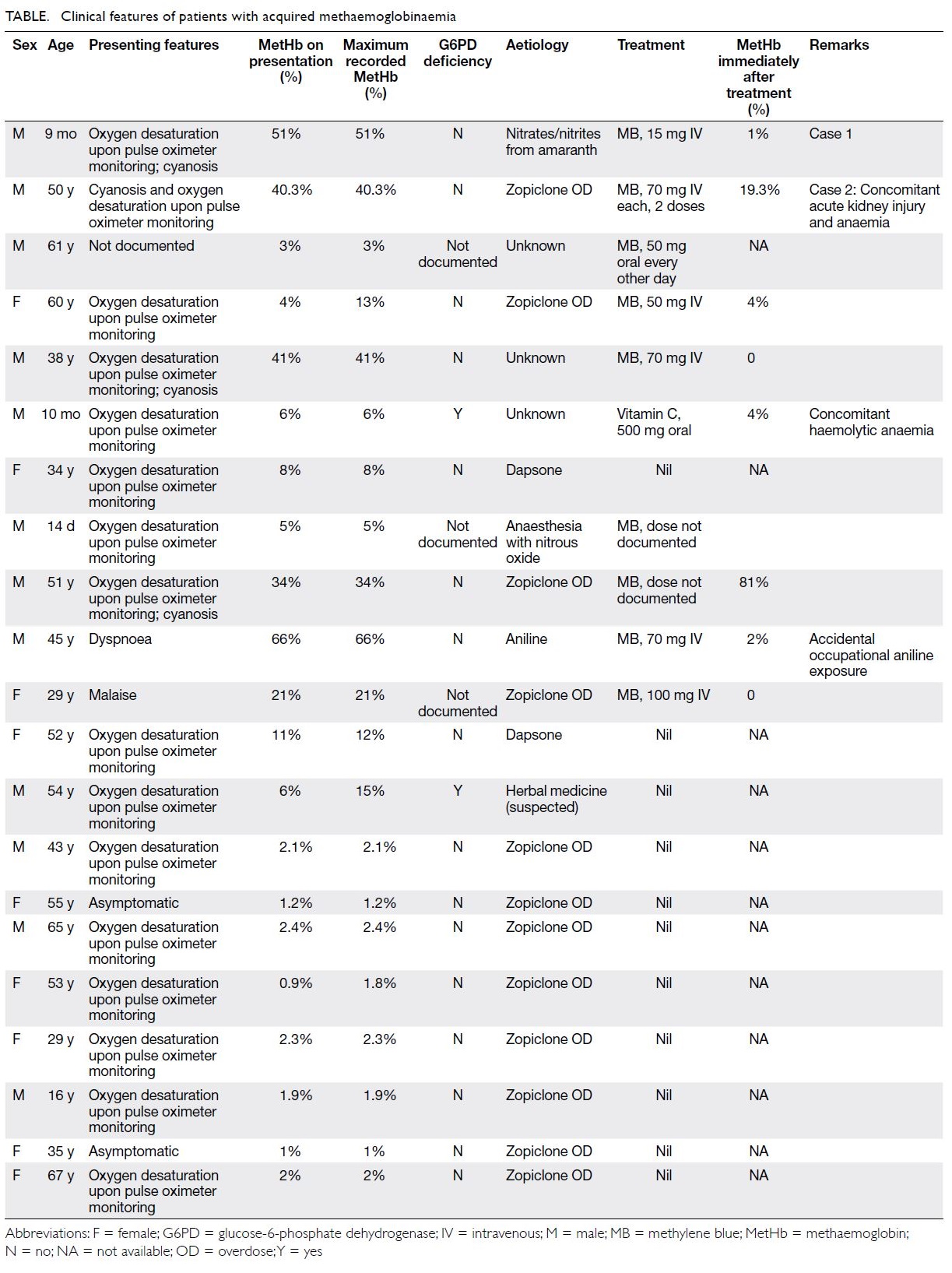
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Clinical experience in diagnosis and management
of acquired methaemoglobinaemia: a case report and retrospective review
TT Chan, MB, BS, MPH1; William CY Leung, MB, BS2; CK Chan, MB, BS FHAKM (Emergency Medicine)3; Heinz KT Lo, MB, BS4; Winnie WY Tso, MB, BS, FHKAM (Paediatrics)5; SH Tsui, MB, BS, FHKAM (Emergency Medicine)1; Thomas SY Chan, FHKCP, FHKAM (Medicine)2; Richard SK Chang, MB, BS, MRCP (HK)2
1 Accident and Emergency Department, Queen Mary Hospital, Hong Kong
2 Department of Medicine, Queen Mary Hospital, Hong Kong
3 Clinical Toxicology Department, United Christian Hospital, Hong Kong
4 Department of Paediatrics, Kwong Wah Hospital, Hong Kong
5 Department of Paediatrics and Adolescent Medicine, The University of Hong Kong, Hong Kong
Corresponding author: Dr Richard SK Chang (changsk@ha.org.hk)
Case reports
Case 1
In June 2008, a 9-month-old boy with unremarkable medical and developmental history presented to the
emergency department with acute cyanosis. The
child had been fed about 200 g of congee containing
rice, vegetables, fish, and egg. He was then noted
to have pallor of face and perioral cyanosis. He
was conscious and irritable and reported to have
vomiting. His respiratory rate was 40 breaths per
minute and heart rate was 190 beats per minute.
Pulse oximetry showed 90% oxygen saturation on
100% oxygen via a face mask. Physical examination
was otherwise unremarkable. He was admitted to
the paediatric intensive care unit. During blood
sampling, his blood was noted to be chocolate brown.
While on 100% oxygen, his arterial blood gas showed
a pH value of 7.33, PaO2 51.8 kPa, PaCO2 4.2 kPa,
and bicarbonate 17 mmol/L. Blood methaemoglobin
(MetHb) level was 51%. He was treated with
intravenous methylene blue 15 mg (about 1.5 mg/kg)
and urine was observed to be light blue (Fig). His
cyanosis resolved within 1 hour after treatment and blood oxygen saturation returned to normal without
oxygen supplementation. The MetHb level dropped
to 1% within 30 minutes after treatment. Mutation
screen of cytochrome B5 reductase gene (CYB5R3)
was negative for the patient and both parents.
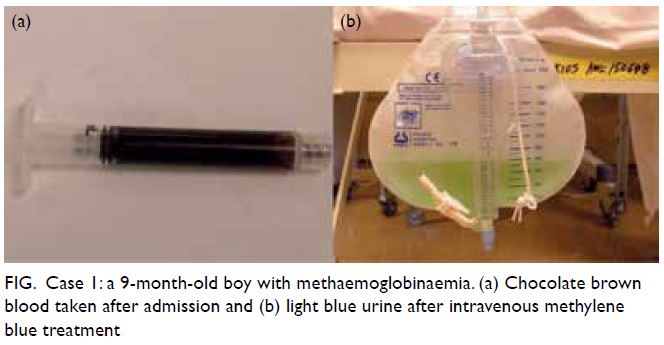
Figure. Case 1: a 9-month-old boy with methaemoglobinaemia. (a) Chocolate brown blood taken after admission and (b) light blue urine after intravenous methylene blue treatment
A high urinary nitrate level of 315 mg/L
(reference range, 41.0-55.6 mg/L) was detected.
Toxicology showed high nitrate and nitrite content
in the congee consumed by the infant, 150 mg/L and
47.7 mg/L, respectively. The uncooked vegetable
Amaranthus remnant contained nitrate but at a
safe level. Water used for cooking had come from
a regular tap, not from a well that could otherwise
have been a source of contamination.
The advised daily intake of nitrates and nitrites
is 0 to 3.7 mg/kg and 0 to 0.07 mg/kg body weight,
respectively. Our patient weighted 9.2 kg and his
single-day consumption of nitrates and nitrites
was 34 mg and 0.644 mg, respectively. When he
consumed 200 g of congee, he consumed 31.8 mg
of nitrates and 9.74 mg of nitrites in one meal. The
prescribed advised daily intake is for adults; a lower
limit should apply for children. The patient has
been followed up for 5 years with no recurrence of
methaemoglobinaemia.
Case 2
In November 2018, a 50-year-old man with a history
of schizophrenia was admitted following massive
zopiclone overdose. He had taken more than 100
tabs of the hypnotic of 7.5 mg each. There was
no evidence of carbon monoxide inhalation. His
presenting symptoms were dizziness, nausea, and
reduced urine output. He was noted to be cyanotic
and with oxygen saturation of 85% on 100% oxygen.
During blood sampling, his blood was noted to be
chocolate brown. Serum MetHb level was 40.3%
initially. Methylene blue 70 mg (around 1 mg/kg)
as a slow intravenous bolus was administered in the emergency department and 1 hour later serum MetHb
level had fallen to 19.3%. Carboxyhaemoglobin was
3.8%. He was admitted to the adult intensive care
unit and received a further dose of intravenous
methylene blue 70 mg. His saturation improved to
96% on pulse oximetry on oxygen supplementation
at 1 litre per minute. Repeat measurement of MetHb
showed it to be <0.1%. Carboxyhaemoglobin level
peaked at 10% and gradually decreased. Urine was
deep green in colour after methylene blue treatment.
He also developed acute kidney injury (creatinine
level peaked at 1239 μmol/L) that required
haemodialysis and a normochromic normocytic
anaemia (trough haemoglobin level 6.2 g/dL).
Haptoglobin level was low, indicative of haemolytic
anaemia. Although evidence of oxidative haemolysis,
such as Heinz bodies, was not documented on blood
film examination, zopiclone-induced oxidative
stress was probably the cause of haemolysis.
Renal ultrasonography showed only mild renal
parenchymal disease. His condition stabilised after
an 11-day stay in the intensive care unit.
Retrospective analysis
To supplement the above cases, we searched
through the computerised medical record system
of our institution and identified all in-patients with
International Classification of Diseases-9 code of
289.7, ‘Methemoglobinemia’, from January 2000
to December 2018. We identified 21 patients with
acquired methaemoglobinaemia, aged 14 days to
67 years (Table). The most common cause was
deliberate self-harm by overdosing with zopiclone.
The most common clinical presentation was
unexplained desaturation on pulse oximetry despite
oxygen supplementation. Nine patients received
methylene blue. One patient with coexisting glucose-6-phosphate dehydrogenase (G6PD) deficiency was
given ascorbic acid. Response to methylene blue was
prompt in all cases, with MetHb falling to a non-toxic
level soon after treatment.
Discussion
Infants or young children may be more susceptible
to methaemoglobinaemia as the haemoglobin
enzymes, such as cytochrome-b5 MetHB reductase,
are immature. As in Case 1, nitrates in vegetables can
cause methaemoglobinaemia, and this underlying
cause is preventable. Nitrates are commonly found
in agricultural products and well water, and can be
formed by bacteria from nitrates. Home-prepared
vegetables should be avoided in infants younger than
3 months of age.1 Farmers should avoid excessive
use of nitrate-containing products during vegetable
production, as suggested by agricultural guidelines.2
Well water should be monitored for nitrate
contamination. Nitrate content can be converted to nitrite due to improper storage in a flask. Nitrate
can also be reduced to nitrite by nitrate reductase
intrinsic to plants or bacteria. Thus, proper washing,
cooking and storage of food rich in nitrates is
essential.
Medication is a main cause of acquired
methaemoglobinaemia. Zopiclone overdose
accounted for a significant portion in our series.
As a non-benzodiazepine hypnotic, zopiclone is
widely prescribed. Massive zopiclone overdose is
relatively more common in Hong Kong because it
can be obtained without a prescription. Intentional
overdose has been reported in those attempting
suicide.3 Zopiclone is known to be associated with
methaemoglobinaemia especially following massive
overdose, and with haemolytic anaemia and kidney
injury. The elevated carboxyhaemoglobin in Case
2 was possibly endogenous due to haemolysis, as
metabolism of haem via haem oxidase produces one
molecule of CO per molecule of haem degraded.4
Other substances that can lead to
methaemoglobinaemia include dapsone, nitrogen,
and alanine. They induce oxidative stress in
erythrocytes. In susceptible subjects who lack
antioxidative enzymatic activity, the ferrous ion in
the haem group is oxidised to a ferric state, forming
MetHb that is incapable of binding oxygen. The
oxygen dissociation curve is shifted to the left;
oxygen desaturation, around 85%, is common in
methaemoglobinaemia. The underlying mechanism
is related to the absorption of light wavelengths
employed by emitter and detector of the pulse
oximeter.
Clinical presentations of methaemoglobinaemia
include both central and peripheral cyanosis,
dizziness, headache, dyspnoea, and malaise.
In extreme cases, coma or death may occur if
MetHb exceeds 30% to 40%. G6PD deficiency
can exacerbate the methaemoglobinaemia and
concomitant haemolysis. Vigilance is needed for
clinical diagnosis. Unexplained cyanosis or poor
oxygen saturation should prompt clinicians to
consider methaemoglobinaemia. Medication and
dietary histories, chemical contacts, recent medical
or dental procedures and occupational exposure
are conducive to diagnosis. Treatment mainly
depends on the level of MetHb. For low levels, no
specific treatment is needed. Methylene blue is
usually indicated if MetHb exceeds 20%. It acts as
a cofactor to increase erythrocyte reduction of
MetHb to haemoglobin in the presence of NADPH.
Therapeutic response is usually prompt. In our
series, MetHb level dropped in all cases to below 20%
after a single dose of methylene blue. Methylene blue
should be used with caution in patients with G6PD
deficiency as haemolysis can result from repeated
use. An excessive cumulative dose of methylene blue
can also potentially induce haemolysis. Ascorbic acid, an antioxidant, may be used as an alternative,
either orally or intravenously.5 It works by scavenging
free radicals and protects cells against oxidative
stress, acting as a co-factor for NADP reductase and
directly reducing MetHb.
In conclusion, methaemoglobinaemia can
affect a wide age range of patients and be due to a
variety of agents. Effective treatment exists and the
response is usually satisfactory.
Author contributions
Concept or design: All authors.
Acquisition of data: TT Chan, RSK Chang.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: TT Chan, RSK Chang.
Critical revision for important intellectual content: All authors.
Acquisition of data: TT Chan, RSK Chang.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: TT Chan, RSK Chang.
Critical revision for important intellectual content: All authors.
All authors had full access to the data, contributed to the
study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patients were treated in accordance with the Declaration
of Helsinki, and provided informed consent for all treatments
and procedures. The retrospective analysis of patient data was
approved by the University of Hong Kong/Hospital Authority
Hong Kong West Cluster institutional review board (Ref
UW 19-205) and the requirement for patient consent for
publication was waived.
References
1. Greer FR, Shannon M, American Academy of Pediatrics
Committee on Nutrition, American Academy of
Pediatrics Committee on Environmental Health. Infant
methemoglobinemia: the role of dietary nitrate in food and
water. Pediatrics 2005;116:784-6. Crossref
2. European Commission. Encouraging low-input farming
in the EU. Available from: https://ec.europa.eu/info/food-farming-fisheries/sustainability/environmental-sustainability/low-input-farming. Accessed 8 Aug 2019.
3. Chan TY. Zopiclone induced methemoglobinemia and hemolytic anemia. Int J Clin Pharmacol Ther 2014;52:402-6. Crossref
4. Hampson NB. Carboxyhemoglobin elevation due to hemolytic anemia. J Emerg Med 2007;33:17-9. Crossref
5. Lee KW, Park SY. High-dose vitamin C as treatment of methemoglobinemia. Am J Emerg Med 2014;32:936. Crossref