Hong Kong Med J 2021 Oct;27(5):380–3 | Epub 18 Oct 2021
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
LETTER TO THE EDITOR
Serological response to mRNA and inactivated
COVID-19 vaccine in healthcare workers in Hong Kong: decline in antibodies 12 weeks after two doses
Jonpaul ST Zee, FRCPath, FHKAM (Medicine)1,2; Kristi TW Lai, MMedsc (HKU)1; Matthew KS Ho, MMedSc (HKU)1; Alex CP Leung, MMedSc (HKU)1; LH Fung, MPhil3; WP Luk, MPhil3; LF Kwok, BSc (Nursing)4; KM Kee, MPH4; Queenie WL Chan, BScN, FHKAN (Medicine-Infection Control)2; SF Tang, FHKCPath, FHKAM (Pathology)1,2; Edmond SK Ma, MD, FRCPath1; KH Lee, MMedSc (HKU), FHKAM (Community Medicine)5; CC Lau, MB, BS, FHKAM (Emergency Medicine)5; Raymond WH Yung, MB, BS, FHKCPath1,2,5
1 Department of Pathology, Hong Kong Sanatorium & Hospital, Hong Kong
2 Infection Control Team, Hong Kong Sanatorium & Hospital, Hong Kong
3 Medical Physics and Research Department, Hong Kong Sanatorium & Hospital, Hong Kong
4 Quality and Safety Division, Hong Kong Sanatorium & Hospital, Hong Kong
5 Hospital Administration, Hong Kong Sanatorium & Hospital, Hong Kong
Corresponding author: Dr Jonpaul ST Zee (jonpaul.st.zee@hksh.com)
To the Editor—We previously reported serological
findings of 302 healthcare workers (HCWs) who
completed two doses of mRNA (BNT162b2/Comirnaty; Fosun-BioNTech Pharma) and inactivated COVID-19 vaccine (CoronaVac; Sinovac
Life Sciences, Beijing, China).1 Both vaccines were found to be immunogenic in the majority of HCWs.
The BNT162b2 resulted in a 11-fold higher level of
anti-spike IgG (Abbott SARS-CoV-2 IgG II Quant
assay, mean=11572.6 AU/mL vs 1005.2 AU/mL;
P<0.001) and a higher surrogate neutralising
antibody (sNAb) [GenScript cPass SARS-CoV-2
Surrogate Virus Neutralization Test Kit] positive
rate (100% vs 94.4%; P<0.001).
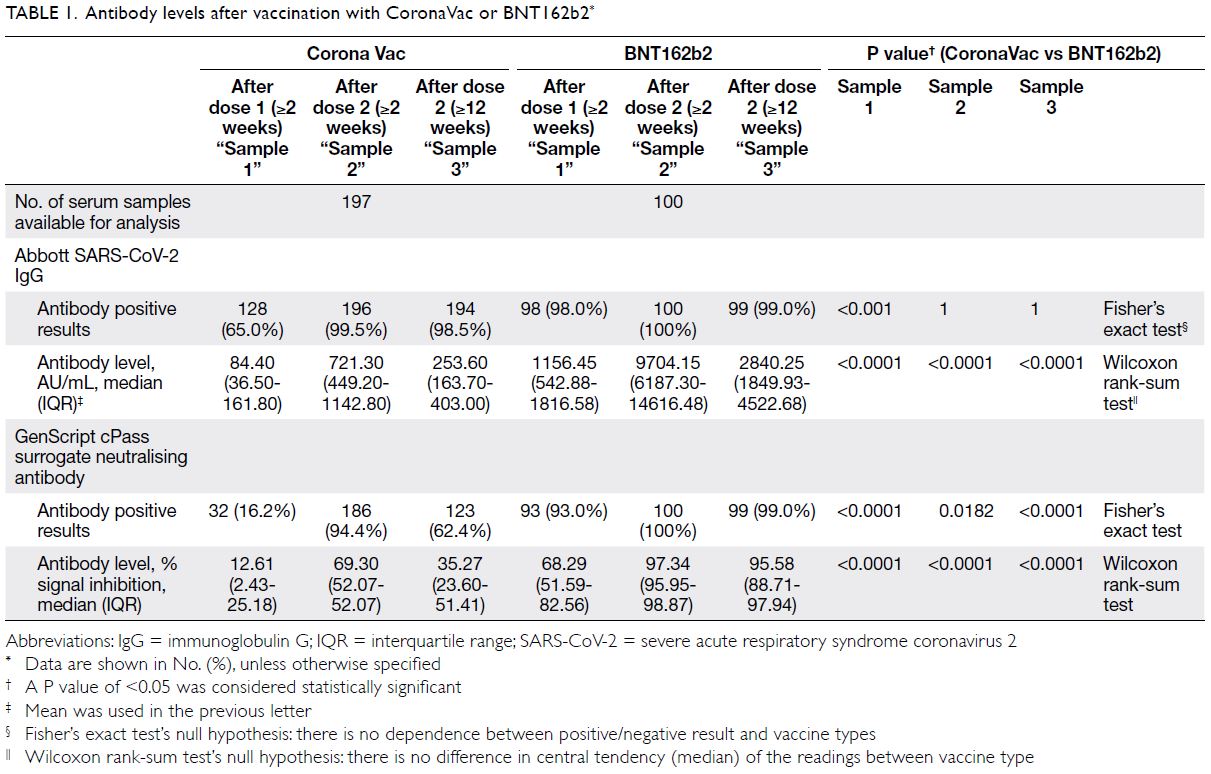
We report week 12 serological data of our
cohort. Among 197 CoronaVac and 100 BNT162b2
recipients, baseline characteristics of the two vaccine arms were comparable except sex (60.9%
and 38% female in CoronaVac and BNT162b2,
respectively) [online supplementary Table 1]. There
was no difference in anti-spike immunoglobulin G
(IgG) positive rate at week 12 (98.5% in CoronaVac
vs 99% in BNT162b2; P=1) [Table 1]. Waning of IgG
level was observed in both vaccine arms with a larger
magnitude of decline in BNT162b2 (-72% vs -64.6%;
P<0.001). Despite the more pronounced decline,
the median anti-spike IgG of BNT162b2 remained
11-fold higher than that of CoronaVac at week 12
(2840.25 AU/mL vs 253.60 AU/mL; P<0.001).
Decline in sNAb was also observed in both
arms but the magnitude was significantly smaller
in BNT162b2 (-28.3% in CoronaVac vs -2.3% in
BNT162b2; P<0.001). Using the manufacturer’s
positive cut-off at 30% signal inhibition or above, significantly more CoronaVac recipients had lost
their sNAb at week 12 (94.4% sNAb positive at week
2, 62.4% at week 12) whereas 99% of BNT162b2
recipients remained sNAb positive. Throughout the
three time points, BNT162b2 arm had higher levels
of anti-spike IgG and sNAb (P<0.001) [Fig].
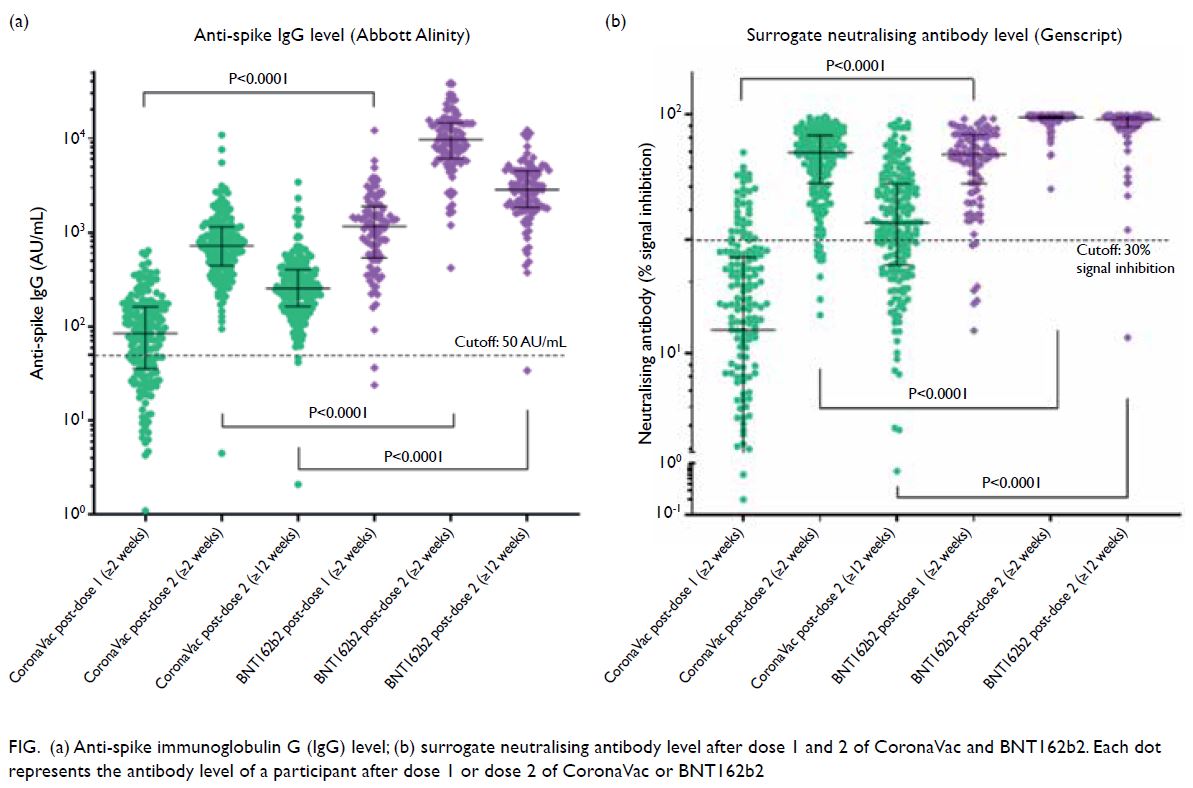
Figure. (a) Anti-spike immunoglobulin G (IgG) level; (b) surrogate neutralising antibody level after dose 1 and 2 of CoronaVac and BNT162b2. Each dot represents the antibody level of a participant after dose 1 or dose 2 of CoronaVac or BNT162b2
Among the 286 HCWs who had positive
sNAb after two doses of vaccine, 64 had lost their
sNAb while 222 had sustained sNAb at week 12.
Sustained sNAb at week 12 were associated with
younger age, BNT162b2 and higher antibody at
any time point (Table 2). Multivariate logistic
regression analysis showed that only higher IgG and
sNAb level at 2 weeks after the second dose were
significantly associated with sustained sNAb at week
12 (online supplementary Table 2).
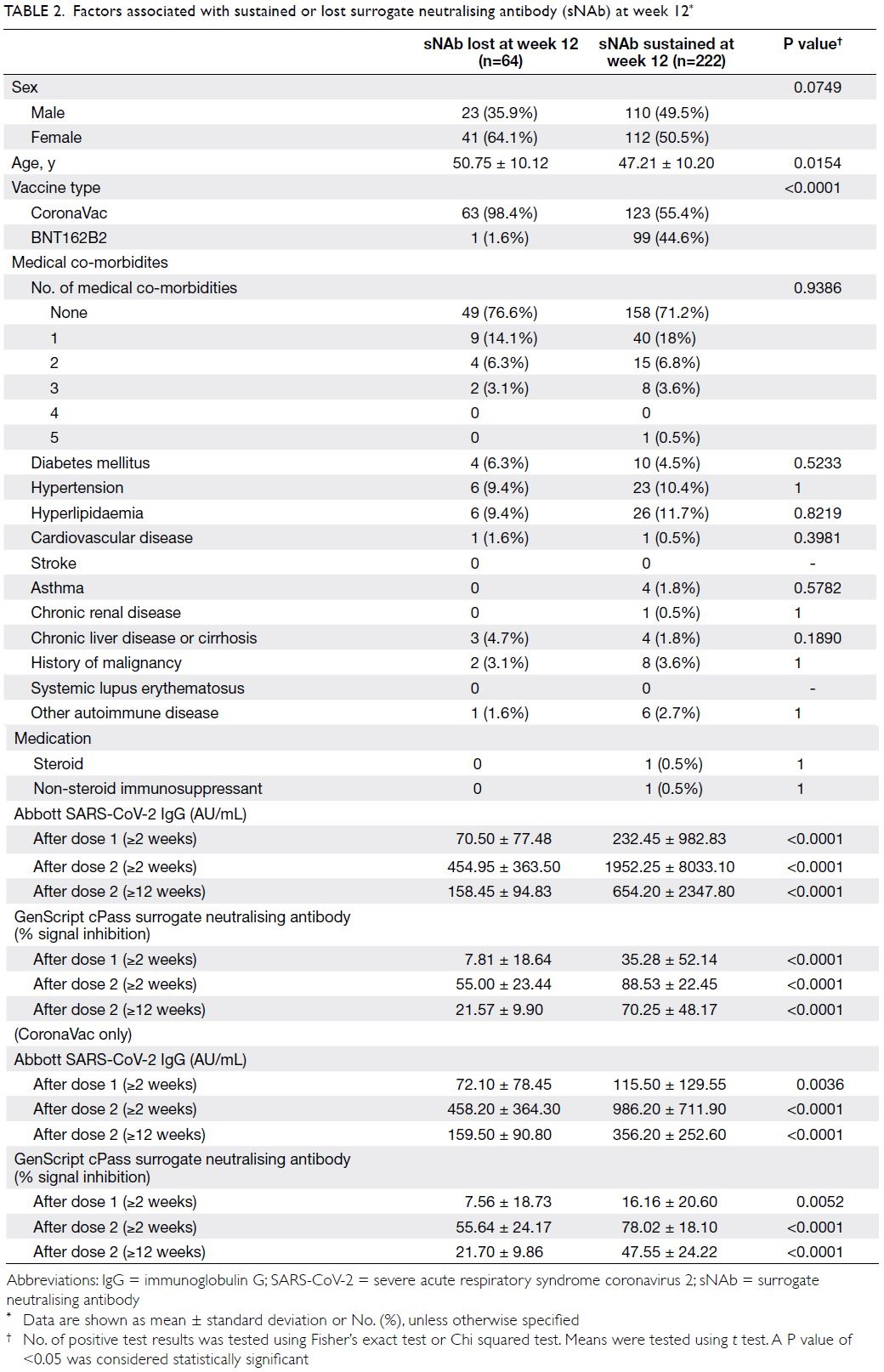
Table 2. Factors associated with sustained or lost surrogate neutralising antibody (sNAb) at week 12
These results demonstrate rapid antibody
decline after both mRNA and inactivated vaccine
with a more durable sNAb in the BNT162b2 arm.
However, further studies are needed to clarify the
impact of waning antibody on vaccine efficacy and
protection against severe infection.
Author contributions
Concept or design: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: JST Zee.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: JST Zee.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors acknowledge the excellent work and contributions by staff at the Clinical Pathology Laboratory of Hong Kong Sanatorium & Hospital.
Funding/support
This letter received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study obtained ethics approval (Ref RC-2021-07) from the Research Ethics Committee of the Hong Kong Sanatorium & Hospital Medical Group.
Reference
1. Zee JS, Lai KT, Ho MK, et al. Serological response to mRNA and inactivated COVID-19 vaccine in healthcare workers in Hong Kong: preliminary results. Hong Kong
Med J 2021;27:312-3. Crossref