibitors (TKIs) and, more recently, immune
checkpoint inhibitors (ICIs). In 2019, members of
two medical societies in Hong Kong formed an expert
panel to establish a set of consensus statements for
the management of metastatic RCC.3 Since then,
the treatment landscape has continued to change
with the addition of two evidence-based ICI-TKI combination therapies: nivolumab/cabozantinib and
pembrolizumab/lenvatinib.4 5 There is also increasing
research concerning the role of adjuvant ICI in the
treatment of advanced RCC after nephrectomy.6
Considering these advances, the same expert panel
met to review recent evidence and reassess their
positions regarding the management of advanced
and metastatic RCC through panel votes on a series
of key clinical questions, with the aim of providing
treatment recommendations for physicians in Hong
Kong.
Methods
The meeting was held on 22 June 2021; the expert panel included 12 clinicians (6 clinical oncologists
and 6 urologists) who had extensive experience
managing patients with RCC in the public or
private healthcare sectors. Prior to the meeting, the
panel identified 15 key clinical questions (online supplementary Appendix) regarding the surgical
and systemic treatment of advanced or metastatic
clear cell, sarcomatoid, and non-clear cell RCCs
in various risk categories. The panel reviewed
randomised controlled trials, observational studies,
systematic reviews/meta-analyses, and international
clinical guidelines that addressed these clinical
questions. Prior to the meeting, review materials
had been identified through a search of the PubMed
database for publications from January 2020 to May 2021 using the key words ‘metastatic/advanced
+ renal cell carcinoma’; the search results were
supplemented with additional articles solicited by the
panellists. At the meeting, the panellists voted on the
15 questions, then discussed relevant clinical
evidence and practical considerations for real-world
clinical practice. The full voting record for each
question is provided in the online supplementary Appendix.
Results
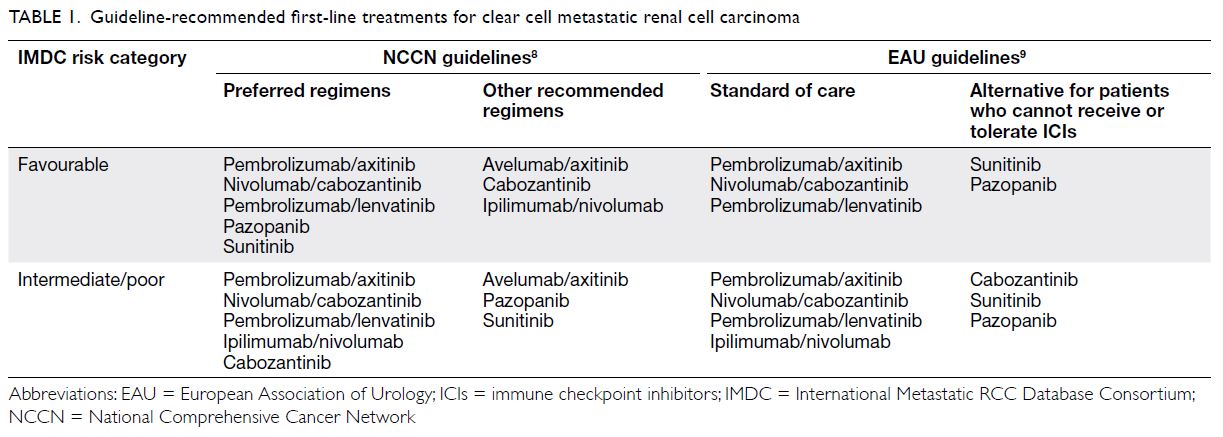
First-line systemic therapies for clear cell
metastatic renal cell carcinoma
Current published evidence
To decide on a treatment strategy for clear cell metastatic RCC, the International Metastatic
RCC Database Consortium (IMDC) risk category7
remains a key consideration. Current international
guidelines largely recommend ICI-containing
combination treatment as the standard of care
for metastatic RCC in all IMDC risk categories
(Table 1).8 9 In phase III open-label randomised
trials, the recommended ICI-containing regimens
significantly improved progression-free survival
(PFS), overall survival (OS), and objective response
rates (ORRs), when compared with sunitinib as first-line
treatment for metastatic RCC in the respective
primary study populations: for intermediate/poor-risk patients, ipilimumab/nivolumab10; and
for intention-to-treat patients, pembrolizumab/axitinib,11 nivolumab/cabozantinib,4 and
pembrolizumab/lenvatinib5 (Table 2). Post-hoc
analyses showed that ipilimumab/nivolumab and
nivolumab/cabozantinib were associated with
better health-related quality of life compared with
sunitinib12 13; there were no significant differences in
health-related quality of life between sunitinib and
pembrolizumab/axitinib or between sunitinib and
pembrolizumab/lenvatinib.14 15
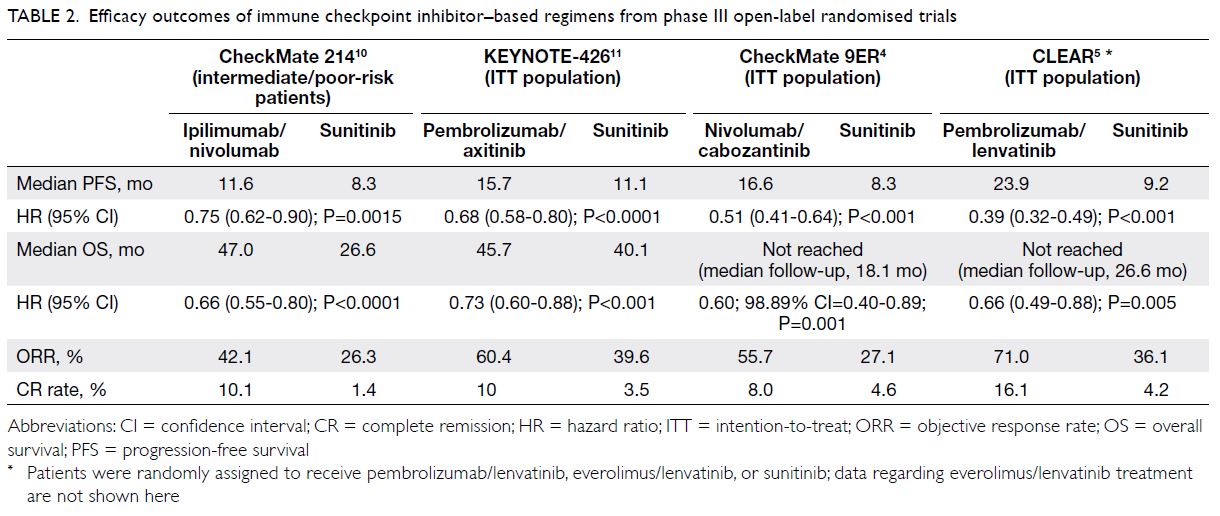
Table 2. Efficacy outcomes of immune checkpoint inhibitor–based regimens from phase III open-label randomised trials
Recommendations from the expert panel
Based on the available evidence and insights from the expert panel, ICI-ICI (ie, ipilimumab/nivolumab)
and ICI-TKI combinations each have specific
advantages and disadvantages (Table 3 4 5 10 11), which
should be considered when selecting a treatment regimen.
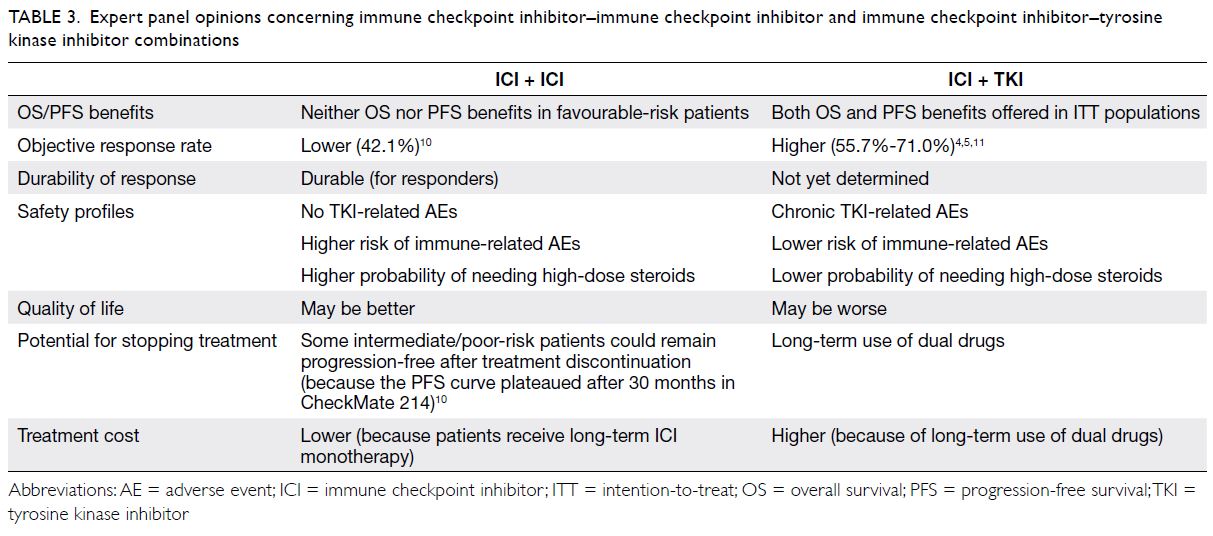
Table 3. Expert panel opinions concerning immune checkpoint inhibitor–immune checkpoint inhibitor and immune checkpoint inhibitor–tyrosine kinase inhibitor combinations
According to the panel consensus, the IMDC risk category and burden of disease or presence of
symptoms were regarded as the most important
patient/disease factors when selecting the first-line
treatment regimen for advanced or metastatic clear
cell RCC. Efficacy (primarily OS, followed by PFS
and ORR) and toxicity were regarded as the most
important treatment-related factors when selecting a
treatment regimen. Figure 1 illustrates the treatment
algorithm recommended by the panel.
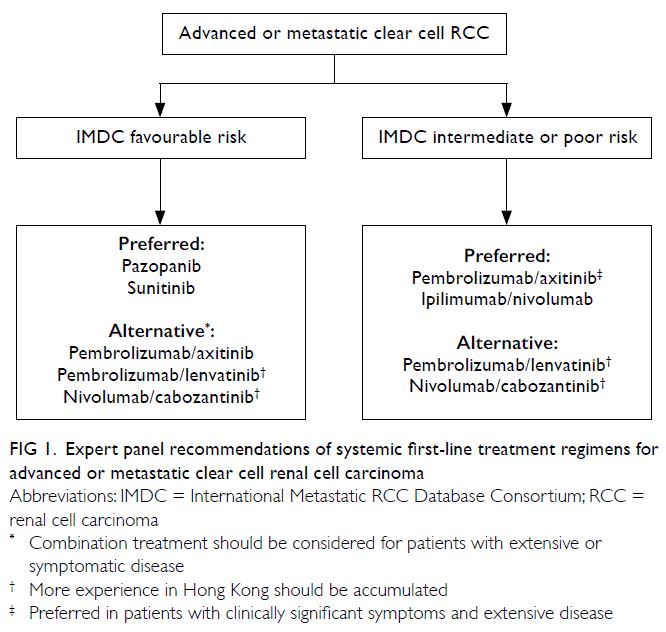
Figure 1. Expert panel recommendations of systemic first-line treatment regimens for advanced or metastatic clear cell renal cell carcinoma
For IMDC favourable-risk advanced or
metastatic clear cell RCC, TKI monotherapy
(pazopanib or sunitinib) was regarded as the
preferred treatment regimen. In subgroup analyses
of phase III open-label randomised trials, the OS
benefits of ICI-TKI combinations were uncertain
in favourable-risk patients, but these results should
be interpreted cautiously because the numbers of
participants were limited in each subgroup.4 5 16
Despite the uncertain OS benefits, ICI-TKI
combinations provided significant PFS and ORR
benefits compared with sunitinib; therefore, they
may remain useful in favourable-risk patients,
particularly patients with extensive or symptomatic
disease who desire treatment with a higher ORR.
For IMDC intermediate/poor-risk advanced
or metastatic clear cell RCC, ICI-based combination
treatment (preferably pembrolizumab/axitinib
or ipilimumab/nivolumab) is recommended. In
patients with clinically significant symptoms and
extensive disease, pembrolizumab/axitinib may
be preferred because it appeared to offer stronger
antitumour activity (ORR, 60.4%; stable disease rate,
22.9%; progressive disease rate, 11.3%) compared
with ipilimumab/nivolumab, according to the
KEYNOTE-426 study.11 In the CheckMate 214 study,
approximately 20% of patients experienced disease
progression after treatment with ipilimumab/nivolumab.10
With respect to newer ICI-TKI combinations (ie, pembrolizumab/lenvatinib and nivolumab/cabozantinib), there is a need to accumulate
additional experience in Hong Kong. The optimal
dose and tolerability profile of lenvatinib, particularly
in Asian patients, should be further investigated; in
the CLEAR study, 70% of patients required dose
reductions for lenvatinib.5 For cabozantinib, there
is a lack of flexibility in dose manipulation; only
60 mg, 40 mg, and 20 mg were available for use in
the CheckMate 9ER study.4 In contrast, the dosage
of axitinib is readily adjustable; 1-mg increments
or reductions can be implemented depending on
patient tolerability.
In public hospitals in Hong Kong, ICIs for the treatment of RCC remain self-financed, whereas TKI
monotherapy (ie, axitinib, pazopanib, and sunitinib)
is supported by the Safety Net programme.17
Adjuvant treatment after nephrectomy in
patients with advanced renal cell carcinoma
Current evidence regarding adjuvant
pembrolizumab treatment
Nephrectomy is the standard of care for localised RCC; however, patients with advanced RCC are
at risk of disease recurrence, and thus the use
of adjuvant treatment warrants investigation. In
the KEYNOTE-564 phase III trial, patients with
high-risk, fully resected clear cell RCC (M0 or M1
without evidence of disease) were randomised to
receive adjuvant pembrolizumab or placebo.6 At the
median follow-up interval of 24 months, adjuvant
pembrolizumab significantly improved disease-free
survival compared with placebo (77.3% vs 68.1% at
24 months; hazard ratio [HR]=0.68, 95% confidence
interval [CI]=0.53-0.87; P=0.002 [two-sided]). While
the OS data were immature, there was a trend
in favour of adjuvant pembrolizumab (96.6% vs
93.5% at 24 months; HR=0.54; 95% CI=0.30-0.96).
These results suggest that adjuvant pembrolizumab
can prevent relapse after surgery in patients with
advanced RCC.
Recommendations from the expert panel
The panellists noted that the use of adjuvant systemic
treatment after nephrectomy depends on patient
preference after a discussion of the benefits and
risks. The limitations of adjuvant treatment include
the lack of clear markers of efficacy, the risks of
overtreatment and toxicity (particularly in older and
frailer patients), and the potential for fewer available
treatment regimens in patients who experience
disease recurrence. Compared with adjuvant TKI,
adjuvant ICI may be associated with fewer adverse
effects and better quality of life, offering new
treatment opportunities for high-risk patients (eg,
with nodal metastases). Further studies are needed
to investigate the clinical benefit of adjuvant ICI in distinct patient subgroups (eg, patients with
non-clear cell RCC or bone oligometastases) and
to explore a risk-adapted approach for optimising
patient selection.
Treatment remains investigational for
patients who develop metastatic disease after
receiving adjuvant pembrolizumab. The panellists
favoured TKI monotherapy (pazopanib or
sunitinib), particularly for patients with a short
relapse-free period (eg, <6 months) after adjuvant
pembrolizumab treatment. They noted that patients
with a longer relapse-free period may receive ICI-based
combination treatment; for example, the
antitumour activity of pembrolizumab/lenvatinib in
ICI-pre-treated patients with clear cell metastatic
RCC (ORR, 55.8%) was demonstrated in a phase
I/IIb study.18
Treatment for advanced or metastatic
renal cell carcinoma with sarcomatoid de-differentiation
The standard of care for sarcomatoid RCC has not been determined. Consistent with the previous
consensus statement, the panellists favoured an
ICI-containing combination for the treatment of
metastatic RCC with sarcomatoid de-differentiation,
which is generally within the IMDC intermediate/poor-risk category. Compared with other RCCs
that lack sarcomatoid features, sarcomatoid
RCCs have higher programmed death-ligand 1
expression; thus, they may be more responsive to ICI
immunotherapies.19 In subgroup analyses of phase
III randomised studies, ICI-containing regimens
offered OS, PFS, and ORR benefits compared with
sunitinib in patients who had metastatic RCC
with sarcomatoid de-differentiation.20 Phase III
randomised trials dedicated to the treatment of
sarcomatoid RCC are expected.
Cytoreductive nephrectomy
Consistent with the previous consensus statement, the panellists favoured systemic treatment, rather
than upfront cytoreductive nephrectomy (CN), for
the management of de novo metastatic RCC.
The CN candidacy in IMDC favourable-risk
patients remains unclear, particularly in the ICI era.
Several panellists noted that CN may be irrelevant
to this patient population because most will have
already undergone nephrectomy or decided to
avoid nephrectomy based on age and performance
status, considering that the time from their diagnosis
until systemic treatment is ≥1 year. However, when
immediate systemic treatment is not required,
upfront CN with metastasectomy may be considered
for patients with asymptomatic primary tumours
and limited metastases confined to the lung. There is
also preliminary evidence to support the use of CN combined with ICI immunotherapy in patients with
pathologically favourable tumour characteristics.
An analysis of the United States National Cancer
Database found that, in patients with metastatic
RCC, the combination of CN (primarily in the
upfront setting) and ICI immunotherapy improved
median OS (not reached vs 11.6 months; HR=0.23,
P<0.001) compared with ICI immunotherapy
alone.21 Because ICI-based combination treatment is increasingly used, the role and sequence of CN warrant prospective validation.
The panellists recommended deciding whether
to perform CN in IMDC intermediate-risk patients
based on the extent of disease and symptoms.
Upfront CN may be considered for patients with
solitary or limited metastases (oligometastases).
Otherwise, delayed CN may be considered for
patients who respond well to systemic treatment.
Further studies are required to explore the patient
selection and optimal timing for CN in the context
of ICI immunotherapy.
The panellists recommended avoiding CN in
IMDC poor-risk patients, considering their low life
expectancy (7-8 months) and poor prognosis, as
well as the potential for surgical complications and
impacts on quality of life. Retrospective data from
the IMDC demonstrated that poor-risk patients did
not experience survival benefits from CN.22
Treatment for advanced or metastatic non-clear
cell renal cell carcinoma
The standard of care for metastatic non-clear cell
RCC remains unclear, particularly considering the
heterogeneity among subtypes. Based on the current
evidence, the panellists favoured TKI monotherapy
(cabozantinib or sunitinib). In a randomised open-label
phase II trial, patients with metastatic papillary
RCC were randomly assigned to receive sunitinib,
cabozantinib, crizotinib, or savolitinib; only
cabozantinib improved median PFS compared with
sunitinib (9.0 vs 5.6 months; HR=0.60 [95% CI=0.37-0.97], one-sided P=0.019).23 The antitumour activity of sunitinib in metastatic non-clear cell RCC has
been demonstrated in prospective studies.24 25
Subsequent treatment for advanced or
metastatic clear cell renal cell carcinoma
after progression on first-line systemic treatment
While the optimal sequence of treatment remains
unclear, the principle of choosing a subsequent
treatment (Fig 2) is consistent with the previous
consensus statement. In patients who demonstrated
progression after ICI-based combination treatment,
the panellists favoured TKI monotherapy, primarily
cabozantinib; its antitumour activity in patients
with prior exposure to ICIs has been demonstrated in large retrospective studies.26 27 In patients who
demonstrated progression after first-line TKI
monotherapy, the panellists favoured nivolumab
or cabozantinib based on prospective evidence,28,29
which was described in the previous consensus
statement.
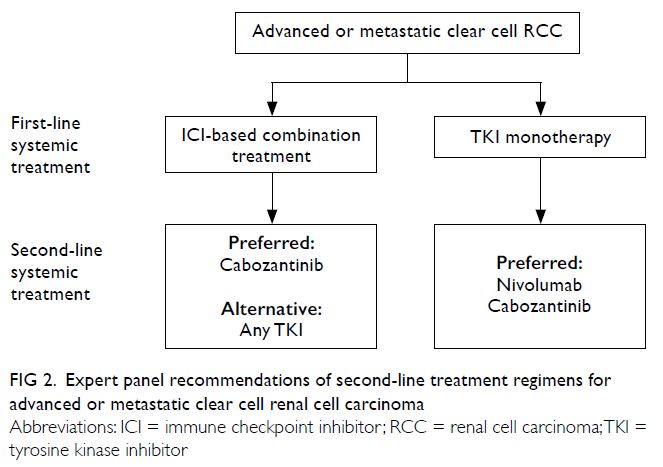
Figure 2. Expert panel recommendations of second-line treatment regimens for advanced or metastatic clear cell renal cell carcinoma
Conclusions
The treatment landscape for advanced and metastatic RCC is evolving. More ICI-based combination
regimens have recently been shown to offer survival
benefits, compared with TKI monotherapy, as first-line
systemic treatment in patients with metastatic
clear cell RCC. There is increasing evidence to
support the feasibility of adjuvant ICI treatment after
surgery in patients with advanced RCC. This article
has summarised recent evidence and insights from
an expert panel on a series of key clinical questions,
with the goal of optimising the management of
advanced and metastatic RCC in Hong Kong. These
recommendations are expected to undergo regular
review and updating, considering that several crucial
areas (eg, the role of CN combined with ICI-based
treatment, the standard of care for RCCs with
sarcomatoid features or non-clear cell histology,
and the optimal sequence of systemic treatments)
require further investigation.
Author contributions
All authors contributed to the concept and/or design of the
study, acquisition of the data, analysis and/or interpretation
of the data, drafting of the manuscript, and critical revision
of the manuscript for important intellectual content. All
authors have had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank Best Solution Company Limited, Hong Kong, for providing medical writing/editorial support, which was funded by the Hong Kong Urological Association.
Funding/support
Medical writing/editorial support and the panel meeting for voting and discussions were funded by the Hong Kong
Urological Association.
References
1. Hospital Authority, Hong Kong SAR Government. Hong Kong Cancer Registry. Available from: https://www3.
ha.org.hk/cancereg/. Accessed 7 Jul 2021.
2. Cairns P. Renal cell carcinoma. Cancer Biomark 2010;9:461-73. Crossref
3. Poon DM, Chan CK, Chan K, et al. Consensus statements
on the management of metastatic renal cell carcinoma
from the Hong Kong Urological Association and the Hong
Kong Society of Uro-Oncology 2019. Asia Pac J Clin Oncol
2021;17(Suppl 3):27-38. Crossref
4. Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2021;384:829-41. Crossref
5. Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021;384:1289-300. Crossref
6. Choueiri TK, Tomczak P, Park SH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 2021;385:683-94. Crossref
7. Heng DY, Xie W, Regan MM, et al. Prognostic factors
for overall survival in patients with metastatic renal cell
carcinoma treated with vascular endothelial growth factor-targeted
agents: results from a large, multicenter study. J
Clin Oncol 2009;27:5794-9. Crossref
8. National Comprehensive Cancer Network. NCCN guidelines kidney cancer. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1440. Accessed 28 Jun 2021.
9. European Association of Urology. EAU guidelines on renal cell carcinoma. Available from: https://uroweb.org/guideline/renal-cell-carcinoma/. Accessed 18 Jun 2021.
10. Motzer RJ, Escudier B, McDermott DF, et al. Survival
outcomes and independent response assessment with
nivolumab plus ipilimumab versus sunitinib in patients
with advanced renal cell carcinoma: 42-month follow-up
of a randomized phase 3 clinical trial. J Immunother
Cancer 2020;8:e000891. Crossref
11. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab (pembro)
plus axitinib (axi) versus sunitinib as first-line therapy for
advanced clear cell renal cell carcinoma (ccRCC): results
from 42-month follow-up of KEYNOTE-426. J Clin Oncol
2021;39 (Suppl 15):4500. Crossref
12. Cella D, Grünwald V, Escudier B, et al. Patient-reported
outcomes of patients with advanced renal cell carcinoma
treated with nivolumab plus ipilimumab versus sunitinib
(CheckMate 214): a randomised, phase 3 trial. Lancet
Oncol 2019;20:297-310. Crossref
13. Cella D, Choueiri TK, Blum SI, et al. Patient-reported outcomes of patients with advanced renal cell carcinoma (aRCC) treated with first-line nivolumab plus cabozantinib
versus sunitinib: the CheckMate 9ER trial. J Clin Oncol
2021;39(Suppl 6):285. Crossref
14. Bedke J, Rini B, Plimack E, et al. Health-related
quality-of-life (HRQoL) analysis from KEYNOTE-426:
pembrolizumab (pembro) plus axitinib (axi) vs sunitinib
for advanced renal cell carcinoma (RCC). Proceedings
of the 35th Annual EAU Congress–Virtual; 2020 July 19;
Game changing session 4.
15. Motzer RJ, Porta C, Alekseev B, et al. Health-related
quality-of-life (HRQoL) analysis from the phase 3 CLEAR
trial of lenvatinib (LEN) plus pembrolizumab (PEMBRO)
or everolimus (EVE) versus sunitinib (SUN) for patients
(pts) with advanced renal cell carcinoma (aRCC). J Clin
Oncol 2021;39(Suppl 15):4502. Crossref
16. Powles T, Plimack ER, Soulières D, et al. Pembrolizumab
plus axitinib versus sunitinib monotherapy as first-line
treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label,
phase 3 trial. Lancet Oncol 2020;21:1563-73. Crossref
17. Hospital Authority, Hong Kong SAR Government. Drug
formulary management. Available from: https://www.ha.org.hk/hadf/en-us/. Accessed 9 Jul 2021.
18. Lee CH, Shah AY, Rasco D, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study
111/KEYNOTE-146): a phase 1b/2 study. Lancet Oncol
2021;22:946-58. Crossref
19. Blum KA, Gupta S, Tickoo SK, et al. Sarcomatoid renal cell
carcinoma: biology, natural history and management. Nat
Rev Urol 2020;17:659-78. Crossref
20. Buonerba C, Dolce P, Iaccarino S, et al. Outcomes
associated with first-line anti-PD-1/ PD-L1 agents vs.
sunitinib in patients with sarcomatoid renal cell carcinoma:
a systematic review and meta-analysis. Cancers (Basel)
2020;12:408. Crossref
21. Singla N, Hutchinson RC, Ghandour RA, et al. Improved
survival after cytoreductive nephrectomy for metastatic renal cell carcinoma in the contemporary immunotherapy
era: an analysis of the National Cancer Database. Urol
Oncol 2020;38:604.e9-17. Crossref
22. Heng DY, Wells JC, Rini BI, et al. Cytoreductive
nephrectomy in patients with synchronous metastases
from renal cell carcinoma: results from the International
Metastatic Renal Cell Carcinoma Database Consortium.
Eur Urol 2014;66:704-10. Crossref
23. Pal SK, Tangen C, Thompson IM Jr, et al. A comparison
of sunitinib with cabozantinib, crizotinib, and savolitinib
for treatment of advanced papillary renal cell carcinoma: a
randomised, open-label, phase 2 trial. Lancet 2021;397:695-703. Crossref
24. Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus
sunitinib for patients with metastatic non-clear cell
renal cell carcinoma (ASPEN): a multicentre, open-label,
randomised phase 2 trial. Lancet Oncol 2016;17:378-88. Crossref
25. Tannir NM, Jonasch E, Albiges L, et al. Everolimus versus
sunitinib prospective evaluation in metastatic non-clear
cell renal cell carcinoma (ESPN): a randomized multicenter
phase 2 trial. Eur Urol 2016;69:866-74. Crossref
26. Iacovelli R, Ciccarese C, Facchini G, et al. Cabozantinib
after a previous immune checkpoint inhibitor in metastatic
renal cell carcinoma: a retrospective multi-institutional
analysis. Target Oncol 2020;15:495-501. Crossref
27. McGregor BA, Lalani AA, Xie W, et al. Activity of
cabozantinib after immune checkpoint blockade in
metastatic clear-cell renal cell carcinoma. Eur J Cancer
2020;135:203-10. Crossref
28. Motzer RJ, Escudier B, George S, et al. Nivolumab
versus everolimus in patients with advanced renal cell
carcinoma: Updated results with long-term follow-up of
the randomized, open-label, phase 3 CheckMate 025 trial.
Cancer 2020;126:4156-67. Crossref
29. Choueiri TK, Escudier B, Powles T, et al. Cabozantinib
versus everolimus in advanced renal cell carcinoma
(METEOR): final results from a randomised, open-label,
phase 3 trial. Lancet Oncol 2016;17:917-27. Crossref