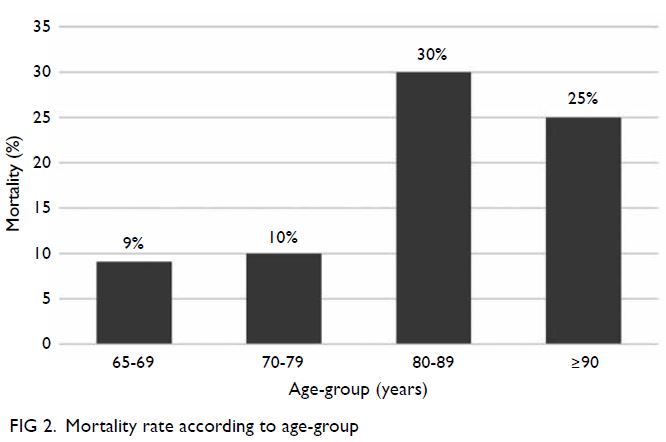Hong Kong Med J 2022 Jun;28(3):215–22 | Epub 10 Jun 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Clinical course and mortality in older patients
with COVID-19: a cluster-based study in Hong Kong
Ellen Maria YY Tam, FHKCP, FHKAM (Medicine); YK Kwan, FHKAM (Medicine), FRCP (Edin); YY Ng, FHKCP, FHKAM (Medicine); PW Yam, FHKAM (Medicine), FRCP (Glasg)
Department of Medicine and Geriatrics, Tuen Mun Hospital, Hong Kong
Corresponding author: Dr Ellen Maria YY Tam (ellentam123@gmail.com)
Abstract
Introduction: Compared with previous waves of the
coronavirus disease 2019 (COVID-19) pandemic in
Hong Kong, the third wave involved a greater number
of frail older patients. Because local healthcare
policy required hospitalisation for all older adults
with COVID-19, we aimed to investigate the clinical
course and outcomes in such patients.
Methods: This retrospective observational study
included all patients aged ≥65 years who were
admitted to Tuen Mun Hospital for management
of COVID-19 between 1 July 2020 and 31 August
2020. We reviewed baseline characteristics, clinical
presentation, laboratory results, complications, and
outcomes. We also investigated the associations of
age and Clinical Frailty Scale (CFS) score with in-patient
mortality.
Results: In total, 101 patients were included
(median age, 73 years); 52.5% were men and 85%
had at least co-morbid chronic disease. The most
common symptoms were fever (80.2%) and cough
(63.4%). Fifty-two patients (51.5%) developed
hypoxia, generally on day 8 (interquartile range,
5-11) after symptom onset. Of the 16 patients who required intensive care unit support, 13 required
mechanical ventilation. The overall mortality rate
was 16.8%. Patients aged 65-69, 70-79, 80-89, and
≥90 years had mortality rates of 9.1%, 10%, 30%, and
25%, respectively. Patients with CFS scores of 1-2,
3-4, 5-6, and ≥7 had mortality rates of 5.7%, 14.7%,
23.5%, and 40%, respectively. A linear relationship
was confirmed between the two mortality trends.
Conclusion: Clinical deterioration was common
in older patients with COVID-19; their overall
mortality rate was 16.8%. Mortality increased
linearly with both age and CFS score.
New knowledge added by this study
- Clinical deterioration occurred in >50% of older patients (aged ≥65 years) with coronavirus disease 2019 (COVID-19).
- The median time to hypoxia was 8 days after symptom onset.
- Age and frailty each had a linear relationship with in-patient mortality.
- Frail older patients had less favourable COVID-19 outcomes.
- Frailty screening should be performed universally in older adults with COVID-19 to enable early risk stratification, regardless of presenting symptoms.
Introduction
Hong Kong faced a third wave of the coronavirus
disease 2019 (COVID-19) pandemic, from July to
September 2020. Whereas the first two waves mainly
consisted of imported cases and generally affected
younger patients, the third wave mainly consisted
of local cases and their respective epidemiological
associations. There were multiple outbreaks in
residential care homes for older adults. The overall
mortality rate increased from 0.69% in late June 2020
to 2% in late October 2020.1
Multiple studies have shown that advanced age and co-morbidities are risk factors for mortality in
patients with COVID-19.2 3 4 Observational studies
focused on older patients have reported in-hospital
mortality rates of 19.2% to 35.9%.5 6 However, findings
in other countries might not be generalisable to
Hong Kong because of considerable variations in
disease surveillance, hospitalisation thresholds, and
treatment guidelines worldwide. Therefore, an in-depth
study of older adults with COVID-19 in Hong
Kong is needed.
In 2020, Hong Kong had one of the highest rates
of COVID-19–related hospitalisation worldwide. The local healthcare policy required hospitalisation
of all patients aged ≥65 years who had COVID-19;
those patients were then admitted to isolation wards,
regardless of disease severity. This unique situation
enabled us to perform a comprehensive review of
the clinical course and outcomes of older patients
with COVID-19 in Hong Kong. We compared
mortality rates among age-groups and frailty levels
to determine whether such factors had predictive
value for survival.
Methods
Study design and data collection
This retrospective observational study included
patients aged ≥65 years who were admitted to Tuen
Mun Hospital, Hong Kong, for management of
polymerase chain reaction–confirmed COVID-19
between 1 July 2020 and 31 August 2020. Cases
were identified from the hospital’s Infectious
Disease Team database. We excluded patients who
had previously been discharged for COVID-19 and
readmitted for other causes, as well as patients who
had not been discharged by 31 October 2020 (ie, the
date of study commencement).
Hospitalised cases were managed in accordance
with standardised practices; routine nursing and
medical care were provided under the supervision of
infectious disease specialists. Each patient’s clinical
data (ie, baseline characteristics, co-morbidities,
clinical presentation, laboratory findings, treatment, clinical outcomes, and complications) were retrieved
from electronic medical records. The 2007 version
of the Clinical Frailty Scale (CFS) was used to assess
frailty with scores from 1 (very fit) to 9 (terminally
ill).7 The CFS scores were retrospectively derived
on the basis of patient co-morbidities, premorbid
mobility, and levels of function; these factors were
determined using clinical notes, medical and nursing
admission assessments, and allied health records.
Presenting symptoms and onset dates were reported
by patients or their caregivers. Chronic heart disease
was defined as any ischaemic or valvular heart
disease, arrhythmia, and/or heart failure. Chronic
respiratory disease was defined as asthma, chronic
obstructive pulmonary disease, bronchiectasis, and/or obstructive sleep apnoea. Chronic kidney disease
was defined as chronic kidney disease stage ≥3a.
Viral load was determined by the cycle threshold
(CT) value in polymerase chain reaction analysis
of specimens from the respiratory tract; this value
reflected the number of amplification cycles required
to produce a detectable amount of viral RNA and was
inversely proportional to the viral load. Laboratory
results were recorded at baseline unless otherwise
specified.
Outcomes
Primary outcomes were the clinical course and
outcomes of patients, including their clinical
presentation, laboratory findings, treatment, clinical
deterioration (defined as hypoxia onset, mechanical
ventilation requirement, or intensive care unit [ICU]
admission), complications (eg, acute kidney injury,
liver impairment, superinfection, thromboembolic,
and acute ischaemic events), and in-patient
mortality. We compared these findings between
survivors and non-survivors. Secondary outcomes
were the mortality rates according to age-group and
frailty level.
Hypoxia was defined as oxygen desaturation
that resulted in a need for supplemental oxygen.
In accordance with the KDIGO (Kidney Disease:
Improving Global Outcomes) 2012 acute kidney
injury guideline,8 acute kidney injury was defined
as an increase in serum creatinine by >26.5 mmol/L
within 48 hours or an increase to ≥1.5-fold above
baseline, where baseline presumably occurred within
the previous 7 days. Liver impairment was defined as
an increase of >3-fold above the upper normal limit
of serum alanine aminotransferase. Superinfection
was defined as secondary bacterial, viral, or fungal
infection that occurred ≥48 hours after admission.
Statistical analysis
Statistical analyses were performed using SPSS
(Window version 22.0; IBM Corp, Armonk [NY],
Unite States). All continuous variables in this study
had skewed distributions using the Kolmogorov–Smirnov test and were expressed as medians with
interquartile ranges (IQRs), while categorical
variables were expressed as numbers with percentages
(%). The Mann-Whitney U test was used to compare
non-parametric continuous data between groups.
As appropriate, the Chi squared test or Fisher’s exact
test was used to compare categorical variables. The
Cochran–Armitage trend test was used to assess
mortality trends. All statistical tests were two-sided
and P<0.05 was considered indicative of statistical
significance.
Results
Study population and baseline characteristics
During the study period, 427 patients were
admitted to Tuen Mun Hospital for management of
COVID-19. After the exclusion of paediatric patients and adults aged <65 years (n=323), as well as older
adults who had not yet been discharged by the study
date (n=3), 101 patients were included in the study.
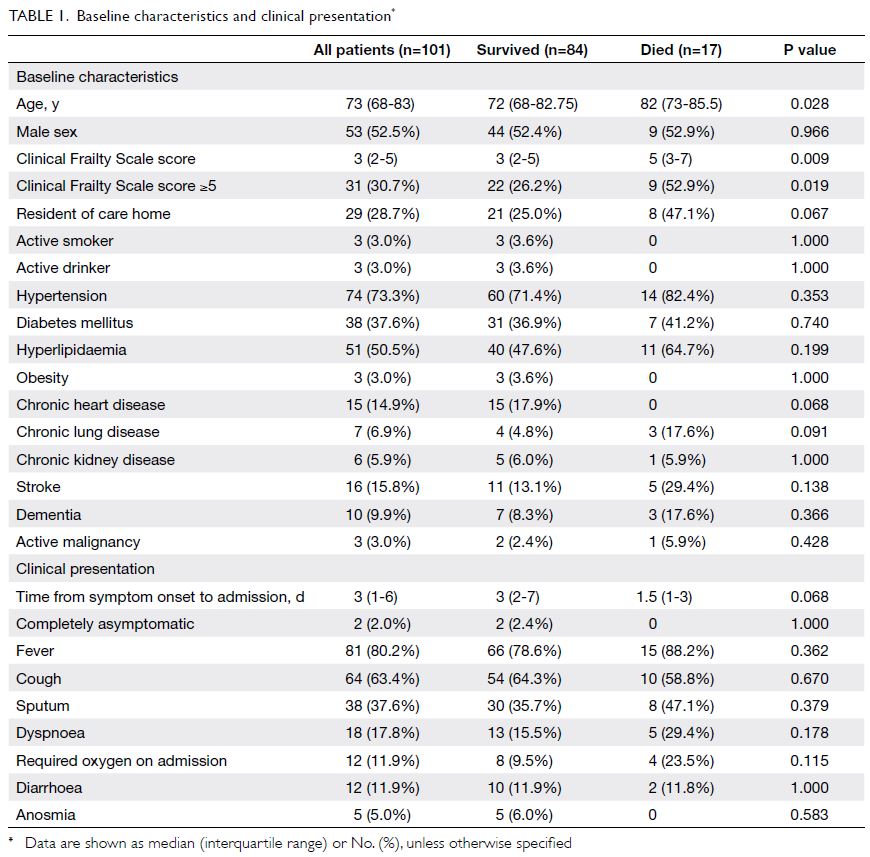
Baseline patient characteristics are shown in
Table 1. The median age was 73 years (range, 65-96);
99% of patients were Chinese, 52.5% were men, and
28.7% were old age home residents. Furthermore,
30.7% had at least mild frailty (CFS score ≥5).
Overall, 85% of the older patients had at least one
co-morbid chronic disease, including hypertension
(73.3%); diabetes mellitus (37.6%); hyperlipidaemia
(50.5%); chronic heart (14.9%), lung (6.9%), or kidney
(5.9%) diseases; stroke (15.8%); dementia (9.9%);
obesity (3%); and active malignancy (3%).
Presentation and laboratory findings
Patients were generally admitted 3 days (IQR, 1-6)
after symptom onset (Table 1). Only 4% of patients were asymptomatic on admission, while only 2%
of patients remained completely asymptomatic
throughout the course of disease. Common
presenting symptoms included fever (80.2%),
cough (63.4%), sputum (37.6%), dyspnoea (17.8%),
diarrhoea (11.9%), and anosmia (5%). Overall, 11.9%
of patients required oxygen support on admission.
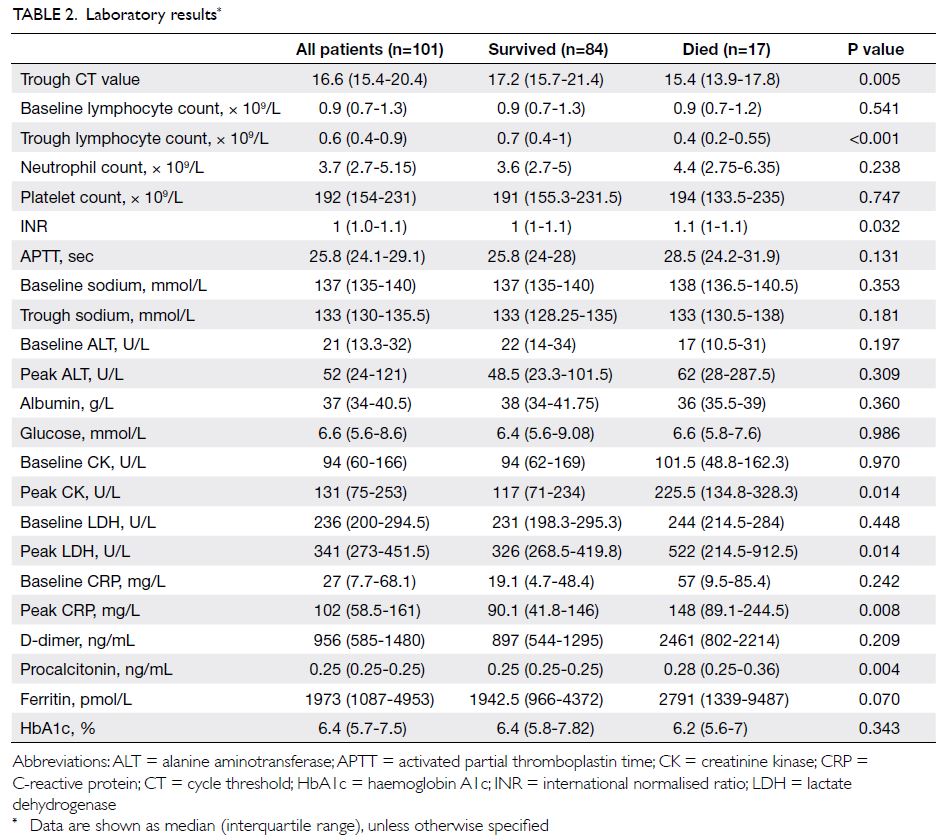
Laboratory findings are shown in Table 2. The
median trough CT value was 16.6. Lymphopenia and
hyponatraemia were common; the median trough
lymphocyte count and sodium level were 0.6 × 109/L
and 133 mmol/L, respectively. Elevated levels of
lactate dehydrogenase, C-reactive protein, D-dimer,
and ferritin were also common.
Treatment
Antiviral drugs were administered to 86.1% of
patients, while antibiotics were administered
to 83.2% of patients. During the study period,
combined administration of lopinavir-ritonavir and
interferon beta-1b was the most commonly used COVID-19-specific antiviral treatment approach.
Other COVID-19 treatments (eg, systemic steroid,
remdesivir, tocilizumab, convalescent plasma,
and extracorporeal blood purification) were
administered in accordance with each patient’s
clinical indications. Systemic steroid treatment was
administered to 48.5% of patients; it was mostly
administered to patients who developed hypoxia.
Overall, 4% of patients received convalescent
plasma, 8% required renal replacement therapy, and
1% required extracorporeal membrane oxygenation.
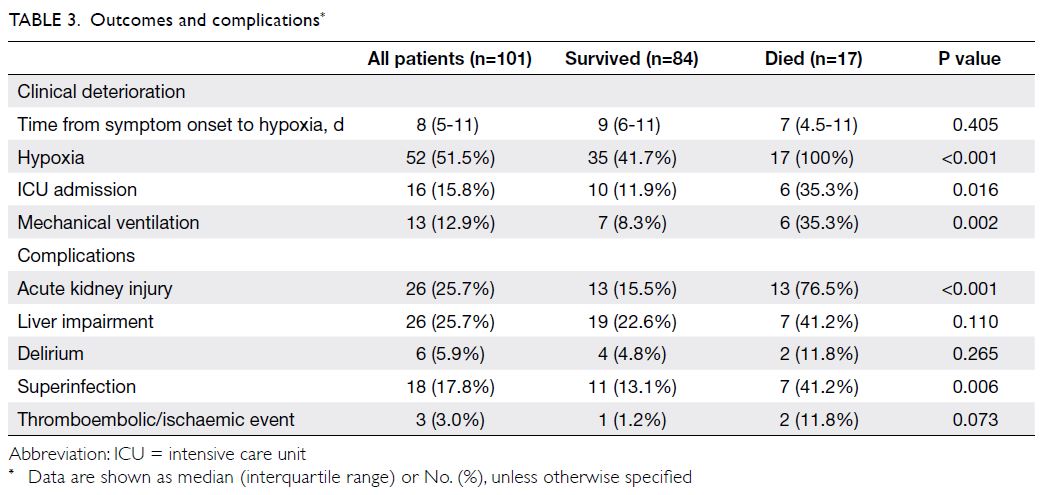
Clinical outcomes and complications
Clinical deterioration occurred in more than half
of the older patients. Fifty-two patients (51.5%)
developed hypoxia, generally on day 8 (IQR, 5-11)
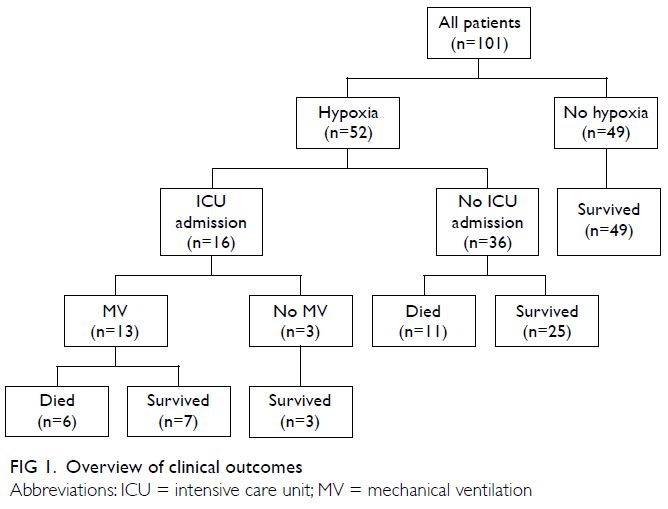
after symptom onset. The outcomes of the 52 patients
who developed hypoxia are shown in Figure 1;
among the 16 patients who received ICU support, 13
required mechanical ventilation and six died. Three
patients did not require mechanical ventilation after ICU admission; all three survived. Thirty-six patients
with hypoxia were admitted to general wards
because they were not candidates for ICU admission
or did not require intensive care; of these 36 patients,
25 survived and 11 died. All 11 patients who died
without ICU support had a do-not-resuscitate
order and thus did not receive cardiopulmonary
resuscitation. Among the 49 patients who did not
develop hypoxia, all survived. The overall mortality
rate was 16.8% (n=17); the mortality rate among
patients who developed hypoxia was 32.7%. Among
all ICU patients and among ICU patients who
required mechanical ventilation, the mortality rates
were 37.5% and 46.2%, respectively.
Acute kidney injury and liver impairment
each occurred in 25.7% of patients (Table 3).
Superinfection occurred in 17.8% of patients, while
delirium occurred in 5.9% of patients. Three patients
(3%) experienced thromboembolic or ischaemic
events: deep vein thrombosis, acute ischaemic
stroke, and acute myocardial infarction (n=1 each).
The median time from admission to discharge was
18.5 days (IQR, 12-26), while the median time from
admission to death was 15 days (IQR, 10-30).
Comparison of survivors and non-survivors
Patients who died during the index admission were
older (median age, 72 vs 82 years, P=0.028) and had
greater frailty (median CFS score 3 vs 5, P=0.009)
[Table 1]. A higher viral load was observed in non-survivors
(trough CT value 17.2 vs 15.4, P=0.005).
Non-survivors also had a lower trough lymphocyte
count (0.7 vs 0.4 × 109/L, P<0.001); a higher
international normalised ratio (1 vs 1.1, P=0.032);
and higher peak levels of creatinine kinase (117 vs
225.5 U/L, P=0.014), lactate dehydrogenase (326
vs 522 U/L, P=0.014), C-reactive protein (90.1 vs
148 mg/L, P=0.008), and procalcitonin (0.25 vs 0.28 ng/mL, P=0.004) [Table 2]. More non-survivors
had acute kidney injury (15.5% vs 76.5%, P<0.001)
and superinfection (13.1% vs 41.2%, P=0.006)
[Table 3].
Impacts of age and frailty on mortality
The mortality rates in patients aged 65-69, 70-79,
80-89, and ≥90 years were 9.1% (3/33), 10% (3/30),
30% (9/30), and 25% (2/8), respectively (Fig 2).
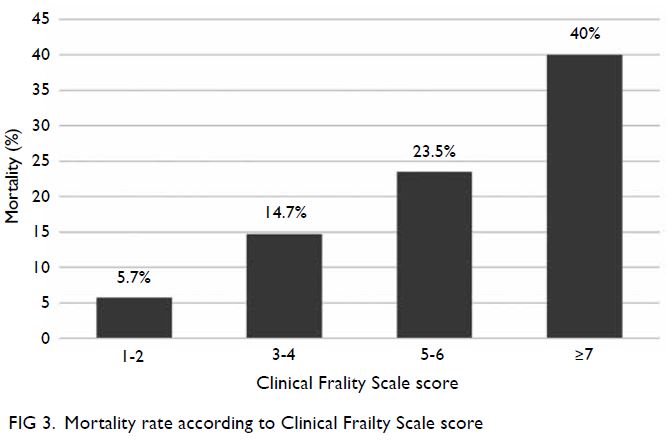
Patients who were very fit and well (CFS score 1-2)
had a mortality rate of 5.7% (2/35); patients who were
managing well or vulnerable (CFS score 3-4) had a
mortality rate of 14.7% (5/34); patients with mild
to moderate frailty (CFS score 5-6) had a mortality
rate of 23.5% (4/17); and patients with at least severe
frailty (CFS score ≥7) had a mortality rate of 40% (6/15) [Fig 3]. The Cochran–Armitage trend test
showed that mortality linearly increased with both
age (P=0.031) and CFS score (P=0.003).
Discussion
As of 31 October 2020, there were >5300 COVID-19
cases in Hong Kong; the median age was 43 and the
overall case fatality rate was 2%.1 Previous studies
have shown that mortality is much higher among
older patients. A large prospective cohort study of
20 000 hospitalised patients with COVID-19 in the
United Kingdom (median age, 74 years) revealed a
mortality rate of 26%.3 Another cohort study of 5700
hospitalised patients with COVID-19 in New York
revealed a mortality rate of 32.7% among the 1425
patients aged >60 years.9 Because hospitalisation
is required for older adults (aged ≥65 years) with
COVID-19 in Hong Kong, our in-patient mortality of 16.8% can be regarded as a close approximation
of the case fatality rate for this age-group; this was
significantly higher than the case fatality rate in
the general population. The broad hospitalisation
requirement for older adults in Hong Kong may also
explain the substantially lower mortality rate in this
study, compared with studies in countries where
only patients with severe disease were hospitalised.
Our findings suggest that older patients tend
to have symptomatic COVID-19. Fever occurred
in >80% of patients; only 2% of patients remained
completely asymptomatic throughout the course of
disease. A meta-analysis of 41 studies by He et al,10
which involved >50 000 patients from all age-groups,
revealed that the pooled percentage of
asymptomatic COVID-19 was 15.6%—this was
much higher than the rate in the present study. In
addition to the possible effects of age differences, the
high rate of symptoms reported in this study could
also be related to the early identification and active
screening of high-risk patients (eg, patients who had
contact with positive cases and were placed under
close medical surveillance in quarantine centres).
We observed some differences between
survivors and non-survivors in terms of baseline
patient characteristics, laboratory findings, and
complications. Non-survivors were significantly
older and had greater frailty; they also had a
higher viral load, lower lymphocyte count, higher
inflammatory marker levels, and higher incidences
of acute kidney injury and superinfection. Because
of sample size limitations, we did not perform
multivariate analyses of each factor potentially
associated with mortality; however, we observed
some trends. For example, death occurred in 29% of
patients with at least mild frailty (CFS score ≥5) and
33.3% of patients who required supplemental oxygen
on admission; these features might be early indicators
of poor prognosis. Furthermore, death occurred in
50% of patients with acute kidney injury and 38.9%
of patients with superinfection. Such complications
could also be indicators of poor prognosis because
the associated mortality rates were not negligible.
In this study, patients generally showed clinical
deterioration on day 8 after symptom onset. This is
consistent with the findings by Zhou et al2 in Wuhan,
where the times from illness onset to dyspnoea and
sepsis were 7 and 9 days, respectively. Additionally,
the overall rate of deterioration was high among
older patients, such that 51.5% developed hypoxia
during the course of disease. This was comparable
to the results of a study by Mostaza et al,6 in which
exacerbation of dyspnoea occurred in 43% of 400
older patients. These high rates are a cause for
concern because older patients with COVID-19 are
reportedly more susceptible to silent hypoxia,11 12
which may be missed without close monitoring;
thus, subsequent treatment may be delayed.
In this study, a do-not-resuscitate order had
been issued for each of the 11 hypoxic patients who
died after a lack of ICU support. These patients
constituted 10.9% of all older patients in the study;
they were substantially older and had greater frailty,
both of which were contra-indications for ICU
admission. The care team was able to promptly
identify these patients and involve them (and/or
their families) in advanced care planning. Because
resources are limited during the COVID-19
pandemic, it is important to identify patients at risk
of deterioration, as well as patients with poor reserve
who are unlikely to survive the disease. In the early
stages of the global pandemic, some countries
proposed age limits for access to intensive care
because of crises in their healthcare systems; such
proposals created ethical dilemmas and allegations
of ageism.13 14 Frailty screening was proposed to
replace the age criterion for resource allocation13;
accordingly, we compared its association with in-patient
mortality to the association of age with in-patient
mortality.
Frailty has been defined as an ageing-related
decline in physiological reserve, which leads
to increased vulnerability to stress. It has been
associated with poor clinical outcomes in older
adults7 15 16 and has been used to predict chest
infection–related mortality.17 The CFS is a simple
nine-point tool for assessing frailty. Compared with
non-frail patients (CFS score 1-4), at least mild frailty
(CFS score ≥5) has been independently associated
with all-cause mortality in hospitalised patients.18
A few studies have shown a relationship
between frailty and COVID-19–related mortality. In
a European multicentre cohort study of in-patients
with COVID-19, Hewitt et al19 found that the hazard
ratio for mortality increased with increasing CFS
score; compared with CFS score 1-2, the adjusted
hazard ratios were 1.55 for CFS score 3-4, 1.83 for
CFS score 5-6, and 2.39 for CFS score 7-9. Disease
outcome was more accurately predicted by frailty
than by age or co-morbidity. Moreover, mortality
rates in patients with CFS scores 5-6 and ≥7 were
30.9% and 41.5%, respectively; these were broadly
similar to our findings. In the United Kingdom,
Brill et al20 conducted a study of very old patients
with COVID-19; they found a significantly higher
CFS score (but not significantly older age) among
patients who died than among patients who survived.
The results of both above studies are consistent with
our findings.
In this study, we observed a substantial increase
in mortality, from approximately 10% in patients
aged 65-79 years to approximately 30% in patients
aged 80-89 years. However, the mortality rate
reached a plateau and did not increase with further
increases in age. In contrast, mortality progressively
increased with increasing frailty, from 5.7% in patients who were fit and well (CFS score 1-2) to 40%
in patients with at least severe frailty (CFS score ≥7).
Although both age and frailty had linear statistical
relationships with mortality, the linearity was more
pronounced for frailty. Our findings support the use
of frailty screening at admission for all older patients
with COVID-19; this early assessment can predict
adverse outcomes, regardless of initial symptoms
and disease severity. Rather than age alone, frailty
and age should be considered together when making
decisions about resuscitation and advanced care
planning.
A notable strength of this study was that it
provided a comprehensive overview of the clinical
course and outcomes in all older patients with
COVID-19, over a wide range of disease severity,
because of the non-selective hospitalisation policy in
Hong Kong. Because all admitted older adults were
included in the study, there was no selection bias.
Furthermore, because patients who had not been
discharged by the study date were excluded from the
study, data were available for all clinical outcomes
among the included patients.
There were some limitations in this study. First,
it had a small sample size. Tuen Mun Hospital was
the only designated centre in the New Territories
West Cluster in Hong Kong that provided acute
care during the index admission for patients with
COVID-19; it covered a population of >1 million.
Although this was a cluster-based study, the sample
size was small and certain statistical tests could not
be performed because they were underpowered.
Future multicentre or multi-cluster studies may
yield more comprehensive results. Second, the CFS
score was determined in a retrospective manner;
it might have been limited by the availability of
functional assessment data from electronic records.
While assessments of patients under geriatric care
are usually comprehensive, evaluations might have
been incomplete for patients who were new to the
Hospital Authority. To minimise potential errors, the
scores were separately determined by two geriatric
specialists, then stratified into four categories
of CFS score. Although dedicated prospective
assessments are preferable, previous studies have
shown that retrospectively determined CFS scores
have high precision and reliability, compared with
prospectively determined scores.21 Third, some data
were missing. For example, effective reporting of
disease symptoms and onset might be difficult for
dependent older adults; moreover, some blood tests
(eg, procalcitonin, ferritin, and D-dimer) were not
performed for some patients. Finally, the results of this
study might not be generalisable to other countries
or centres because the management of patients
with COVID-19 largely depends on local practices.
Hospitalisation rates, treatment thresholds, and
therapeutic regimens may considerably vary around the world. Thus, our findings should be carefully
interpreted and compared with the results of other
studies.
Conclusion
Clinical deterioration was common in older patients with COVID-19. Mortality was high with respect
to the overall case fatality rate. Linear relationships
with mortality were observed for both age and frailty.
Author contributions
Concept or design: EMYY Tam, YK Kwan.
Acquisition of data: EMYY Tam, YK Kwan, YY Ng.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: EMYY Tam.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: EMYY Tam, YK Kwan, YY Ng.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: EMYY Tam.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Declaration
An abstract of this study was submitted to the Hospital Authority Convention 2021.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the New Territories West Cluster Research Ethics Committee (Ref No: NTWC/REC/20135).
References
1. Centre for Health and Protection, Department of Health,
Hong Kong SAR Government. Latest situation on
cases on COVID-19 (as of 31 October 2020). Available
from: https://www.chp.gov.hk/files/pdf/local_situation_covid19_en_20201031.pdf Accessed 31 Oct 2020.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors
for mortality of adult inpatients with COVID-19 in Wuhan,
China: a retrospective cohort study. Lancet 2020;395:1054-62. Crossref
3. Docherty AB, Harrison EM, Green CA, et al. Features
of 20 133 UK patients in hospital with covid-19
using the ISARIC WHO Clinical Characterisation
Protocol: prospective observational cohort study. BMJ
2020;369:m1985. Crossref
4. Wu Z, McGoogan JM. Characteristics of and important
lessons from the coronavirus disease 2019 (COVID-19)
outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and
Prevention. JAMA 2020;323:1239-42. Crossref
5. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in
elderly patients: characteristics and prognostic factors
based on 4-week follow-up. J Infect 2020;80:639-45. Crossref
6. Mostaza JM, García-Iglesias F, González-Alegre T, et al.
Clinical course and prognostic factors of COVID-19
infection in an elderly hospitalized population. Arch
Gerontol Geriatr 2020;91:104204. Crossref
7. Dalhousie University. Clinical Frailty Scale. Available from:
https://www.dal.ca/sites/gmr/our-tools/clinical-frailtyscale.
html. Accessed 31 Oct 2020.
8. Khwaja A. KDIGO clinical practice guidelines for acute
kidney injury. Nephron Clin Pract 2012;120:179-84. Crossref
9. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting
comorbidities, and outcomes among 5700 patients
hospitalized with COVID-19 in the New York City area.
JAMA 2020;323:2052-9. Crossref
10. He J, Guo Y, Mao R, Zhang J. Proportion of asymptomatic
coronavirus disease 2019: a systematic review and metaanalysis.
J Med Virol 2021;93:820-30. Crossref
11. Tobin MJ, Laghi F, Jubran A. Why COVID-19 silent
hypoxemia is baffling to physicians. Am J Respir Crit Care
Med 2020;202:356-60. Crossref
12. Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht
BN. The pathophysiology of ‘happy’ hypoxemia in
COVID-19. Respir Res 2020;21:198. Crossref
13. Cesari M, Proietti M. COVID-19 in Italy: ageism and
decision making in a pandemic. J Am Med Dir Assoc
2020;21:576-7. Crossref
14. Ayalon L, Chasteen A, Diehl M, et al. Aging in times of
the COVID-19 pandemic: avoiding ageism and fostering
intergenerational solidarity. J Gerontol B Psychol Sci Soc
Sci 2021;76:e49-52. Crossref
15. Fried LP, Tangen CM, Walston J, et al. Frailty in older
adults: evidence for a phenotype. J Gerontol A Biol Sci
Med Sci 2001;56:M146-56. Crossref
16. Kundi H, Wadhera RK, Strom JB, et al. Association of frailty
with 30-day outcomes for acute myocardial infarction,
heart failure, and pneumonia among elderly adults. JAMA
Cardiol 2019;4:1084-91. Crossref
17. Luo J, Tang W, Sun Y, et al. Impact of frailty on 30-day
and 1-year mortality in hospitalised elderly patients
with community-acquired pneumonia: a prospective
observational study. BMJ Open 2020;10:e038370. Crossref
18. Bagshaw SM, Stelfox HT, McDermid RC, et al. Association
between frailty and short- and long-term outcomes among
critically ill patients: a multicentre prospective cohort
study. CMAJ 2014;186:E95-102. Crossref
19. Hewitt J, Carter B, Vilches-Morgara A, et al. The effect of
frailty on survival in patients with COVID-19 (COPE): a
multicentre, European, observational cohort study. Lancet
Public Health 2020;5:e444-51.
20. Brill SE, Jarvis HC, Ozcan E et al. COVID-19: a retrospective
cohort study with focus on the over-80s and hospital-onset
disease. BMC Med 2020;18:194. Crossref
21. Stille K, Temmel N, Hepp J, Herget-Rosenthal S. Validation
of the clinical frailty scale for retrospective use in acute
care. Eur Geriatr Med 2020;11:1009-15. Crossref