Hong Kong Med J 2022 Apr;28(2):133–9 | Epub 12 Apr 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Patient acceptance of transvaginal sonographic
endometrial thickness assessment compared
with hysteroscopy and biopsy for exclusion of
endometrial cancer in cases of postmenopausal
bleeding
Linda WY Fung, FHKAM (Obstetrics and Gynaecology), FHKCOG; Eva CW Cheung, FHKAM (Obstetrics and Gynaecology), FRCOG; Alyssa SW Wong, FHKAM (Obstetrics and Gynaecology), FRCOG; Daljit S Sahota, PhD; Terence TH Lao, MD, FRCOG
Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Dr Linda WY Fung (lindafung@cuhk.edu.hk)
Abstract
Introduction: Available examinations for women
with postmenopausal bleeding include transvaginal
sonography to measure endometrial thickness
(TVS-ET), and invasive endometrial assessment
using hysteroscopy/endometrial biopsy. However,
selection of the examination method seldom
involves consideration of patient preferences. The
aim of this study was to examine patient preferences
for the method used to investigate postmenopausal
bleeding.
Methods: Women were asked to complete an
interviewer-administered structured survey before
they underwent clinical investigations at a university
gynaecology unit from June 2016 to June 2017.
Using the standard gamble approach, women were
asked to choose between invasive assessment by
hysteroscopy/endometrial biopsy (gold standard)
or TVS-ET with a risk of missing endometrial
cancer. The risk of missing endometrial cancer
during TVS-ET was varied until each woman was
indifferent to either option.
Results: The median detection rate for endometrial
cancer required using TVS-ET was 95% (interquartile
range=80%-99.9%). In total, 200 women completed
the survey, and 77 (38.5%) women required TVS-ET to have a 99.9% detection rate for endometrial
cancer. Prior hysteroscopy experience was the only
factor that influenced the women’s decisions: a
significantly higher detection rate was required by
this patient group than by patients without previous
hysteroscopy experience (P=0.047).
Conclusions: A substantial proportion of women
would accept TVS-ET alone for the investigation
of postmenopausal bleeding. In the era of patientcentred
care, clinicians should incorporate
patient preferences and enable women to make
informed choices concerning the management of
postmenopausal bleeding.
New knowledge added by this study
- We assessed patient preferences for the investigational approach used to exclude endometrial cancer in Hong Kong women with postmenopausal bleeding.
- In our study population, most women would select transvaginal sonography to measure endometrial thickness (TVS-ET) if the endometrial cancer detection rate were >95%; if the TVS-ET detection rate were ≤95%, the women would select the more invasive hysteroscopy/endometrial biopsy approach.
- Nearly 40% of the women required TVS-ET to detect nearly all endometrial cancers before they would select TVS-ET as the sole investigational approach.
- Using an endometrial thickness cut-off value of 3 mm, a substantial proportion of women would accept TVS-ET alone for the investigation of postmenopausal bleeding.
- Women with previous hysteroscopy experience prefer hysteroscopic assessment unless TVS-ET alone can achieve a nearly identical rate of endometrial cancer detection.
- Clinicians should incorporate patient preferences concerning the investigation of postmenopausal bleeding to enable an informed choice about invasive testing to exclude endometrial cancer.
Introduction
Endometrial cancer is among the most common
gynaecological malignancies worldwide. Among
women with endometrial cancer, 90% initially report
postmenopausal bleeding (PMB).1 2 3 4 5 Approximately
10% of postmenopausal women are estimated to
experience PMB.1 Generally, there is no harmful
underlying cause of PMB; however, women with
recurrent PMB require medical assessment
to distinguish between benign aetiology (eg,
vaginal atrophy, uterine fibroids, and polyps) and
endometrial cancer. Endometrial assessment is
needed to exclude underlying malignancy.2 6 7 8 9 10
The endometrium can be examined non-invasively,
using transvaginal sonography (TVS)
to measure endometrial thickness (TVS-ET);
alternatively, it can be examined invasively via
blinded undirected endometrial sampling, saline
infusion sonography, or diagnostic hysteroscopy.11 12 13
Although both TVS-ET and blinded endometrial
sampling are recommended as first-line
investigations,2 6 7 14 15 16 17 18 the gold standard approach for
PMB investigation remains diagnostic hysteroscopy
with visually guided endometrial sampling; this
allows direct visualisation of the uterine cavity and
histological investigation.19
Importantly, hysteroscopy is invasive and
carries risks of complications such as infection, bleeding, uterine perforation, and visceral injury to
the cervix or nearby organs (eg, bladder and bowel);
it cannot be performed in women with cervical
stenosis.20 Additionally, some women report that
hysteroscopy is uncomfortable and painful within
an out-patient or office setting; thus, hysteroscopy,
cervical dilation, and uterine curettage have been
performed under general anaesthesia in such cases.
Although TVS-ET has become an established
investigational tool, there remains a lack of
consensus concerning the endometrial thickness (3,
4, or 5 mm) that constitutes ‘abnormal’. Our previous
study of 4300 women with PMB demonstrated that
3% of women with PMB and endometrial thickness
≤3 mm had endometrial cancer.21
Patient preference regarding investigation
approach is an important component of the decision
care pathway. Individual women must balance the
risks associated with an invasive procedure (eg,
diagnostic hysteroscopy) with the risk of missing an
endometrial cancer diagnosis if they select a non-invasive
assessment (eg, TVS-ET). To our knowledge,
the nature of this balance has not been assessed. The
aim of the present study was to determine the extent
to which women with PMB would accept the risk of
missing endometrial cancer if they were to undergo
TVS-ET as the first investigation of PMB.
Methods
This cross-sectional study was conducted in a
tertiary centre in Hong Kong from June 2016 to
June 2017. Women referred by either primary
or secondary healthcare providers to the One-stop
PMB Clinic for assessment and management
were invited to participate in the study. Patient
assessments included history taking, physical
examination, pelvic ultrasound to measure
endometrial thickness and screen for other pelvic
pathologies, Pap smear (for women without recent
Pap smear records), endometrial sampling with
or without hysteroscopy. Women were excluded if
they had <1 year of amenorrhea; had a prior TVS
finding of endometrium thickness ≥5 mm; were aged
≥70 years; had dementia or mental retardation;
and/or were unable to read or understand Chinese.
Prior to their clinic consultation, study
participants completed a structured interview that
was administered by an independent interviewer.
Women were asked to first read an information
leaflet regarding PMB, which described the risk of
endometrial cancer, possible investigation options,
and the risks associated with each option. The leaflet
and interviewer explained that hysteroscopy and
endometrial biopsy were expected to achieve a 100%
detection rate, but these methods involved risks of
pain, bleeding, infection, and uterine perforation
related to uterine cavity exploration. The leaflet and
interviewer also explained that TVS-ET did not require entry into uterine cavity but would potentially
miss some cases of endometrial cancer. The leaflet
and interviewer did not disclose the percentage of
endometrial cancers that would fail to be detected by
TVS-ET. After they had read the leaflet, women were
asked to complete a study questionnaire regarding
their sociodemographic characteristics and their
personal and family histories of gynaecological
cancer; they also completed the Chinese version of
the 20-item State-Trait Anxiety Inventory to measure
their trait and state anxiety levels. The women’s
state and trait scores were categorised as above or
below the scale midpoint. Women then underwent
assessment of utilities regarding examination by
either hysteroscopy or TVS-ET and the possibility
of a missed cancer diagnosis, using the standard
gamble technique.22
The standard gamble technique is the gold
standard method used to determine utility towards
a particular health state when a risk is involved.
Individuals are asked to choose whether they prefer
to have a certain guaranteed option or health state
with a guaranteed outcome and no risk, or whether
they would prefer an alternative option which entails
some risk. The risks for the two health states are
varied until the individual becomes indifferent to
either option. At the point of indifference, the ‘utility’
for the health state under consideration is considered
equal to ‘p’, while the utility of the alternative health
state is considered equal to ‘1–p’.
Women were first asked to complete a standard
gamble related to blindness, thereby ensuring that
they understood the process. Subsequently, they were
asked to complete a standard gamble to test their
preferences towards the investigations of PMB. Each
woman was asked to choose between the following
tests: (1) TVS-ET, which is less invasive but involves
some risk of missing endometrial cancer (probability
of 1–p), or (2) an invasive test with hysteroscopy
and endometrial biopsy, which detects 100% of all
cancers but carries the risks described during the
structured interview. To determine the level of
acceptance of missing endometrial cancer during
TVS-ET, the women were initially informed that the
assumed detection rate of the TVS-ET was 75%; this
detection rate was then increased in 5% intervals to
90%, then in 1% intervals to 98%, and finally in 0.1%
intervals to 99.9%. We recorded the stated detection
rate at which the woman was indifferent to either
option. The missed endometrial cancer rate that
women would accept to avoid an invasive procedure
was defined as 1–detection rate.
Sociodemographic characteristics, past and
current gynaecological history findings, and anxiety
levels are presented as mean ± standard deviation or
median and interquartile range; qualitative variables
are presented as absolute frequency and percentage.
The acceptable rate of endometrial cancer detection by TVS-ET alone, as an alternative to invasive
hysteroscopy/biopsy, is presented as median and
interquartile range. Differences in scores among
sociodemographic groups were compared using the
Mann-Whitney U test. SPSS software (Windows
version 20; IBM Corp, Armonk [NY], United States)
was used for all statistical analyses. A P value of
<0.05 was considered statistically significant.
Results
During the study period, 202 women agreed to
participate in the study; 200 of these women
completed the questionnaires and the standard
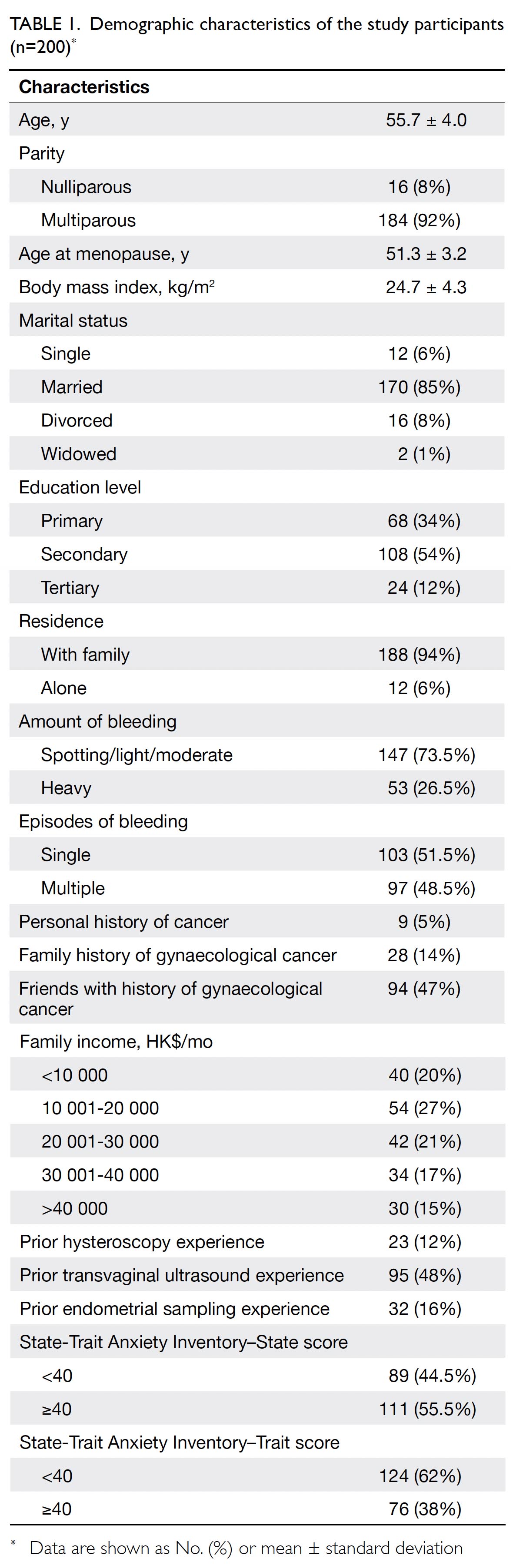
gamble assessments. Table 1 summarises the
sociodemographic, obstetric and gynaecological
histories, and anxiety levels of these 200 women.
Overall, 11 (5.5%) of the 200 women were
subsequently diagnosed with cancer or an atypical
endometrium: nine had endometrial cancer, one had
cervical cancer, and one had atypical hyperplasia.
Among 42 patients who underwent Pap smears in
our clinic, smear results showed atypical glandular
cells in two patients with endometrial cancer, while
four patients with endometrial cancer had a shift in
vaginal flora suggestive of bacterial vaginosis; the
remaining smear results were normal.
The median endometrial cancer detection rate
or utility that women would require for selection of
TVS-ET to avoid invasive hysteroscopy examination
was 95% (interquartile range=80%-99.9%). Overall,
77 (38.5%) women required TVS-ET to have a 99.9%
detection rate for endometrial cancer. Thus, 38.5% of
the women in our cohort would require TVS-ET to
be comparable with diagnostic hysteroscopy before
they would accept TVS-ET as the sole method for
examination of the endometrium and uterine cavity.
Table 2 summarises the results of univariate
analysis of the relationships between patient
characteristics and the TVS-ET endometrial cancer
detection rate. Women with previous hysteroscopy
experience required the endometrial detection rate
by TVS-ET to be significantly higher than did women
without previous hysteroscopy experience (P=0.047).
There were no significant differences in required
endometrial cancer detection rates by TVS-ET
among other sociodemographic characteristics, past
and current obstetric and gynaecological histories,
and state or trait anxiety (Table 2).
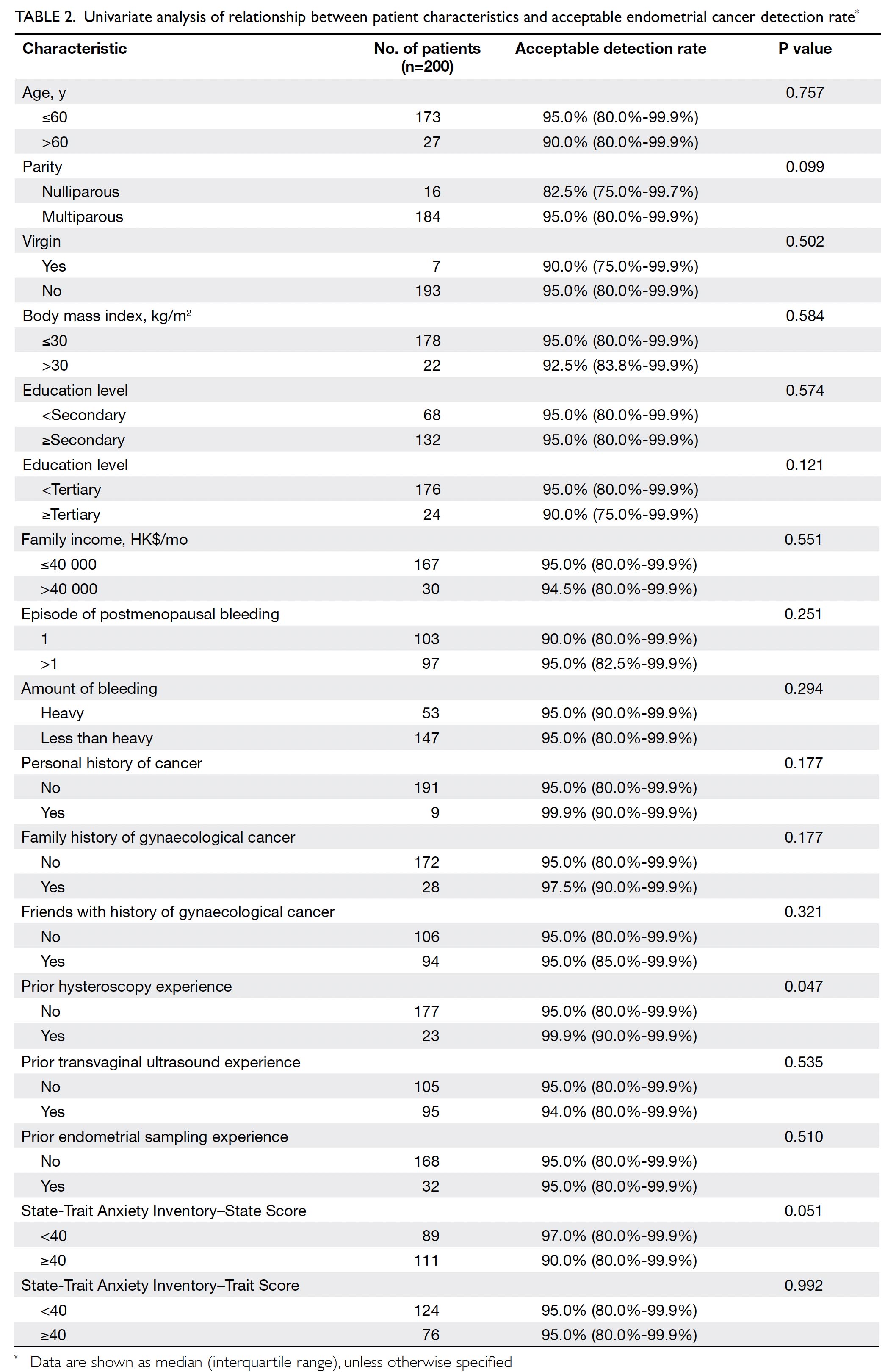
Table 2. Univariate analysis of relationship between patient characteristics and acceptable endometrial cancer detection rate
Discussion
To our knowledge, this study is the first to utilise
the standard gamble technique to evaluate patient
preference with regard to approaches used for the
investigation of PMB. Specifically, we assessed the
extent to which women would prefer to avoid an
invasive investigation (eg, hysteroscopy and biopsy)
if a non-invasive alternative were available. Our findings suggested that TVS-ET would need to
detect approximately 95% of endometrial cancers
(or miss approximately 5% of endometrial cancers)
for women to select TVS-ET with the intention
of avoiding an invasive investigation. However,
our analysis also suggested that nearly 40% of the
participants required TVS-ET to detect nearly
all endometrial cancers before they would select
TVS-ET as the sole investigational approach.
There are sparse published data concerning
patient preferences for the investigation of PMB.
Our literature review revealed a single study by
Timmermans et al.23 However, that study was
limited to 39 participants and the results were
obtained via telephone survey. In contrast to our
protocol, Timmermans et al23 only assessed patient
preferences after the women’s investigations had
been completed; thus, their reported clinical
experiences and preferences might have been
biased. In the present study, we adopted the
standard gambling approach which enabled a more
quantitative analysis of patient willingness to select
a different investigational approach. The standard
gamble method is the gold standard approach for
assessment of preferences in an uncertain situation24;
it can be used to express the outcomes of different
choices. It has been used previously to explore the
acceptable risk of miscarriage after a high-risk Down
syndrome screening test25 26 27 28; it has also been used to
explore patient preferences concerning the risks of
other medical treatments.
Currently, endometrial thickness cut-off
values in endometrial pathology or cancer screening
differ among hospitals.2 6 19 The most commonly
used cut-off endometrial thickness value is 4 mm2.
Our study population of postmenopausal women
accepted an endometrial cancer detection rate of
95% when using TVS-ET alone, with the intention
of avoiding the more invasive procedure of
hysteroscopy/endometrial biopsy. In our previous
study, TVS-ET offered endometrial cancer detection
rates of 97%, 94.1%, and 93.5% using 3 mm, 4 mm, and
5 mm as respective cut-off values.21 Thus, a TVS-ET
cut-off of 3 mm would generally be consistent with
the endometrial cancer detection accuracy that
women in our study required for TVS-ET to be used
as the sole investigational approach. In our study
population, women with previous hysteroscopy
experience required TVS-ET to have higher
detection rates; hence, they preferred hysteroscopic
assessment.
There were some limitations in our study.
First, women aged ≥70 years were excluded because
we presumed that they would have difficulty
understanding the standard gamble technique and/or completing the study questionnaires without
assistance. Second, although our sample size
was sufficient to assess our primary goal, it was inadequate for subgroup analysis. Larger studies
are needed to explore the relationships of specific
patient characteristics with the acceptable rate of
endometrial cancer detection by TVS-ET alone,
particularly in relation to factors such as personal
history of cancer or precancerous conditions.
Finally, our findings concerning the acceptable rate
of endometrial cancer detection by TVS-ET reflect
the preferences of women who participated in our
study; they may not be generalisable to populations
with different sociodemographic characteristics or
clinical management pathways.
Conclusions
Clinicians should incorporate patient preferences
concerning the investigation of PMB to enable an
informed choice about invasive testing to exclude
endometrial cancer. Our study population accepted
an endometrial cancer detection rate of 95% by
TVS-ET alone; this rate could be used to guide the
design of future PMB investigation strategies.
Author contributions
Concept or design: LWY Fung, ECW Cheung, ASW Wong, DS Sahota.
Acquisition of data: LWY Fung, ECW Cheung, ASW Wong, DS Sahota.
Analysis or interpretation of data: LWY Fung, DS Sahota.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: LWY Fung, ECW Cheung, ASW Wong, DS Sahota.
Analysis or interpretation of data: LWY Fung, DS Sahota.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors acknowledge the clinical care provided
by gynaecologists and nursing staff at the One-stop
Postmenopausal Bleeding Clinic, Prince of Wales Hospital.
We thank Miss Jennifer SF Tsang for her help with database
management.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Ethics approval was obtained in August 2015 from The Joint Chinese University of Hong Kong–New Territories East
Cluster Clinical Research Ethics Committee (CREC Ref
2015.437).
References
1. Astrup K, Olivarius Nde F. Frequency of spontaneously occurring postmenopausal bleeding in the general
population. Acta Obstet Gynaecol Scand 2004;83:203-7. Crossref
2. American College of Obstetricians and Gynaecologists.
ACOG Committee Opinion No. 426: The role of
transvaginal ultrasonography in the evaluation of
postmenopausal bleeding. Obstet Gynecol 2009;113:462-4. Crossref
3. Chandavarkar U, Kuperman JM, Muderspach LI, Opper N,
Felix JC, Roman L. Endometrial echo complex thickness
in postmenopausal endometrial cancer. Gynaecol Oncol
2013;131:109-12. Crossref
4. Jacobs I, Gentry-Maharaj A, Burnell M, et al. Sensitivity of
transvaginal ultrasound screening for endometrial cancer
in postmenopausal women: a case-control study within the
UKCTOCS cohort. Lancet Oncol 2011;12:38-48. Crossref
5. Hong Kong Cancer Registry, Hospital Authority, Hong
Kong SAR Government. Leading cancer sites in Hong
Kong. Available from: http://www3.ha.org.hk/cancereg/.
Accessed 5 Jun 2015.
6. Renaud MC, Le T, SOGC-GOC-SCC Policy and
Practice Guidelines Committee; Special Contributors.
Epidemiology and investigations for suspected endometrial
cancer. J Obstet Gynaecol Can 2013;35:380-1. Crossref
7. Institute of Obstetricians and Gynaecologists, Royal
College of Physicians of Ireland and Directorate of
Clinical Strategy and Programmes, Health Service
Executive. Clinical Practice Guideline. Investigation of
Postmenopausal Bleeding. Guideline No. 26, Version 1.0.
2013. Available from: https://rcpi-live-cdn.s3.amazonaws.
com/wp-content/uploads/2016/05/21.-Investigation-of-Postmenopausal-Bleeding.pdf. Accessed 5 Jun 2015.
8. Ewies AA, Musonda P. Managing postmenopausal
bleeding revisited: what is the best first line investigation
and who should be seen within 2 weeks? A cross-sectional
study of 326 women. Eur J Obstet Gynaecol Reprod Biol
2010;153:67-71. Crossref
9. Salman MC, Bozdag G, Dogan S, Yuce K. Role of
postmenopausal bleeding pattern and women’s age in
the prediction of endometrial cancer. Aust N Z J Obstet
Gynaecol 2013;53:484-8. Crossref
10. Tarling R, Gale A, Martin-Hirsch P, Holmes L,
Kanesalingam K, Dey P. Experiences of women referred for
urgent assessment of postmenopausal bleeding (PMB). J
Obstet Gynaecol 2013;33:184-7. Crossref
11. van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic
evaluation of the endometrium in postmenopausal
bleeding: an evidence-based approach. Maturitas
2011;68:155-64. Crossref
12. Dimitraki M, Tsikouras P, Bouchlariotou S, et al. Clinical
evaluation of women with PMB. Is it always necessary
an endometrial biopsy to be performed? A review of the
literature. Arch Gynaecol Obstet 2011;283:261-6. Crossref
13. Clark TJ, Barton PM, Coomarasamy A, Gupta JK, Khan KS.
Investigating postmenopausal bleeding for endometrial
cancer: cost-effectiveness of initial diagnostic strategies.
BJOG 2006;113:502-10. Crossref
14. Karlsson B, Gransberg S, Wikland M, et al. Transvaginal
ultrasonography of the endometrium in women with
postmenopausal bleeding—a Nordic multicenter study.
Am J Obstet Gynecol 1995;172:1488-94. Crossref
15. Ferrazzi E, Torri V, Trio D, Zannoni E, Filiberto S,
Dordoni D. Sonographic endometrial thickness: a useful
test to predict atrophy in patients with postmenopausal
bleeding. An Italian multicenter study. Ultrasound Obstet Gynaecol 1996;7:315-21. Crossref
16. Smith-Bindman R, Kerlikowske K, Feldstein VA, et al.
Endovaginal ultrasound to exclude endometrial cancer and
other endometrial abnormalities. JAMA 1998;280:1510-7. Crossref
17. Gupta JK, Chien PF, Voit D, Clark TJ, Khan KS.
Ultrasonographic endometrial thickness for diagnosing
endometrial pathology in women with postmenopausal
bleeding: a meta-analysis. Acta Obstet Gynaecol Scand
2002;81:799-816. Crossref
18. Timmermans A, Opmeer BC, Khan KS, et al. Endometrial
thickness measurement for detecting endometrial cancer
in women with postmenopausal bleeding: a systematic
review and meta-analysis. Obstet Gynaecol 2010;116:160-7. Crossref
19. Investigation of Post-Menopausal Bleeding. A National
Clinical Guideline. Scottish Intercollegiate Guidelines
Network; 200
20. Genovese F, D’Urso G, Di Guardo F, et al. Failed diagnostic
hysteroscopy: analysis of 62 cases. Eur J Obstet Gynecol
Reprod Biol 2020;245:193-7. Crossref
21. Wong AS, Lao TT, Cheung CW, et al. Reappraisal of
endometrial thickness for the detection of endometrial
cancer in postmenopausal bleeding: a retrospective cohort
study. BJOG 2016;123:439-46. Crossref
22. Froberg DG, Kane RL. Methodology for measuring health-state
preferences–II: Scaling methods. J Clin Epidemiol
1989;42:459-71. Crossref
23. Timmermans A, Opmeer BC, Veersema S, Mol BW. Patients’ preferences in the evaluation of postmenopausal
bleeding. BJOG 2007;114:1146-9. Crossref
24. van Osch SM, Stiggelbout AM. The construction of
standard gamble utilities. Health Econ 2008;17:31-40. Crossref
25. Chan YM, Sahota DS, Chan OK, Leung TY, Lau TK.
Miscarriage after invasive prenatal diagnostic procedures:
how much risk our pregnant women are willing to take?
Prenat Diagn 2009;29:870-4. Crossref
26. Chan YM, Sahota DS, Leung TY, Choy KW, Chan OK,
Lau TK. Chinese women’s preferences for prenatal
diagnostic procedure and their willingness to trade
between procedures. Prenat Diagn 2009;29:1270-6. Crossref
27. Chan YM, Leung TY, Chan OK, Cheng YK, Sahota DS.
Patient’s choice between a non-invasive prenatal test and
invasive prenatal diagnosis based on test accuracy. Fetal
Diagn Ther 2014;35:193-8. Crossref
28. Chan YM, Chan OK, Cheng YK, Leung TY, Lao TT,
Sahota DS. Acceptance towards giving birth to a child with
beta-thalassemia major—a prospective study. Taiwan J
Obstet Gynecol 2017;56:618-21. Crossref