Hong Kong Med J 2022 Jun;28(3):230–8 | Epub 7 Jun 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Prognostic implication of the neoadjuvant rectal
score and other biomarkers of clinical outcome in Hong Kong Chinese patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiotherapy
Sandy SK Ho, FHKAM (Medicine), FHKCP1 #, Sophie SF Hon, FRCSEd (Gen)2 #; Esther Hung, FHKCR, FHKAM (Radiology)3; Janet FY Lee, FRCSEd (Gen)4; Frankie Mo, PhD5; Macy Tong, FHKCR, FHKAM (Radiology)5; Cathy So, MBChB5; Simon Chu, FRCSEd (Gen)4; Dennis CK Ng, FRCSEd (Gen)6; Daisy Lam, FHKCR, FHKAM (Radiology)7; Carmen Cho, FHKCR, FHKAM (Radiology)3; Tony WC Mak, FRCSEd (Gen)4; Simon SM Ng, FRCSEd (Gen)4; Kaori Futaba, FRCS (Eng)4; Joyce Suen, FHKCR, FHKAM (Radiology)7; KF To, FHKCPath, FHKAM (Pathology)8; Anthony WH Chan, FHKCPath, FHKAM (Pathology)8; William WK Yeung, FHKCR, FHKAM (Radiology)9; Brigette BY Ma, FRACP, MD5
1 Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong
2 Department of Surgery, Alice Ho Miu Ling Nethersole Hospital, Hong Kong
3 Department of Imaging and Interventional Radiology, Prince of Wales Hospital, Hong Kong
4 Department of Surgery, Prince of Wales Hospital, Hong Kong
5 State Key Laboratory in Translational Oncology in South China, Sir YK Pao Centre for Cancer, Department of Clinical Oncology, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong
6 Department of Surgery, North District Hospital, Hong Kong
7 Department of Clinical Oncology, Prince of Wales Hospital, Hong Kong
8 Department of Anatomical and Cellular Pathology, The Chinese University of Hong Kong, Hong Kong
9 Private Practice
# Co-first authors
Corresponding author: Prof Brigette BY Ma (brigette@clo.cuhk.edu.hk)
Abstract
Background: Neoadjuvant chemoradiotherapy
is a standard treatment for locally advanced rectal
cancer, for which pathological complete response
is typically used as a surrogate survival endpoint.
Neoadjuvant rectal score is a new biomarker that
has been shown to correlate with survival. The main
objectives of this study were to investigate factors
contributing to pathological complete response, to
validate the prognostic significance of neoadjuvant
rectal score, and to investigate factors associated
with a lower neoadjuvant rectal score in a cohort of
Hong Kong Chinese.
Methods: Data of patients with locally advanced
rectal cancer who received neoadjuvant
chemoradiotherapy from August 2006 to October
2018 were retrieved from hospital records and
retrospectively analysed.
Results: Of 193 patients who had optimal response to
neoadjuvant chemoradiotherapy and surgery, tumour
down-staging was the only independent prognostic
factor that predicted pathological complete response
(P<0.0001). Neoadjuvant rectal score was associated
with overall survival (hazard ratio [HR]=1.042, 95%
confidence interval [CI]=1.021-1.064; P<0.0001),
disease-free survival (HR=1.042, 95% CI=1.022-1.062; P<0.0001), locoregional recurrence-free
survival (HR=1.070, 95% CI=1.039-1.102; P<0.0001)
and distant recurrence-free survival (HR=1.034,
95% CI=1.012-1.056; P=0.002). Patients who had
pathological complete response were associated with
a lower neoadjuvant rectal score (P<0.0001), but
pathological complete response was not associated
with survival. For patients with intermediate
neoadjuvant rectal scores, late recurrences beyond
72 months from diagnosis were observed.
Conclusion: Neoadjuvant rectal score is an independent prognostic marker of survival and
disease recurrence in a cohort of Hong Kong Chinese patients who received neoadjuvant chemoradiotherapy for locally advanced rectal
cancer.
New knowledge added by this study
- Neoadjuvant rectal (NAR) score is a validated prognostic marker of survival for patients with locally advanced rectal cancer. A lower NAR score is associated with subsequent achievement of pathological complete response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer.
- Although pathological complete response is a surrogate endpoint of survival in clinical trials of neoadjuvant therapy for locally advanced rectal cancer, the present study failed to confirm this in a cohort of Chinese patients.
- The NAR score should be incorporated as a study endpoint in clinical trials of neoadjuvant therapy for Chinese patients with locally advanced rectal cancer.
- The NAR score should be prospectively evaluated as a prognostic indicator in identifying patients who might benefit from more intensive adjuvant treatment.
- Moreover, the results of the present study suggest that longer follow-up for ≥72 months may be needed for patients with intermediate NAR scores.
Introduction
Early-stage rectal cancer is primarily treated with
total mesorectal excision surgery, while ‘high-risk’
rectal cancers can be treated with neoadjuvant
short-course radiotherapy alone or concurrent
chemotherapy and long-course radiotherapy
(neoadjuvant chemoradiotherapy; NCRT).1 High-risk
rectal cancer is defined as the presence of T3
or T4 disease, node-positive disease, the presence of
close or involved circumferential resection margin CRM) by staging magnetic resonance imaging
(MRI) and/or low-lying tumours involving the anal
sphincters.1 Randomised phase III trials have shown
that neoadjuvant is more effective than adjuvant
chemoradiotherapy, as it can improve disease-free
survival (DFS), local tumour control, sphincter
preservation and has better treatment compliance
with fewer adverse drug effects.2 3 4 Furthermore,
the addition of 5-fluorouracil (5FU) to neoadjuvant
radiotherapy has been shown to be more effective
than radiotherapy alone with higher rates of
pathological complete response (pCR) and lower
local relapse rate.5
Historically, NCRT has been associated with
15% to 27% pCR rates that have been associated
with progression-free survival and overall survival
(OS).6 Other prognostic markers such as the
presence of tumour down-staging in terms of T
stage and N stage,7 tumour regression grading
based on pathological and radiological criteria6 8
and CRM status9 have all been evaluated in clinical
studies and correlated with predict survival and
risk of cancer recurrence. However, a recently
published meta-analysis has failed to show pCR rate
as a significant surrogate marker of 5-year OS—an
important primary endpoint in randomised trials, in
patients with locally advanced rectal cancer (LARC)
undergoing NCRT.10 Therefore, a new endpoint
known as the neoadjuvant rectal (NAR) score has
been developed as a prognostic factor and study
endpoint for clinical research in LARC. This is a
composite endpoint consisting of both clinical and
pathological information on T stage and N stage
obtained before and after NCRT and has been
validated in prospective clinical trials in Western
populations.11 12 The NAR score has also been shown
to better predict OS in clinical trials on rectal cancer
than pCR.11
The primary objective of the present study
was to validate the prognostic significance of
NAR score and pCR in a cohort of Hong Kong
Chinese patients with LARC in terms of OS, DFS, locoregional recurrence-free survival (LRFS) and
distant recurrence-free survival (DRFS). The second
objective was to investigate associations between
NAR score (or pCR) and known prognostic factors
such as CRM status, tumour location, extramural
vascular invasion (EMVI) and other treatment-related
factors. The third objective was to investigate
factors that might predict a lower NAR score.
Methods
The data of patients with LARC who were referred
to the local multidisciplinary Lower Gastrointestinal
Tumour Board and then underwent NCRT at
the Prince of Wales Hospital, Hong Kong, from
August 2006 to October 2018 were extracted from
hospital records and retrospectively evaluated. Data
were also retrieved from the records of the Lower
Gastrointestinal Tumour Board meetings
and the surgical new case database from the Prince
of Wales Hospital.
Patient selection
Eligible patients had histologically confirmed LARC as defined by the presence of T3 or T4 tumour;
or node-positive disease, and/or the presence of
threatened CRM, and/or low-lying tumours involving
the anal sphincters. All eligible patients underwent
MRI and whole-body computed tomography (CT)
scan staging before and after NCRT. Patients were
excluded from the study who had distant metastasis
at the time of diagnosis; who were not fit for NCRT
or surgery due to poor performance status and/or
presence of serious medical co-morbidities; or who
had not completed the full course of NCRT.
Outline of oncological treatment,
surgery, magnetic resonance imaging and pathological examination
All treatment decisions were jointly made by the Lower Gastrointestinal Tumour Board. At
baseline, all patients underwent MRI staging and
also systemic staging with contrast CT scan and/or positron emission tomography–CT imaging.
Magnetic resonance imaging staging was determined
by MRI radiologists and reported in a standardised
format that contained information on T stage and N
stage, presence of EMVI, CRM status and tumour
regression grade response criteria.13 For patients
with MRI reports which did not contain the relevant
data, the MRI scans were assessed retrospectively in
order to obtain the study information.
All patients were treated according to the
institutional radiotherapy protocol at the Prince
of Wales Hospital, as represented by a long-course
pelvic radiotherapy up to a total dose of 45 Gy at
1.8 Gy per day, five fractions per week for 5 weeks
with boost 5.4 Gy at 1.8 Gy per day for three fractions. The majority of patients received concurrent
chemotherapy with bolus intravenous 5FU and
leucovorin that were given at week 1 and week 5 of
radiotherapy, followed by adjuvant chemotherapy
with 5FU and leucovorin or oxaliplatin-based
chemotherapy.14 Some patients also received
neoadjuvant (modified) FOLFOXIRI regimen
followed by concurrent capecitabine during pelvic
radiotherapy as part of a prospective clinical trial.15
All patients underwent total mesorectal excision
surgery with curative intent, and pathologists at the
New Territories East Cluster–affiliated hospitals
performed pathological examination on all the
resected surgical specimens. The presence of pCR
was defined as the resolution of all tumour cells in all
resected tissues including the lymph nodes.
Collection of clinical and radiological data
The following data were collected: age, sex, location of tumour from anal verge (defined as the endoscopic
distance from anal verge as ‘low’ [0-5 cm], ‘mid’
[5-10 cm], ‘high’ [>10 cm]), tumour histology,
neoadjuvant and adjuvant chemotherapy and
the overall TNM (tumour, node, and metastasis)
stage, as defined by the American Joint Committee
on Cancer, 8th version. The date at histological
diagnosis, cancer progression, locoregional and/or
distant recurrence and the date of last follow-up
examination or death were collected.
Pre- and post-treatment MRI data were
collected: T stage (T2, T3 or T4), N stage (node
positive or node negative), CRM (non-involved
margin is defined as ≥2 mm; involved margin is
defined as <2 mm from the anticipated surgical
margin). The presence of EMVI was determined in
the MRI scans of 152 patients.
Calculation of neoadjuvant rectal score
The NAR score was calculated according to the
Valentini’s nomograms for survival based on the
following formula16:
NAR = [5pN−3(cT−pT)+12]2 / 9.61,
where cT = clinical T stage before NRCT; pN =
pathological nodal stage after NCRT and surgery;
and pT = pathological T stage after NCRT and
surgery.
The relationship between NAR scores and
clinical outcome were analysed with NAR score
as a continuous variable (24 discrete scores by the
nomograms)16 or in groups based on previous
studies.12 17 The NAR scores were grouped as: ‘low’
(NAR score <8), ‘intermediate’ (NAR score 8-16),
and ‘high’ (NAR score >16), as previously published
in the National Surgical Adjuvant Breast and Bowel
Project ‘R-04’ trial,11 or in quartiles according to the
‘FORWARC’ study.17
Statistical analysis
Overall survival was defined from the time of
diagnosis to the time of death from any cause.
Survival time will be censored at the last date the
patient is known to be alive. Disease-free survival was
defined from the time of diagnosis of rectal cancer to
the time of disease recurrence and death from any
cause. Locoregional recurrence-free survival was
measured from the date of diagnosis to the date of
locoregional recurrence and death from any cause.
Distant recurrence-free survival was measured from
the date of diagnosis to the date of distant metastasis
and death from any cause.
Statistical analysis was performed using the
SPSS (Window version 26; IBM Corp, Armonk [NY],
United States). The Chi squared or Fisher’s exact test
was used for analysing categorical variables, t test
for continuous variables and logistic regression was
used to analyse the relationship between continuous
variables and disease recurrence. Time-to-event
endpoints include OS, DFS, LRFS and DRFS were
estimated using the Kaplan–Meier method and
compared using the log-rank test. Cox proportional
hazards model was used to evaluate any interaction
between time-to-event endpoints and important
covariates. The multivariable Cox regression with stepwise selection method was used to study NAR
score and other prognostic factors. A value of P<0.05
was considered significant. The correlation between
pCR and important covariates was obtained by
using logistic regression. The odds ratio and the
corresponding 95% confidence interval (CI) will be
given.
Results
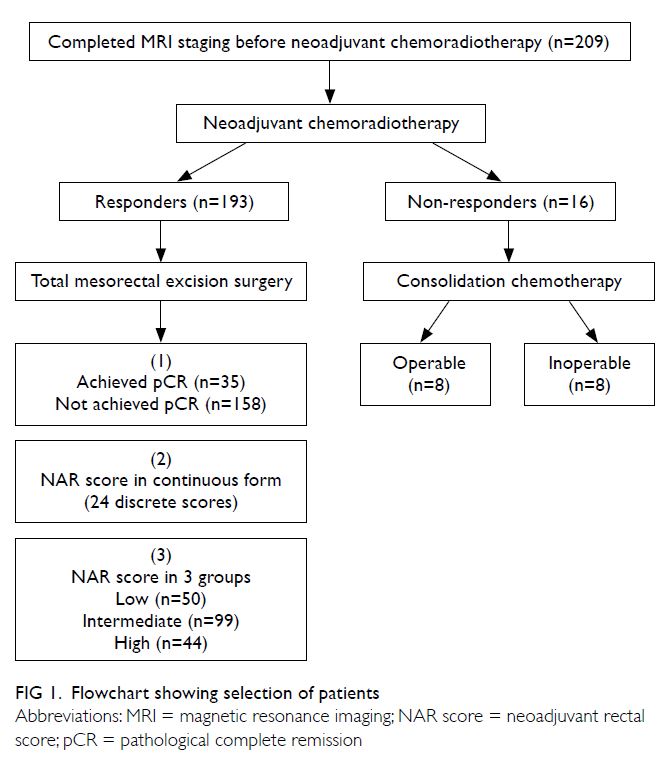
A total of 209 patients were found to be eligible, 16 of whom had suboptimal response to NCRT as
defined by one or more of the following factors:
persistently positive CRM, absence of significant
tumour regression on MRI, or frank radiological
progression (Fig 1). These patients were treated
with consolidation chemotherapy after NCRT and
of whom eight patients responded and underwent
surgery with curative intent. The characteristics
of the remaining 193 patients who had optimal
response after NCRT had a mean age of 62 years,
with a male and female ratio of 2.94:1 (Table 1).
The median follow-up duration for all patients was
47.7 months (range, 42.7-53.5).
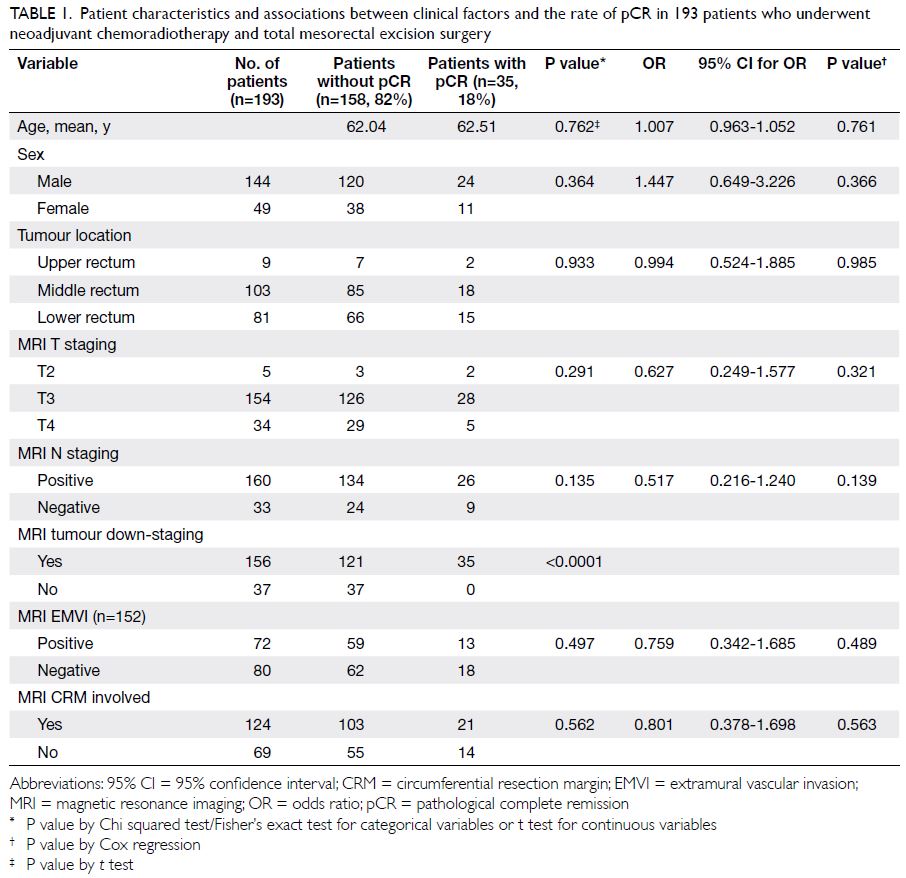
Table 1. Patient characteristics and associations between clinical factors and the rate of pCR in 193 patients who underwent neoadjuvant chemoradiotherapy and total mesorectal excision surgery
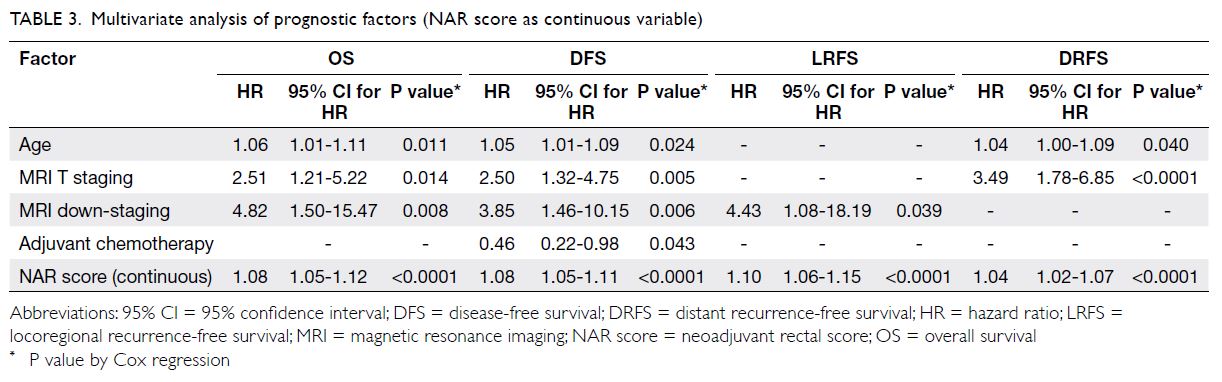
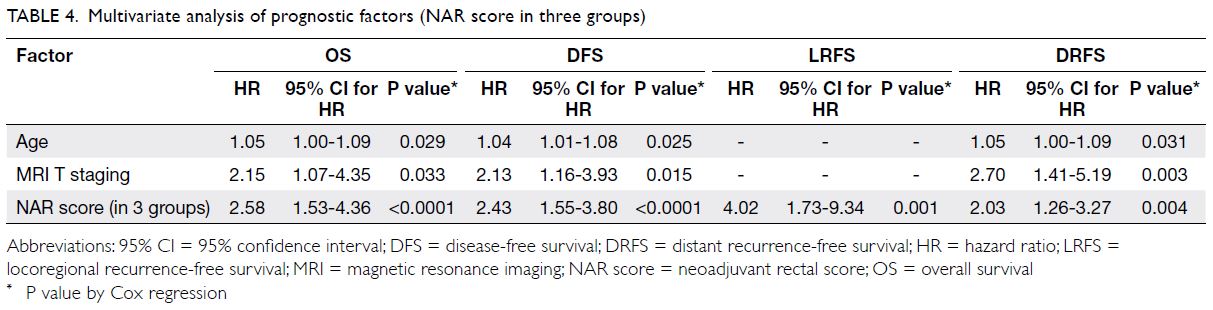
Prognostic significance of the neoadjuvant
rectal score–survival rates
When the NAR score was analysed as 24 discrete
scores by Valentini’s nomograms,16 it was found to
be associated with OS (hazard ratio [HR]=1.042,
95% CI=1.021-1.064; P<0.0001), DFS (HR=1.042,
95% CI=1.022-1.062; P<0.0001), LRFS (HR=1.070,
95% CI=1.039-1.102; P<0.0001) and DRFS
(HR=1.034, 95% CI=1.012-1.056; P=0.002).
To evaluate the effect of NAR score on survival
rates, patients were arbitrarily divided into three
groups according to NAR score: low (score <8; n=50),
intermediate (score 8-16; n=99) and high (score >16;
n=44) [Table 2]. There was a significant difference
among the OS curves of low, intermediate, and
high NAR score groups (P=0.004, Fig 2). Similarly,
there was a significant difference among the DFS
rates of the low, intermediate, and high NAR score
groups (P<0.0001, Fig 3). The DFS was lower for the
intermediate NAR score group than for the low NAR
score group (HR=4.50, 95% CI=1.35-14.95; P=0.014),
whereas the risk of progression was higher for the
high NAR score group than for the low NAR score
group (HR=8.14, 95% CI=2.40-27.65; P=0.001).
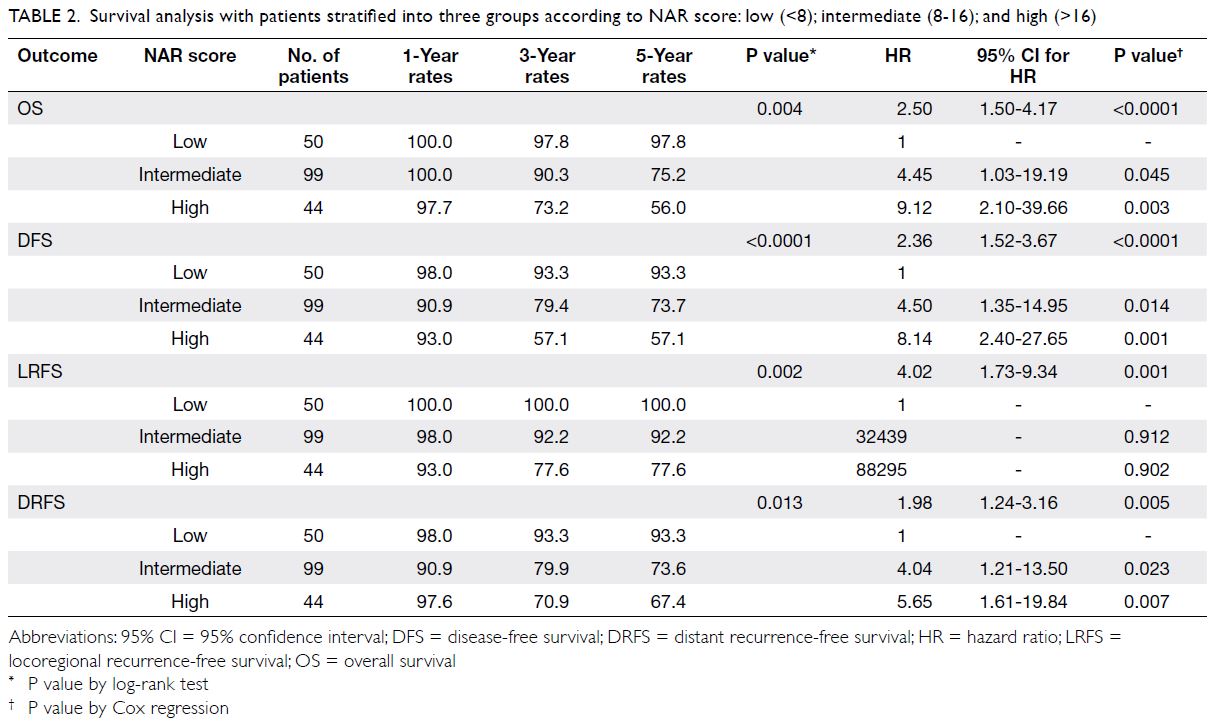
Table 2. Survival analysis with patients stratified into three groups according to NAR score: low (<8); intermediate (8-16); and high (>16)
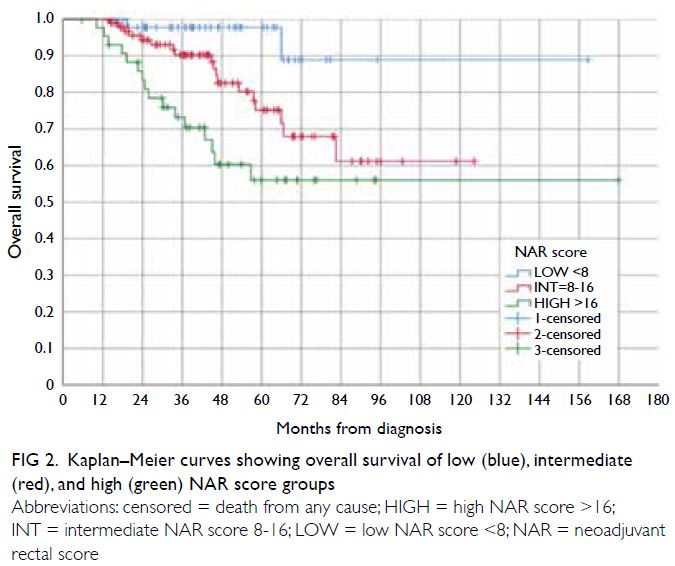
Figure 2. Kaplan–Meier curves showing overall survival of low (blue), intermediate (red), and high (green) NAR score groups
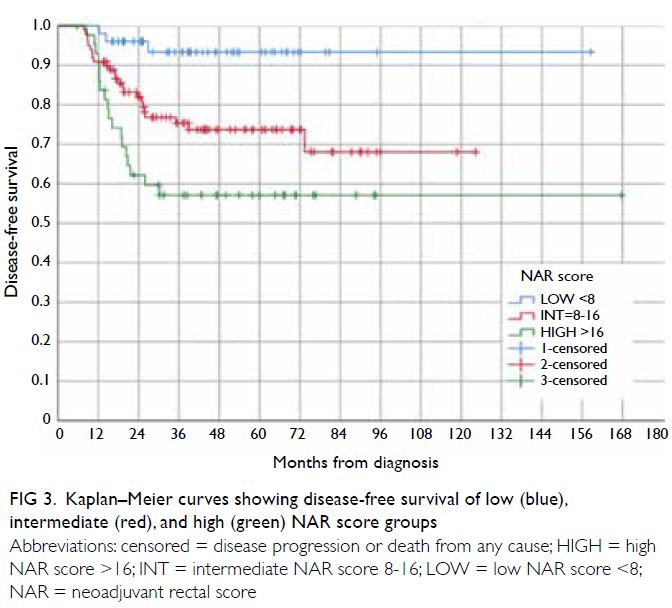
Figure 3. Kaplan–Meier curves showing disease-free survival of low (blue), intermediate (red), and high (green) NAR score groups
There was a significant difference among
the LRFS rates of the low, intermediate, and high
NAR score groups as shown in Figure 4 (P=0.002).
Similarly, as shown in Figure 5, the DRFS rates of
the three NAR score groups showed a statistical
difference (P=0.013). The intermediate NAR score
group had a lower DRFS than the low NAR score
group (HR=4.04, 95% CI=1.21-13.50; P=0.023),
while the high NAR score group had a higher risk of distant recurrence than the low NAR score group
(HR=5.65, 95% CI=1.61-19.84; P=0.007).
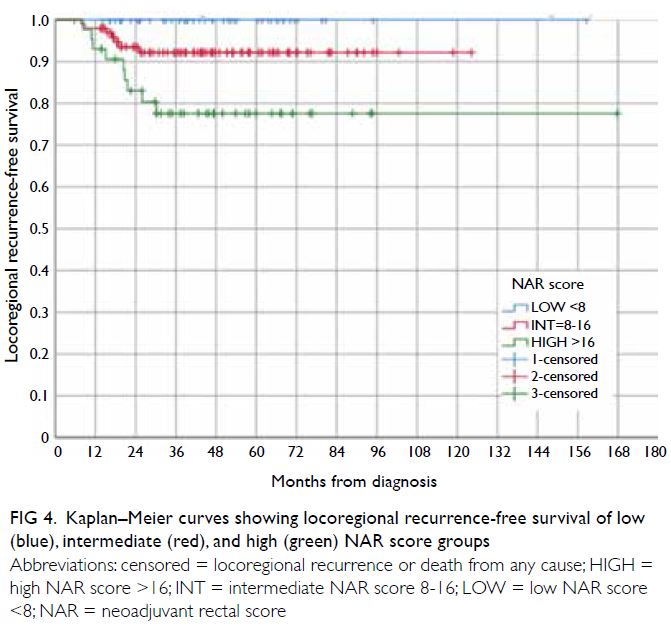
Figure 4. Kaplan–Meier curves showing locoregional recurrence-free survival of low (blue), intermediate (red), and high (green) NAR score groups
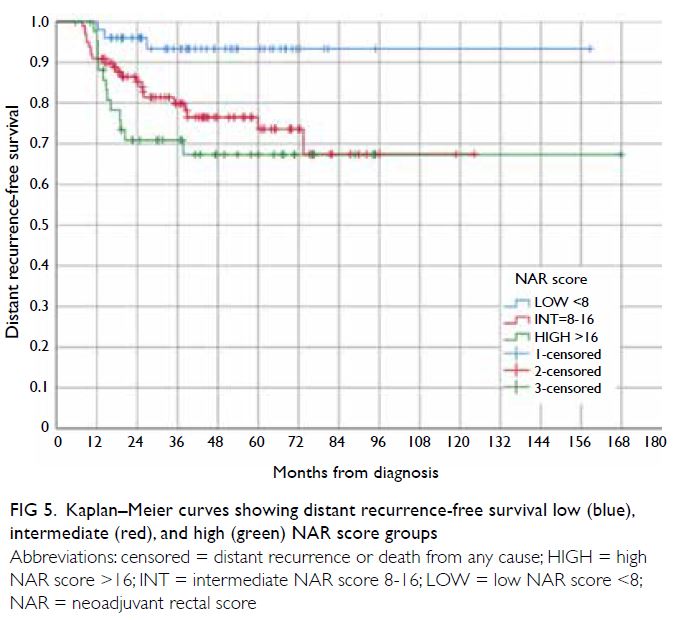
Figure 5. Kaplan–Meier curves showing distant recurrence-free survival low (blue), intermediate (red), and high (green) NAR score groups
Multivariate analysis of neoadjuvant rectal
score and other prognostic factors
The NAR score was an independent prognostic factor for OS, DFS, LRFS and DRFS, irrespective of whether
NAR score was analysed as a continuous variable or
in groups of low, intermediate, and high NAR score
(Tables 3 and 4). Other prognostic markers, such as
age and MRI T stage, were predictive of OS, DFS
and DRFS. The MRI tumour down-staging after
NCRT was an independent prognostic factor for
OS, DFS and LRFS. This study further evaluated
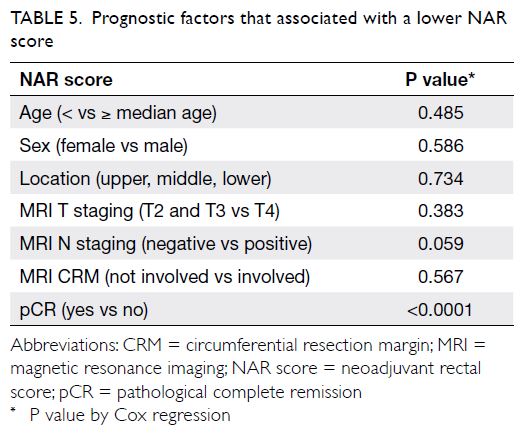
the prognostic factors that might predict a low
NAR score in subgroups of patients after NCRT. Of
all the prognostic factors evaluated, only pCR was
associated with a lower NAR score (NAR score ≤8
or >8) [Table 5].
Prognostic factors that predict pathological
complete response after neoadjuvant
chemoradiotherapy
In the 193 patients who had pCR to NCRT and surgery, MRI tumour down-staging was the only
prognostic factor which was associated with the rate
of pCR (P<0.0001) [Table 1].
Discussion
In the present study, NAR score was found to
be a more power prognostic factor than pCR.
Furthermore, patients who achieved pCR post
NCRT tend to have lower NAR scores. Furthermore,
the results of the present study indicate significant
differences in the rates of OS, DFS, LRFS and DRFS
among patients with low, intermediate, and high
NAR scores in a Hong Kong Chinese population,
which is consistent with observations from a study
in Western populations.12 Several interesting
observations can be made in the survival rates among
the low, intermediate, and high NAR score groups.
The DFS and DRFS curves of the intermediate and
high NAR score groups (Figs 3 and 5) crossed over
around the 1-year mark, demonstrating that survival
of the intermediate group was initially inferior to
the high NAR score group. This trend might be
explained by an imbalance in the sample size of
patients were in the intermediate NAR score group
(n=99) compared with the high NAR score group
(n=44) [Fig 1]. The recurrence rate in the low NAR
score group reached a plateau at around 3 years,
whereas in the intermediate NAR score group, late
recurrences (especially distant recurrence) could occur well over 72 months after diagnosis. Therefore,
this study suggests that longer follow-up duration for
a period beyond 72 months may be needed for the
intermediate NAR score group. This is in contrast
to the recommendation in the European Society
of Medical Oncology guideline which suggests a
follow-up duration of up to 60 months.18
In this study, the NAR score (not pCR) was
found to be an independent prognostic marker for
survival and disease recurrence. It is possible that
NAR score could better reflect the magnitude and
dynamics of tumour regression over time, whereas
pCR could give only dichotomised results observed
at a single time-point after surgery.
There are several limitations to this
retrospective study. The sample size was relatively
small and there was an imbalance in the number
of patients in the intermediate NAR score group
compared with the other groups (Fig 1). Given the
prognostic significance of MRI EMVI in LARC,19
this study included this endpoint in the multivariate
analysis. However, the MRI EMVI status could not be
retrieved for some patients, especially those who had
MRI imaging >5 years ago when this information was
not captured at the time of imaging. Furthermore,
the MRI N stage was only reported as either ‘positive’
or ‘negative’ in terms of nodal involvement without
specifying the exact number of suspicious nodes. The
CRM status and EMVI after NCRT and surgery has
been shown in previous studies to affect prognosis
and alter postoperative management.20 21 However,
information on these two prognostic factors could
not be traced retrospectively, therefore only the
pretreatment MRI CRM and MRI EMVI were
included in the analysis. Nevertheless, the findings
of this study are significant given the multicentre
nature and also relatively long follow-up duration.
Furthermore, it is consistent with the results of
previous studies.12 17
Although NAR score is a consistent and
validated prognostic marker, its determination relies
on the availability of radiological and pathological
assessments after surgery. In clinical practice,
surgeons and oncologists have to rely heavily on MRI
and/or endoscopic findings on assessing response
to NRCT when making decisions on operability
and preoperative consolidation chemotherapy after
NRCT. Nevertheless, the NAR score is useful in the
decision-making process with regard to the need for
intensifying adjuvant chemotherapy and also length
of follow-up duration. A study in Japan showed a
benefit in administering adjuvant chemotherapy to
patients with low NAR score (<16), but not in those
with higher NAR score (≥16).22 Further studies are
needed to individualise adjuvant chemotherapy for
Chinese patients using NAR scores after NCRT
for LARC. Other more novel strategies such as
personalised drug testing using rectal cancer organoid platforms in studying individual response
to NCRT are on the horizon.23
Conclusion
The NAR score is an independent prognostic marker of survival and disease recurrence in a cohort of
Hong Kong Chinese patients who received NCRT for LARC.
Author contributions
Concept or design: BBY Ma, SSK Ho, SSF Hon.
Acquisition of data: SSK Ho, SSF Hon, E Hung, JFY Lee, M Tong, C So, S Chu, DCK Ng, D Lam, C Cho, TWC Mak, SSM Ng, K Futaba, J Suen, KF To, AWH Chan, WWK Yeung, BBY Ma.
Analysis or interpretation of data: F Mo, SSK Ho.
Drafting of the manuscript: BBY Ma, SSK Ho, SSF Hon.
Critical revision of the manuscript for important intellectual content: BBY Ma, SSK Ho, SSF Hon.
Acquisition of data: SSK Ho, SSF Hon, E Hung, JFY Lee, M Tong, C So, S Chu, DCK Ng, D Lam, C Cho, TWC Mak, SSM Ng, K Futaba, J Suen, KF To, AWH Chan, WWK Yeung, BBY Ma.
Analysis or interpretation of data: F Mo, SSK Ho.
Drafting of the manuscript: BBY Ma, SSK Ho, SSF Hon.
Critical revision of the manuscript for important intellectual content: BBY Ma, SSK Ho, SSF Hon.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
This research was presented as a poster and abstract at the ESMO Asia Virtual Congress in 2020.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the New Territories East Cluster–The Chinese University of Hong Kong (NTEC-CUHK) Ethics
Committee (Ref NTEC-2019-0086). The requirement for
patient consent was waived by the ethics board owing to the
retrospective nature of the study.
References
1. Johnson D, Li L, Lee KC, et al. Total neoadjuvant therapy for
high risk rectal cancer in Western and Asian populations—
current evidence and clinical applications. Clin Colorectal Cancer 2022;21:45-54. Crossref
2. Sauer R, Becker H, Hohenberger W, et al. Preoperative
versus postoperative chemoradiotherapy for rectal cancer.
N Engl J Med 2004;351:1731-40. Crossref
3. Sauer R, Liersch T, Merkel S, et al. Preoperative versus
postoperative chemoradiotherapy for locally advanced
rectal cancer: results of the German CAO/ARO/AIO-94
randomized phase III trial after a median follow-up of
years. J Clin Oncol 2012;30:1926-33. Crossref
4. Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease–free survival in
patients with carcinoma of the rectum: NSABP R-03. J Clin
Oncol 2009;27:5124-30. Crossref
5. Gérard JP, Conroy T, Bonnetain F, et al. Preoperative
radiotherapy with or without concurrent fluorouracil and
leucovorin in T3-4 rectal cancers: results of FFCD 9203. J
Clin Oncol 2006;24:4620-5. Crossref
6. Maas M, Nelemans PJ, Valentini V, et al. Long-term
outcome in patients with a pathological complete response
after chemoradiation for rectal cancer: a pooled analysis of
individual patient data. Lancet Oncol 2010;11:835-44. Crossref
7. Fokas E, Liersch T, Fietkau R, et al. Downstage migration
after neoadjuvant chemoradiotherapy for rectal cancer:
the reverse of the Will Rogers phenomenon? Cancer
2015;121:1724-7. Crossref
8. Fokas E, Liersch T, Fietkau R, et al. Tumor regression
grading after preoperative chemoradiotherapy for locally
advanced rectal carcinoma revisited: updated results of the
CAO/ARO/AIO-94 trial. J Clin Oncol 2014;32:1554-62. Crossref
9. Kelly SB, Mills SJ, Bradburn DM, Ratcliffe AA, Borowski DW,
Northern Region Colorectal Cancer Audit Group. Effect of
the circumferential resection margin on survival following
rectal cancer surgery. Br J Surg 2011;98:573-81. Crossref
10. Petrelli F, Borgonovo K, Cabiddu M, Ghilardi M, Lonati V,
Barni S. Pathologic complete response and disease-free
survival are not surrogate endpoints for 5-year survival
in rectal cancer: an analysis of 22 randomized trials. J
Gastrointest Oncol 2017;8:39-48. Crossref
11. George TJ Jr, Allegra CJ, Yothers G. Neoadjuvant rectal
(NAR) score: a new surrogate endpoint in rectal cancer
clinical trials. Curr Colorectal Cancer Rep 2015;11:275-80. Crossref
12. Fokas E, Fietkau R, Hartmann A, et al. Neoadjuvant rectal
score as individual-level surrogate for disease-free survival
in rectal cancer in the CAO/ARO/AIO-04 randomized
phase III trial. Ann Oncol 2018;29:1521-7. Crossref
13. Sclafani F, Brown G. Extramural venous invasion (EMVI)
and tumour regression grading (TRG) as potential
prognostic factors for risk stratification and treatment
decision in rectal cancer. Curr Colorectal Cancer Rep
2016;12:130-40. Crossref
14. Yeung WW, Ma BB, Lee JF, et al. Clinical outcome of
neoadjuvant chemoradiation in locally advanced rectal
cancer at a tertiary hospital. Hong Kong Med J 2016;22:546-55. Crossref
15. Lam G, Tong M, Lee J, et al. A multicenter phase II study
of neoadjuvant FOLFOXIRI followed by concurrent
capecitabine and radiotherapy for high risk rectal cancer:
a final report. Ann Oncol 2019;30(Suppl 9):ix30-41. Crossref
16. Valentini V, van Stiphout RG, Lammering G, et al.
Nomograms for predicting local recurrence, distant
metastases, and overall survival for patients with
locally advanced rectal cancer on the basis of European
randomized clinical trials. J Clin Oncol 2011;29:3163-72. Crossref
17. Deng Y, Cai Y, Zhang J, et al. Validation of neoadjuvant
rectal cancer (NAR) score as a surrogate endpoint for
disease free survival in Chinese FOWARC study. J Clin
Oncol 2019;37(15 Suppl):e15162. Crossref
18. Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer:
ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol 2017;28(suppl 4):iv22-40. Crossref
19. Cho MS, Park YY, Yoon J, et al. MRI-based EMVI positivity
predicts systemic recurrence in rectal cancer patients with
a good tumor response to chemoradiotherapy followed by
surgery. J Surg Oncol 2018;117:1823-32. Crossref
20. Goffredo P, Zhou P, Ginader T, et al. Positive circumferential
resection margins following locally advanced colon cancer
surgery: risk factors and survival impact. J Surg Oncol
2020;121:538-46. Crossref
21. Song KS, Lee DW, Kim B, et al. Differences in prognostic
relevance of rectal magnetic resonance imaging findings
before and after neoadjuvant chemoradiotherapy. Sci Rep
2019;9:10059. Crossref
22. Maeda K, Shibutani M, Tachimori A, et al. Prognostic
significance of neoadjuvant rectal score and indication
for postoperative adjuvant therapy in rectal cancer
patients after neoadjuvant chemoradiotherapy. In Vivo
2020;34:283-9. Crossref
23. Ganesh K, Wu C, O’Rourke KP, et al. A rectal cancer organoid platform to study individual responses to
chemoradiation. Nat Med 2019;25:1607-14. Crossref