Hong Kong Med J 2022;28(6):438-46 | Epub 20 Oct 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Effects of primary granulocyte-colony stimulating
factor prophylaxis on neutropenic toxicity and chemotherapy dose delivery in Chinese patients with breast cancer who received adjuvant docetaxel plus cyclophosphamide chemotherapy: a retrospective cohort study
Carol CH Kwok, MB, ChB1; WH Wong, BSc, MSc1; Landon L Chan, MB, ChB1; Sabrina PY Wong, MB, BS, FCSHK2; F Wang, BMed, PhD3; Martin CS Wong, MD, MPH3; Shelly LA Tse, BMed, PhD3
1 Department of Oncology, Princess Margaret Hospital, Hong Kong
2 Department of Surgery, Princess Margaret Hospital, Hong Kong
3 JC School of Public Health and Primary Care, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Dr Carol CH Kwok (kwokch@ha.org.hk)
Abstract
Introduction: This study was performed to examine
the effects of primary granulocyte-colony stimulating
factor (G-CSF) prophylaxis on neutropenic toxicity,
chemotherapy delivery, and hospitalisation among
Chinese patients with breast cancer in Hong Kong.
Methods: This retrospective study included
patients with breast cancer who received adjuvant
docetaxel plus cyclophosphamide chemotherapy
from November 2007 to October 2013 at Princess
Margaret Hospital. Data were collected regarding the
usage of G-CSF prophylaxis; incidences of grade 3 or
4 neutropenia, febrile neutropenia, non-neutropenic
fever, and infection; hospital admissions, and
chemotherapy dose delivery. Patients who began
to receive G-CSF prophylaxis during the first cycle
of chemotherapy and continued such prophylaxis
in subsequent cycles were regarded as the primary
G-CSF prophylaxis group.
Results: In total, 231 female Chinese patients
with breast cancer were included in the analysis.
Overall, 193 (83.5%) patients received primary
G-CSF prophylaxis. The demographics and tumour
characteristics were comparable between patients
with and without primary G-CSF prophylaxis.
Primary G-CSF prophylaxis significantly reduced
febrile neutropenia incidence from 31.6%
to 14.5% (relative risk=0.45, 95% confidence
interval=0.25-0.81). Primary G-CSF prophylaxis
also significantly reduced the incidence of grade 3 or 4 neutropenia from 57.9% to 24.7% (relative
risk=0.43, 95% confidence interval=0.30-0.62)
and the incidence of febrile neutropenia–related
hospital admission from 31.6% to 12.4% (P=0.025).
Finally, it enabled more patients to receive adequate
chemotherapy dose delivery.
Conclusion: Primary G-CSF prophylaxis effectively
reduced the incidences of grade 3 or 4 neutropenia
and febrile neutropenia, while enabling adequate
chemotherapy dose delivery and reducing hospital
admissions among Chinese patients with breast
cancer who received adjuvant docetaxel plus
cyclophosphamide chemotherapy.
New knowledge added by this study
- Primary granulocyte-colony stimulating factor (G-CSF) prophylaxis was associated with reduced neutropenic toxicity from adjuvant docetaxel plus cyclophosphamide (TC) chemotherapy.
- Our 4-day G-CSF schedule helped to maintain the planned chemotherapy regimen and reduce the rate of hospital admission.
- Routine use of primary G-CSF prophylaxis enabled successful chemotherapy treatment and adequate chemotherapy dose delivery for patients with early breast cancer who received adjuvant TC chemotherapy.
- Primary G-CSF prophylaxis could reduce hospital admissions for the management of febrile neutropenia; it may reduce in-patient bed occupancy and offset hospitalisation costs.
- G-CSF prophylaxis can be extended to patients on all docetaxel-containing regimens of neoadjuvant and adjuvant chemotherapy.
Introduction
Adjuvant chemotherapy significantly improves
disease-free survival and overall survival in patients
with early breast cancer.1 For intermediate-risk
patients who have axillary lymph node-negative
early breast cancer,2 a common regimen comprises
four cycles of doxorubicin plus cyclophosphamide.
In 2009, Jones et al3 published a 7-year follow-up
study of patients with stages I-III operable breast
cancer in the United States; they reported that
superior disease-free survival and overall survival
could be achieved with four cycles of docetaxel
plus cyclophosphamide (TC), compared with four
cycles of doxorubicin plus cyclophosphamide.
Since then, TC has been increasingly regarded as an
alternative chemotherapy regimen to doxorubicin
plus cyclophosphamide for patients with early-stage
breast cancer. However, docetaxel causes significant
myelotoxicity, characterised by high incidences of
grade 3 or 4 neutropenia and febrile neutropenia
(FN). Chemotherapy-induced neutropenia is
a major type of toxicity that limits the dose of cancer therapy; FN is associated with substantial
morbidity, mortality, and financial costs.4 Febrile
neutropenia is considered a medical emergency,
which often requires immediate hospitalisation
and empirical administration of broad-spectrum
antibiotics. Severe (grade 3 or 4) neutropenia or FN
is the most common cause of dose reductions and/or cycle delays that lead to lower chemotherapy
dose intensity; such changes may influence clinical
outcomes, particularly when treatment is intended
to be curative or to prolong survival.5 6
There is substantial evidence that granulocyte-colony
stimulating factor (G-CSF) prophylaxis
reduces the incidence of chemotherapy-associated
FN in patients with diverse malignancies, including
patients with breast cancer who are receiving
chemotherapy and have moderately high/high
FN risk; this prophylaxis can result in fewer
chemotherapy dose reductions or delays.5 7 8 9
Current guidelines consistently recommend G-CSF
prophylaxis during chemotherapy treatment for
patients with cancer who have a high estimated risk
of FN (ie, approximately 20%), as well as patients
with cancer who have a history of FN.5 10 11 12 The
administration of G-CSF should also be used to
facilitate the maintenance of chemotherapy dose
intensity for patients in whom reduced chemotherapy
dose intensity or density is likely to cause a poor
outcome (eg, patients receiving adjuvant or
potentially curative treatment, or patients receiving
treatment to prolong survival).5 10 12
We began using TC chemotherapy in 2007 at
Princess Margaret Hospital, but we encountered a
high incidence of FN. Our initial solution comprised
dose reduction; after the first episode of FN, doses of
chemotherapeutic agents were reduced by 10% to 25%
in subsequent cycles. Then, G-CSF prophylaxis was
administered if the second episode of FN occurred to
avoid further dose reduction and to ensure delivery
of planned chemotherapy; it was also intended to
prevent the occurrence of further FN. This study was
conducted to investigate the effects of primary G-CSF
prophylaxis on neutropenic toxicity, chemotherapy
delivery, and hospitalisation in patients with breast
cancer if G-CSF was administrated from the first
cycle of TC chemotherapy.
Methods
Patient selection
This retrospective cohort study was performed
at Princess Margaret Hospital, Hong Kong. We
reviewed the medical records of female Chinese
patients with breast cancer who received adjuvant
TC chemotherapy from November 2007 to October
2013. Exclusion criteria were as follows: previous
receipt of chemotherapy, mixed TC and doxorubicin
plus cyclophosphamide or other chemotherapy regimens; more than four cycles of TC; failure to
complete four cycles of chemotherapy; and use
of G-CSF after the occurrence of FN. Data were
retrieved from the included patients’ out-patient
and in-patient records, chemotherapy charts, and
discharge summaries.
Tumour characteristics
Tumour staging, histological type, histological
grade, lymphovascular invasion, oestrogen receptor
status, progesterone receptor status, human
epidermal growth factor receptor 2 (HER2) status,
and Ki67 status were extracted from each patient’s
pathology report. Oestrogen and/or progesterone
receptor statuses were considered positive if either
percentage of immunohistochemical staining was
≥1% (ie, an H score of ≥50 or an Allred score of
≥3). The HER2 status was considered positive if the
immunohistochemical score was 3, or if fluorescence
in situ hybridisation showed HER2 gene amplification
if immunohistochemical score was equivocal.
Docetaxel plus cyclophosphamide treatment
protocol
Chemotherapy was initiated within 6 to 8 weeks after
surgery. The chemotherapeutic regimen consisted
of docetaxel 75 mg/m2 and cyclophosphamide
600 mg/m2 administered by intravenous infusion
over 60 minutes and 30 minutes, respectively, on day
1 at 3-week intervals for four cycles; dexamethasone
premedication and standard anti-emetic were
administered during each cycle. In accordance with
standard protocol in the Department of Oncology at
Princess Margaret Hospital, patients were required
to have an Eastern Cooperative Oncology Group
performance status of 0 or 1. At baseline and
before each cycle of chemotherapy, complete blood
counts were performed, along with tests of renal
and hepatic function. To proceed with treatment,
patients were required to have a white blood cell
count of ≥3 × 109/L, an absolute neutrophil count
(ANC) of ≥1.5 × 109/L, and a platelet count of
≥100 × 109/L. For patients with insufficient blood
counts, chemotherapy was deferred for ≥1 week
until counts reached the required levels. For patients
with an elevated alanine transaminase level (ie,
≥1.5-fold above the upper limit of normal), the dose
of docetaxel was reduced by 15%, in accordance with
prescribing information. Complete blood counts
were also checked at nadir (ie, the lowest white blood
cell count or ANC recorded within 21 days of the
first cycle of chemotherapy, typically on day 10 after
chemotherapy) to assess the severity of neutropenia;
based on blood count findings, chemotherapy dosage
was adjusted (if necessary) in subsequent cycles. The
dosage reduction ranged from 10% to 25% according
to the occurrence of grade 4 neutropenia or FN in
prior cycles. Hepatitis status was checked at baseline. Prophylactic antiviral therapy was administered to
patients who had a positive test result for hepatitis
B surface antigen.
Granulocyte-colony stimulating factor use
We suggested G-CSF prophylaxis (on a self-financed
basis during the study period) to each patient who
was scheduled to receive adjuvant TC, unless they
had contra-indications mentioned in the prescribing
information. The intent of G-CSF prophylaxis was to
prevent the occurrence of FN, which would lead to
cycle delay and chemotherapy dose reduction. We
defined primary G-CSF prophylaxis as upfront use
in the first chemotherapy cycle and continuation in
subsequent cycles. The administration of G-CSF after
an episode of FN during a previous chemotherapy
cycle was considered secondary use; patients
who received such treatment were excluded from
the analysis, as indicated in the Patient selection
subsection. Antibiotic treatment was administered
to patients who showed grade 3 or 4 neutropenia at
nadir.13 Neupogen (filgrastim) 30 MU was the form of
G-CSF used during the study period; this treatment
was administered subcutaneously from day 4 to day
7 of each chemotherapy cycle.
Febrile neutropenia and other adverse events
Febrile neutropenia was defined as a single reading
of oral temperature ≥38.3°C or a sustained (≥1 h)
oral temperature of ≥38.0°C, with either an ANC of
<0.5 × 109 cells/L or an ANC of <1.0 × 109 cells/L
and predicted decrease to <0.5 × 109 cells/L over
the next 48 hours.14 Haematological and other non-haematological
adverse events were categorised
and graded in accordance with the National Cancer
Institute’s Common Terminology Criteria for
Adverse Events (version 3.0); they were expressed as
maximum toxicity per patient. Other adverse effects
were reported as grade 3 or 4.
Hospital admission for chemotherapy-related
toxicities
An admission was regarded as a single hospital
admission that occurred between two consecutive
chemotherapy cycles; admissions were recorded
until 1 month after the final cycle of chemotherapy.
If neutropenic fever was diagnosed or suspected,
patients were admitted for isolation, sepsis tests, and
antibiotic treatment; if patients refused admission,
they were prescribed oral antibiotics. If indicated,
patients were also admitted for treatment of
chemotherapy-related adverse effects. Admissions
were categorised according to the primary diagnosis
at the time of admission.
Statistical analyses
Descriptive statistics were used to summarise baseline patient and tumour characteristics,
chemotherapy delivery, and adverse events. The Chi
squared test (or Fisher’s exact test) and Student’s
t test (or Mann–Whitney U test) were used for
comparisons of categorical and continuous variables,
respectively. Multivariate logistic regression models
were used to calculate the relative risk and 95%
confidence interval for the occurrence of FN with
primary G-CSF prophylaxis after adjustment for age.
The total percentage of planned doses received
was calculated as the sum of the percentage of planned
doses over four cycles divided by the number of
cycles of chemotherapy administered. Dose intensity
was calculated as the cumulative dose (mg/m2)
divided by the total duration of chemotherapy (wk).
Planned dose intensity was calculated as the planned
cumulative dose (mg/m2) divided by the planned
treatment duration (wk). Relative dose intensity was
calculated as the ratio of delivered dose intensity to
planned dose intensity. Chemotherapy dose delay
was defined as a delay of ≥3 days from the planned
treatment date. In all statistical analyses, P<0.05 was
considered indicative of statistical significance. All
data analyses were performed using SPSS software
(Windows version 26.0; IBM Corp, Armonk [NY],
United States).
Results
Baseline characteristics of patients according
to primary granulocyte-colony stimulating
factor prophylaxis status
During the initial review, 261 patients were identified
and eight of them presented with synchronous
bilateral breast cancer. In total, 30 patients were
excluded from analysis for the following reasons:
previous receipt of chemotherapy (n=2), mixed
TC and doxorubicin plus cyclophosphamide or
other chemotherapy regimens (eg, epirubicin plus
cyclophosphamide, or cyclophosphamide plus
methotrexate plus 5-fluorouracil) [n=14], or more
than four cycles of TC (n=6); failure to complete four
cycles of chemotherapy (n=3; 1 died of pneumonia, 1
discontinued chemotherapy after one cycle because
of advanced age, and 1 discontinued chemotherapy
after three cycles for unspecified reasons); and use
of G-CSF after the occurrence of FN (n=5). Finally,
231 female Chinese patients with breast cancer
were included in this study; 193 patients (83.5%)
received primary G-CSF prophylaxis (Table 1). Age
at diagnosis, body weight and height, body mass
index, and menopausal and co-morbidity statuses
were comparable between patients with and without
primary G-CSF prophylaxis. The distributions of
tumour stages, histological subtypes, histological
grades, and molecular biomarker statuses were also
similar between the two groups.
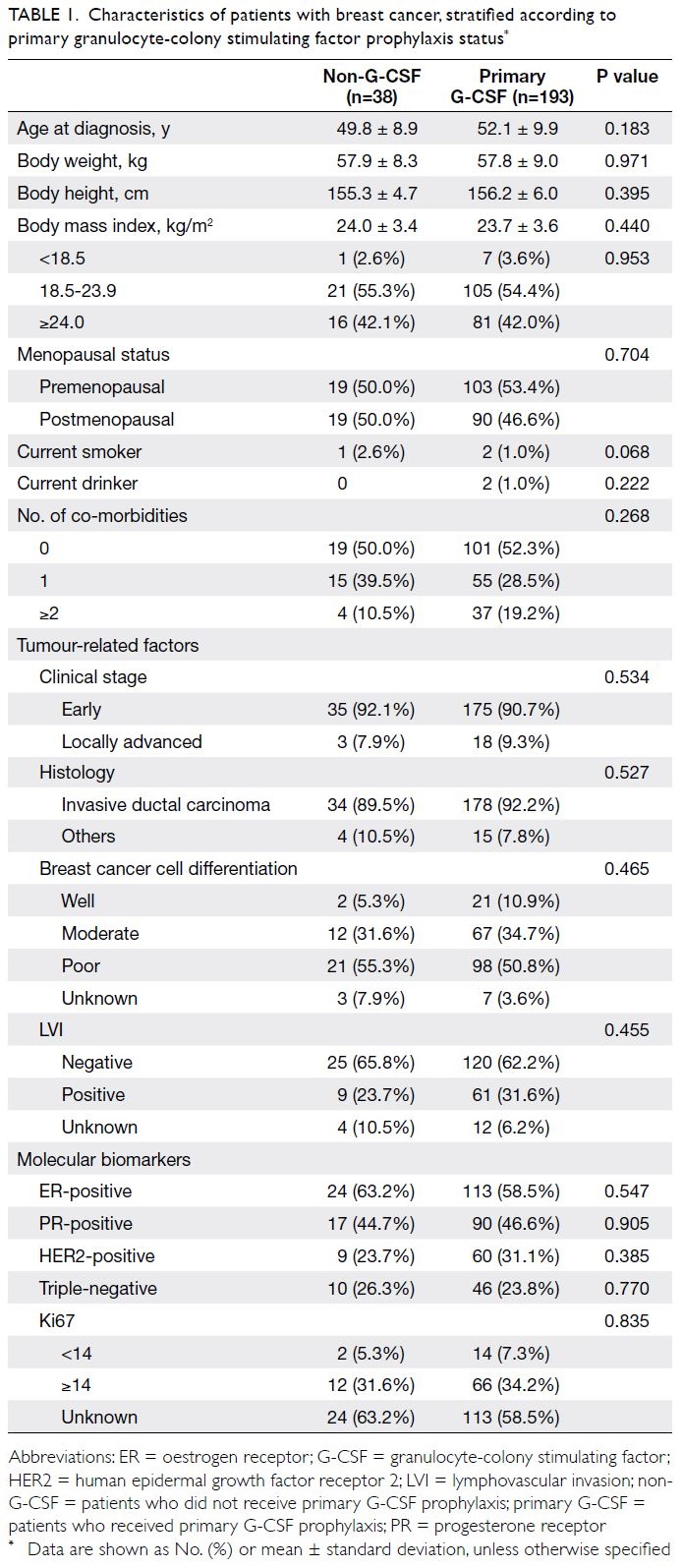
Table 1. Characteristics of patients with breast cancer, stratified according to primary granulocyte-colony stimulating factor prophylaxis status
Development of febrile neutropenia and
other chemotherapy-related toxicities
In total, 106 patients (45.9%) had ≥1 episode of
neutropenia; 69 patients (65.1%) developed grade
3 or 4 neutropenia (Table 2). Among patients with
neutropenia, the incidences of FN were 40.0%
(12/30) in the non-G-CSF group and 36.8% (28/76)
in the G-CSF group. Compared with patients who
did not receive G-CSF prophylaxis, primary G-CSF
prophylaxis was associated with a lower incidence
of FN (31.6% vs 14.5%) and a lower incidence of
grade 3 or 4 neutropenia (57.9% vs 24.7%) [Table 2];
the relative risks were 0.45 (95% confidence
interval=0.25-0.81) and 0.43 (95% confidence interval=0.30-0.62), respectively. However, G-CSF
prophylaxis was not associated with reduced
incidences of non-neutropenic fever and infection.
The incidences of grade 3 or 4 chemotherapy-related
toxicities other than neutropenia were very low
among our patients. Only four episodes of grade
3 or 4 chemotherapy-related adverse events were
observed (one episode of anaemia, one episode of
non-neutropenic leukopenia, and two episodes of
diarrhoea).
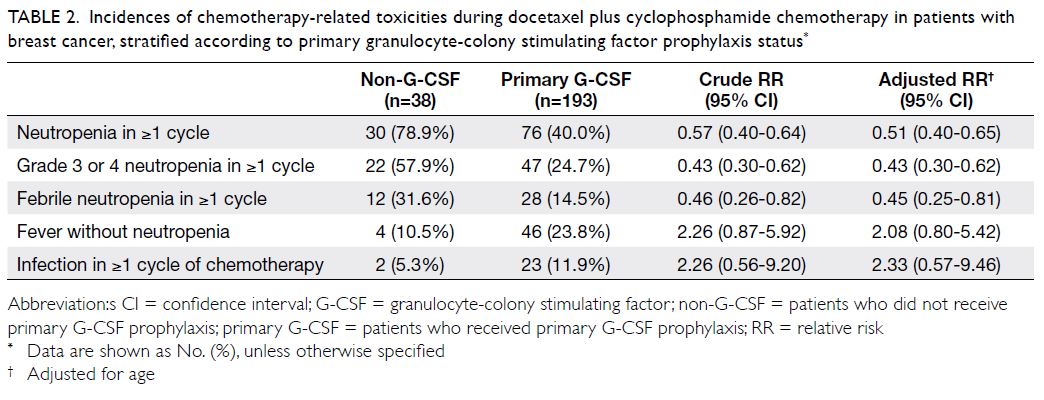
Table 2. Incidences of chemotherapy-related toxicities during docetaxel plus cyclophosphamide chemotherapy in patients with breast cancer, stratified according to primary granulocyte-colony stimulating factor prophylaxis status
Delivery of chemotherapy
Primary G-CSF prophylaxis helped to maintain the planned regimen of TC chemotherapy (Table 3). When compared with the non-G-CSF group, the
proportion of patients who received the standard
dose of TC was higher among patients in the G-CSF
group (63.2% vs 74.6%); moreover, the proportion of
patients with an overall dose deduction of >10% for
docetaxel or cyclophosphamide was lower among
patients in the G-CSF group (18.4% vs 14.0% and
13.2% vs 7.3%, respectively), although the difference
was not statistically significant. However, compared
with the non-G-CSF group, more patients in the
G-CSF group experienced chemotherapy cycle
delays (26.3% vs 56.5%). There were no significant
differences in dose intensity or relative dose
intensity for both docetaxel and cyclophosphamide
between the G-CSF and non-G-CSF group (online
supplementary Table 1).
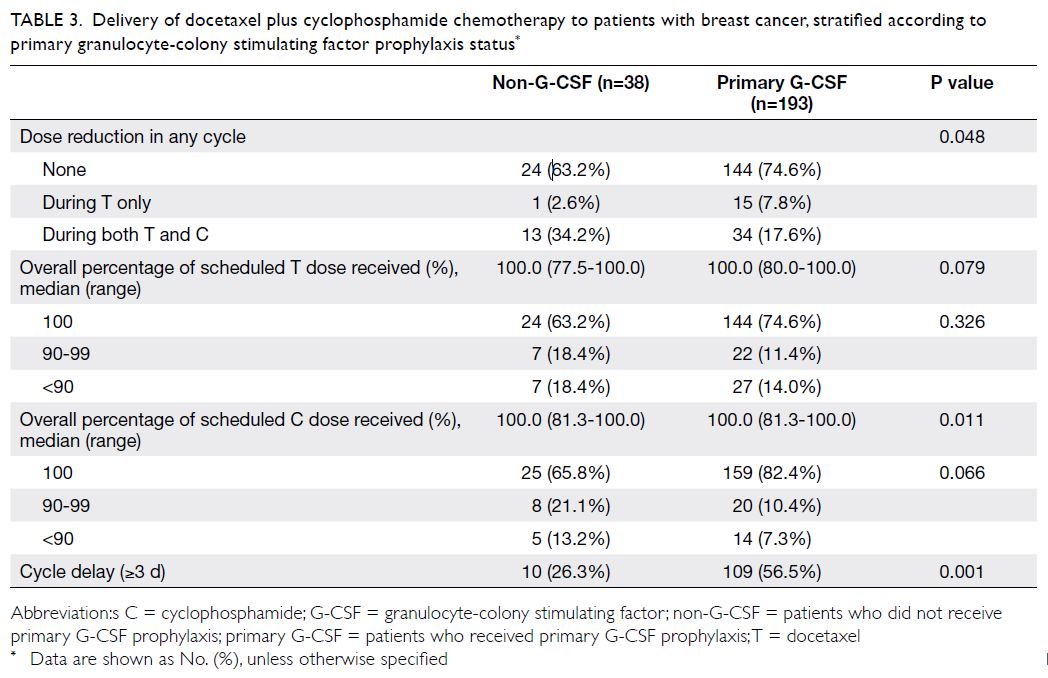
Table 3. Delivery of docetaxel plus cyclophosphamide chemotherapy to patients with breast cancer, stratified according to primary granulocyte-colony stimulating factor prophylaxis status
Rate of hospital admission
Sixty patients had ≥1 hospital admission for a severe
chemotherapy-related adverse event; 36 of these
patients (60%) were diagnosed with FN. Compared
with the non-G-CSF group, the hospital admission
rate was lower in the primary G-CSF prophylaxis
group, particularly in terms of admission for FN
(31.6% vs 12.4%) [Table 4].
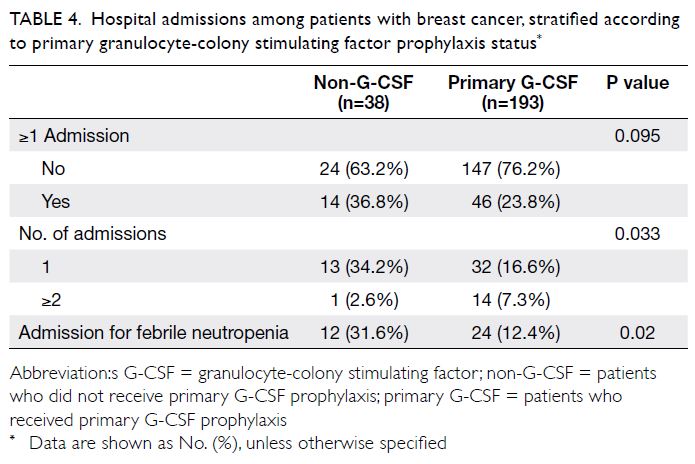
Table 4. Hospital admissions among patients with breast cancer, stratified according to primary granulocyte-colony stimulating factor prophylaxis status
Discussion
Primary granulocyte-colony stimulating
factor prophylaxis reduced febrile
neutropenia and severe neutropenia
To our knowledge, this is the first study to demonstrate
an association between the use of a fixed schedule of
G-CSF prophylaxis (days 4 to 7) and the reduction
of neutropenia and FN incidences in patients with
early-stage breast cancer who received adjuvant
TC chemotherapy. Our study demonstrated that patients who received primary G-CSF prophylaxis
were significantly more likely to maintain their
planned regimen of chemotherapy and have a lower
rate of hospital admission. These findings suggest
that G-CSF prophylaxis can enhance treatment
efficacy and conserve medical resources.
Chinese patients on docetaxel-based
chemotherapy had higher incidences of
neutropenia/febrile neutropenia
Our results are consistent with previous reports that
the FN rate was higher among patients receiving
TC chemotherapy in the absence of G-CSF.3 15 16 17
Rates of myelosuppression and neutropenia during
docetaxel-based chemotherapy were higher in our
Chinese patients compared with those in Caucasian
patients in previous studies.18 19 20 In the original TC
study, incidence of FN was only 5%.3 Inter-individual
and inter-ethnic variations in pharmacokinetics and
pharmacodynamics may be linked to variations in
docetaxel toxicity.21 22 23
Four-day course of granulocyte-colony
stimulating factor prophylaxis was effective
With regard to the schedule of G-CSF prophylaxis,
international guidelines recommend initiation
between 24 and 72 hours after the last day of
chemotherapy, with continuation until sufficient
and stable ANC recovery has been achieved after
nadir.5 10 11 The optimal clinical benefits of filgrastim
have been achieved with approximately 11 daily
injections; ANC recovery typically requires 10 to
11 days.5 Therefore, the median recommended
duration of daily filgrastim injections is 10 to 11
days.5 11 Nevertheless, the chemotherapy schedule
varies in clinical practice.5 24 25 In a previous study,
von Minckwitz et al8 found that daily G-CSF was
most frequently administered at five to seven
doses per cycle. We initiated G-CSF on day 4 in
accordance with the recommendation (mentioned
above) that G-CSF should be initiated between 24
and 72 hours after chemotherapy. According to
the docetaxel prescribing information, a median of
7 days is needed to reach nadir; the median duration
to reach severe neutropenia (<500 cells/mL) is also
7 days. Based on our previous experience concerning
nadir, we examined complete blood counts from
day 7 to day 11; we observed that the duration of
neutrophil nadir for docetaxel was short and the
ANC rapidly rebounded after day 10. Because the
median neutrophil nadir of docetaxel typically
occurs on day 7, the administration of G-CSF until
day 7 would constitute the shortest duration of
G-CSF injection; the increased neutrophil count
as a result of G-CSF injection (due to the effect of
G-CSF) would presumably have a protective effect
during the expected neutrophil nadir period.
In this study, FN occurred after the first
cycle in 28 of 193 (14.5%) patients who received
primary G-CSF prophylaxis; all of those patients
received G-CSF on days 4 to 7. It is important to
consider whether this finding suggests that our
G-CSF schedule was insufficient for FN prevention.
Notably, there have been reports that the initial
episode of neutropenia most frequently occurred
during the first cycle in patients receiving cancer
chemotherapy.6 25 26 The lower apparent risk after
the first cycle presumably results from subsequent
dose reductions and delays, or from the secondary
use of a white blood cell growth factor.6 27 The high
frequency of first-cycle FN has been proposed to
emphasise the need for early (during the first cycle)
initiation of G-CSF to reduce the risk of FN. A longer
duration of G-CSF prophylaxis may further reduce
the incidence of first-cycle FN, but this hypothesis
requires further investigation.
Granulocyte-colony stimulating factor
prophylaxis enabled adequate chemotherapy
dose delivery
We found that primary G-CSF prophylaxis
influenced the incidences of FN and FN-related
hospitalisation and also enabled adequate
chemotherapy dose delivery. Our findings were
similar to the results reported by von Minckwitz et al8;
however, their study involved a comparison of
primary prophylaxis with long-acting pegfilgrastim
to either no G-CSF treatment or any cycle of G-CSF/pegfilgrastim. In the group of patients who received
G-CSF, the longer duration of chemotherapy might
have offset the effect of the higher total percentage
of scheduled chemotherapy doses on dose intensity
and relative dose intensity. These findings were
analogous to the findings in a Cochrane review,
which showed CSF treatment did not help much in
maintaining the planned chemotherapy schedules.9
Additionally, von Minckwitz et al8 reported that
neutropenia prophylaxis influenced chemotherapy
dose reductions (≥15%) but did not affect the
incidence of chemotherapy dose delays (≥3 days).
Similarly, in a study that evaluated the effect of
pegfilgrastim during the first and subsequent cycles
versus placebo, the proportion of patients who
received their planned dose on time (defined as
receiving ≥80% of the planned dose and no dose
≥3 days late) did not significantly differ between the
two groups. The authors of the study concluded that
no difference had been present because patients who
developed FN were allowed to receive pegfilgrastim
in subsequent cycles, which (because of study
design) prevented the identification of a difference
between the pegfilgrastim and placebo groups.26
Although the reduction of FN incidence is
an important clinical outcome, G-CSF prophylaxis might facilitate the maintenance of chemotherapy
dose intensity7; G-CSF has also been used as an
adjunct to achieve moderate increases in dose
intensity. Early clinical trials of patients with solid
malignancies demonstrated a limited survival
benefit for patients who received higher dose
therapy.28 In clinical studies, dose-dense schedules
(ie, with shortened treatment intervals) have
shown increased survival, whereas the benefit in
dose escalation studies has been less consistent.6
Notably, a meta-analysis showed that the receipt of
primary G-CSF prophylaxis was associated with a
modest reduction in all-cause mortality, compared
with the absence of primary prophylaxis.29 Recently
published meta-analyses confirm the survival benefit
of dose-dense chemotherapy.29 30 These provide
supporting evidence that ensuring chemotherapy
dose intensity is an important consideration for
treatment outcome which is particularly relevant in
adjuvant chemotherapy settings. Further studies are
awaited to assess the effects of chemotherapy dose
delivery on survival outcomes in our patients with
early breast cancer.
Our results showed that G-CSF prophylaxis
reduced the rate of hospital admission for FN.
Conventional management of FN involves hospital
admission with intravenous administration of
broad-spectrum antibiotics for 5 to 7 days. The
mean length of hospitalisation for FN may exceed
1 week; patients must be placed in isolation rooms
to undergo numerous diagnostic procedures and
receive intravenous antibiotic support, and there is a
need to consider the potential complications of such
therapy (American Society of Clinical Oncology
guideline 1994, 2000).31 The benefit of reducing
the rate of hospital admission is that it can reduce
demands on the resources of a public healthcare
system with a limited number of in-patient beds.
Additionally, the reduced rate of hospital admission
can help to minimise disruption for patients and
their families, thereby avoiding negative impacts on
quality of life.
Study implications
The patients in this study received treatment from
November 2007 to October 2013. Among the
patients, 14.7% had luminal A–like breast cancer
subtypes according to histopathological criteria
(online
supplementary Table 2); thus far, patients
with such breast cancer subtypes have experienced
minimal benefits from chemotherapy. With the
increasing use of gene expression profiling as a
personalised medicine approach for adjuvant
chemotherapy in patients with hormone receptor–positive, HER2-negative breast cancers, the use of
adjuvant chemotherapy has become increasingly
selective for such patients; nevertheless, it remains important for patients with HER2-positive and
triple-negative breast cancers. The TC regimen is
an important type of adjuvant chemotherapy; it is
recommended within the National Comprehensive
Cancer Network guidelines. Because it is an
anthracycline-free regimen, TC chemotherapy
has been compared with anthracycline-containing
regimens in some large, randomised trials; it has
demonstrated excellent results.32 33 The TC regimen
is considered an efficacious and less-toxic option in
lower-risk patients, as well as patients with known
cardiac disease or pre-existing risk factors for
cardiac toxicity.32 33 Routine G-CSF prophylaxis has
made adjuvant TC chemotherapy safer and more
successful. Currently, the 4-day G-CSF schedule is
widely used for patients in our hospital who receive
other docetaxel-containing regimens, such as TCH
(ie, trastuzumab, carboplatin, and docetaxel) and
docetaxel 100 mg/m2 regimens; the outcomes are
generally positive.
Study limitations
First, this study used a retrospective cohort design
and only included patients from a single centre. Thus,
the overall sample size was moderate and the non-G-CSF group included a small number of patients.
Moreover, because this was an observational study
without randomisation, the number of patients who
received G-CSF prophylaxis substantially differed
from the number of patients who did not receive
such prophylaxis. Our results require confirm in
multicentre studies with diverse patient populations.
Second, indication bias might have been present,
such that patients who received G-CSF might
constitute a distinct group, compared with patients
who did not receive G-CSF. We suggested the use of
primary G-CSF prophylaxis to each patient who was
scheduled to receive adjuvant TC chemotherapy;
most patients accepted this suggestion (193/231).
Among 43 patients who did not receive G-CSF
treatment at the first cycle of chemotherapy, only five
received secondary administration of G-CSF, which
indicated that cost was the main factor influencing
receipt of primary G-CSF prophylaxis. Nevertheless,
we used multivariate logistic regression models
to adjust for potential confounding factors (eg,
baseline differences between the two groups).
Third, incomplete documentation of adverse effects
might have influenced our findings because only
significant adverse effects were recorded for most
study participants. Febrile neutropenia and hospital
admission were the major clinical outcomes recorded
in medical records; therefore, medication-related
adverse effects might have been neglected. Finally, because only short-term toxicity (ie, neutropenic toxicity) was examined in this study, the long-term effects of chemotherapy (eg, neurotoxicity) and G-CSF prophylaxis on quality of life should be addressed in future studies.
Conclusion
Our study demonstrated that the use of 4-day
primary G-CSF prophylaxis can reduce neutropenic
toxicity from adjuvant TC chemotherapy; it enables
successful chemotherapy treatment and facilitates
adequate chemotherapy dose delivery. Further
studies are needed to assess the effects of primary
G-CSF prophylaxis and chemotherapy dose delivery
on survival outcomes in patients with breast
cancer.
Author contributions
Concept or design: CCH Kwok, F Wang, SLA Tse.
Acquisition of data: CCH Kwok, SPY Wong.
Analysis or interpretation of data: WH Wong, CCH Kwok, SLA Tse, F Wang, LL Chan.
Drafting of the manuscript: CCH Kwok.
Critical revision of the manuscript for important intellectual content: CCH Kwok, F Wang, SLA Tse, MCS Wong.
Acquisition of data: CCH Kwok, SPY Wong.
Analysis or interpretation of data: WH Wong, CCH Kwok, SLA Tse, F Wang, LL Chan.
Drafting of the manuscript: CCH Kwok.
Critical revision of the manuscript for important intellectual content: CCH Kwok, F Wang, SLA Tse, MCS Wong.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As the Editor-in-Chief and adviser of the journal, respectively, MCS Wong and SLA Tse were not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Hong Kong Hospital Authority Kowloon West Cluster Research Ethics Committee (Ref: KW/EX-12-068 [53-03]). The requirement for informed consent
was waived.
References
1. Early Breast Cancer Trialists’ Collaborative Group
(EBCTCG). Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year
survival: an overview of the randomized trials. Lancet
2005;365:1687-717. Crossref
2. Goldhirsch A, Wood WC, Gelber RD, et al. Progress and
promise: highlights of the international expert consensus
on the primary therapy of early breast cancer 2007. Ann
Oncol 2007;18:1133-44. Crossref
3. Jones S, Holmes FA, O’Shaughnessy J, et al. Docetaxel with
cyclophosphamide is associated with an overall survival
benefit compared with doxorubicin and cyclophosphamide:
7-year follow-up of US oncology research trial 9735. J Clin
Oncol 2009;27:1177-83. Crossref
4. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH.
Mortality, morbidity, and cost associated with
febrile neutropenia in adult cancer patients. Cancer
2006;106:2258-66. Crossref
5. Aapro MS, Bohlius J, Cameron DA, et al. 2010 Update
of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of
chemotherapy-induced febrile neutropenia in adult
patients with lymphoproliferative disorders and solid
tumours. Eur J Cancer 2011;47:8-32. Crossref
6. Lyman GH. Impact of chemotherapy dose intensity
on cancer patient outcomes. J Natl Compr Canc Netw
2009;7:99-108. Crossref
7. Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of
primary prophylaxis with granulocyte colony-stimulating
factor on febrile neutropenia and mortality in adult cancer
patients receiving chemotherapy: a systematic review. J
Clin Oncol 2007;25:3158-67. Crossref
8. von Minckwitz G, Schwenkglenks M, Skacel T, et al. Febrile
neutropenia and related complications in breast cancer
patients receiving pegfilgrastim primary prophylaxis
versus current practice neutropenia management: Results
from an integrated analysis. Eur J Cancer 2009;45:608-17. Crossref
9. Renner P, Milazzo S, Liu JP, Zwahlen M, Birkmann J,
Horneber M. Primary prophylactic colony-stimulating
factors for the prevention of chemotherapy-induced febrile
neutropenia in breast cancer patients. Cochrane Database
Syst Rev 2012;(10):CD007913. Crossref
10. Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 Update
of recommendations for the use of white blood cell growth
factors: an evidence-based clinical practice guideline. J
Clin Oncol 2006;24:3187-205. Crossref
11. Crawford J, Caserta C, Roila F; ESMO Guidelines Working
Group. Hematopoietic growth factors: ESMO clinical
practice guidelines for the applications. Ann Oncol 2010;21
Suppl 5:v248-51. Crossref
12. Crawford J, Becker PS, Armitage JO, et al. Myeloid Growth
Factors, Version 2.2017, NCCN Clinical Practice Guidelines
in Oncology. J Natl Compr Canc Netw 2017;15:1520-41. Crossref
13. Herbst C, Naumann F, Kruse EB et al. Prophylactic
antibiotics or G-CSF for the prevention of infections
and improvement of survival in cancer patients
undergoing chemotherapy. Cochrane Database Syst Rev
2009;(1):CD007107. Crossref
14. Taplitz RA, Kennedy EB, Bow EJ, et al. Antimicrobial
prophylaxis for adult patients with cancer-related
immunosuppression: ASCO and IDSA clinical practice
guideline update. J Clin Oncol 2018;36:3043-54. Crossref
15. Rayson D, Lutes S, Sellon M, et al. Incidence of febrile
neutropenia during adjuvant chemotherapy for breast
cancer: a prospective study. Curr Oncol 2012;19:e216-8. Crossref
16. Myers R, Higgins B, Jeffrey M, et al. Chemotherapy induced
febrile neutropenia of docetaxel with cyclophosphamide
(TC) for adjuvant therapy of breast cancer in the
community—reality check [abstract 2092]. Cancer Res
2009;69(24 Suppl):2092. Crossref
17. Younis T, Rayson D, Thompson K. Primary G-CSF
prophylaxis for adjuvant TC or FEC-D chemotherapy
outside of clinical trial settings: a systematic review and
meta-analysis. Support Care Cancer 2012;20:2523-30. Crossref
18. Chow LW, Biganzoli L, Leo AD, et al. Toxicity profile
differences of adjuvant docetaxel/cyclophosphamide (TC)
between Asian and Caucasian breast cancer patients. Asia
Pac J Clin Oncol 2017;13:372-8. Crossref
19. Yau TK, Ng TY, Soong IS, et al. Toxicity of docetaxel plus
cyclophosphamide as adjuvant therapy for breast cancer in
Chinese patients—the Hong Kong experience. Asia Pac J
Clin Oncol 2009;5:123-8. Crossref
20. Ye X, Zhai Q, Wang ZY, Du Q, Zhu B, Yu B. Neutropenic
complications in Chinese patients with breast cancer in a
real-world setting. Int J Clin Exp Pathol 2017;10:651-60.
21. Nieuweboer AJ, de Morrée ES, de Graan AJ, Sparreboom
A, de Wit R, Mathijssen RH. Inter-patient variability in
docetaxel pharmacokinetics: a review. Cancer Treat Rev
2015;41:605-13. Crossref
22. Puisset F, Alexandre J, Treluyer JM, et al. Clinical pharmacodynamic factors in docetaxel toxicity. Br J Cancer 2007;97:290-6. Crossref
23. Ling WH, Lee SC. Inter-ethnic differences—how important is it in cancer treatment? Ann Acad Med Singap 2011;40:356-61.
24. Frasci G. Treatment of breast cancer with chemotherapy
in combination with filgrastim: approaches to improving
therapeutic outcome. Drugs 2002;62 Suppl 1:17-31. Crossref
25. Crawford J, Dale DC, Kuderer NM, et al. Risk and timing
of neutropenic events in adult cancer patients receiving
chemotherapy: the results of a prospective nationwide
study of oncology practice. J Natl Compr Canc Netw
2008;6:109-18. Crossref
26. Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and
subsequent cycle use of pegfilgrastim prevents febrile
neutropenia in patients with breast cancer: a multicenter,
double-blind, placebo-controlled phase III study. J Clin
Oncol 2005;23:1178-84. Crossref
27. Lyman GH. Guidelines of the National Comprehensive
Cancer Network on the use of myeloid growth factors
with cancer chemotherapy: a review of the evidence. J Natl
Compr Canc Netw 2005;3:557-71. Crossref
28. Papaldo P, Lopez M, Marolla P, et al. Impact of five
prophylactic filgrastim schedules on hematologic toxicity
in early breast cancer patients treated with epirubicin and
cyclophosphamide. J Clin Oncol 2005;23:6908-18. Crossref
29. Lyman GH, Dale DC, Culakova E, et al. The impact of the
granulocyte colony-stimulating factor on chemotherapy
dose intensity and cancer survival: a systemic review
and meta-analysis of randomized trials. Ann Oncol
2013;24:2475-84. Crossref
30. Early Breast Cancer Trialists' Collaborative Group
(EBCTCG). Increasing the dose intensity of chemotherapy
by more frequent administration or sequential scheduling:
a patient-level meta-analysis of 37 298 women with
early breast cancer in 26 randomized trials. Lancet
2019;393:1440-52. Crossref
31. Ozer H, Armitage JO, Bennett CL, et al. 2000 Update of
recommendations for the use of hematopoietic colony-stimulating
factors: evidence-based, clinical practice
guidelines. American Society of Clinical Oncology Growth
Factors Expert Panel. J Clin Oncol 2000;18:3558-85. Crossref
32. Nitz U, Gluz O, Clemens M, et al. West German Study
PlanB Trial: adjuvant four cycles of epirubicin and
cyclophosphamide plus docetaxel versus six cycles of
docetaxel and cyclophosphamide in HER2-negative early
breast cancer. J Clin Oncol 2019;37:799-808. Crossref
33. Caparica R, Bruzzone M, Poggio F, Ceppi M, de Azambuja E,
Lambertini M. Anthracycline and taxane-based
chemotherapy versus docetaxel and cyclophosphamide
in the adjuvant treatment of HER2-negative breast
cancer patients: a systematic review and meta-analysis
of randomized controlled trials. Breast Cancer Res Treat
2019;174:27-37. Crossref

