Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Management of cytokine release syndrome after
chimeric antigen T-cell therapy for paediatric
relapsed/refractory acute lymphoblastic
leukaemia: a case report
Frankie WT Cheng, MD (CUHK), FHKAM (Paediatrics), 1,2Ben S Li, MD3; Grace KS Lam, FHKCPaed, HKAM (Paediatrics)1,2; KL Hon, MD, FAAP1,2; Gavin Joynt, FRCP (Edin), FHKAM (Anaesthesiology)4; CK Li, MD, FRCPCH1,2,5
1 Department of Paediatrics, Prince of Wales Hospital, Hong Kong
2 Department of Paediatrics and Adolescent Medicine, Hong Kong Children’s Hospital, Hong Kong
3 Department of Hematology & Oncology, Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine,
National Children’s Medical Center, Shanghai, China
4 Department of Anaesthesia and Intensive Care, The Chinese University of Hong Kong, Hong Kong
5 Department of Paediatrics, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Prof CK Li (ckli@cuhk.edu.hk)
Case report
The event-free survival rate of standard risk/low-risk
childhood acute lymphoblastic leukaemia (ALL) is
approaching 90%, but there remains around 10% to
15% of children who suffer relapse.1 Early relapse of
ALL or refractory ALL has a very poor prognosis,
even with haematopoietic stem cell transplantation.
In recent years, chimeric antigen receptor T-cell
(CAR-T) therapy has offered a promising treatment
for relapsed/refractory ALL.2 At present, CAR-T
therapy is not available for ALL patients in Hong
Kong. Cytokine release syndrome (CRS) is one of the
most challenging complications following CAR-T
therapy. We report our experience of four children
prescribed CAR-T therapy for relapsed/refractory
B-cell ALL.
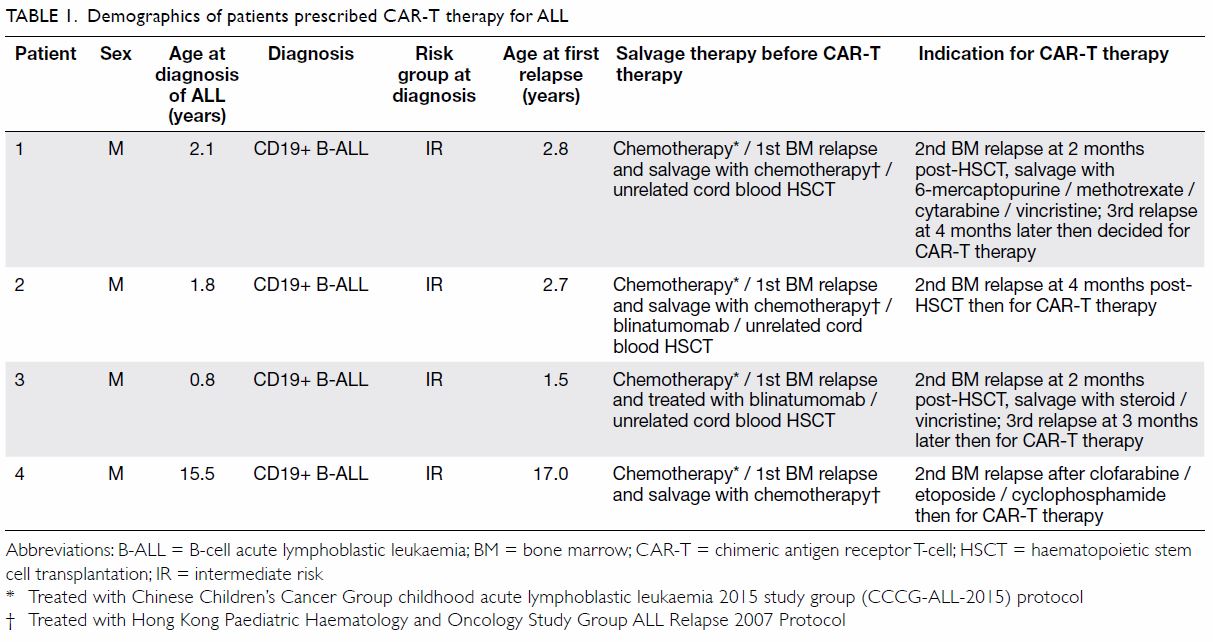
Between June 2018 and March 2019, four
children with relapsed/refractory CD19+ B-cell ALL
(aged 1-17 years at first relapse) received CAR-T
CD19 therapy at a haematology centre in Shanghai,
China. Patients 1, 2, and 3 received autologous
CAR-T cell products and patient 4 received allogeneic
CAR-T cell products. The patients returned to Hong
Kong within 12 hours of CAR-T cell infusion and
were cared for at our centre. Their clinical progress
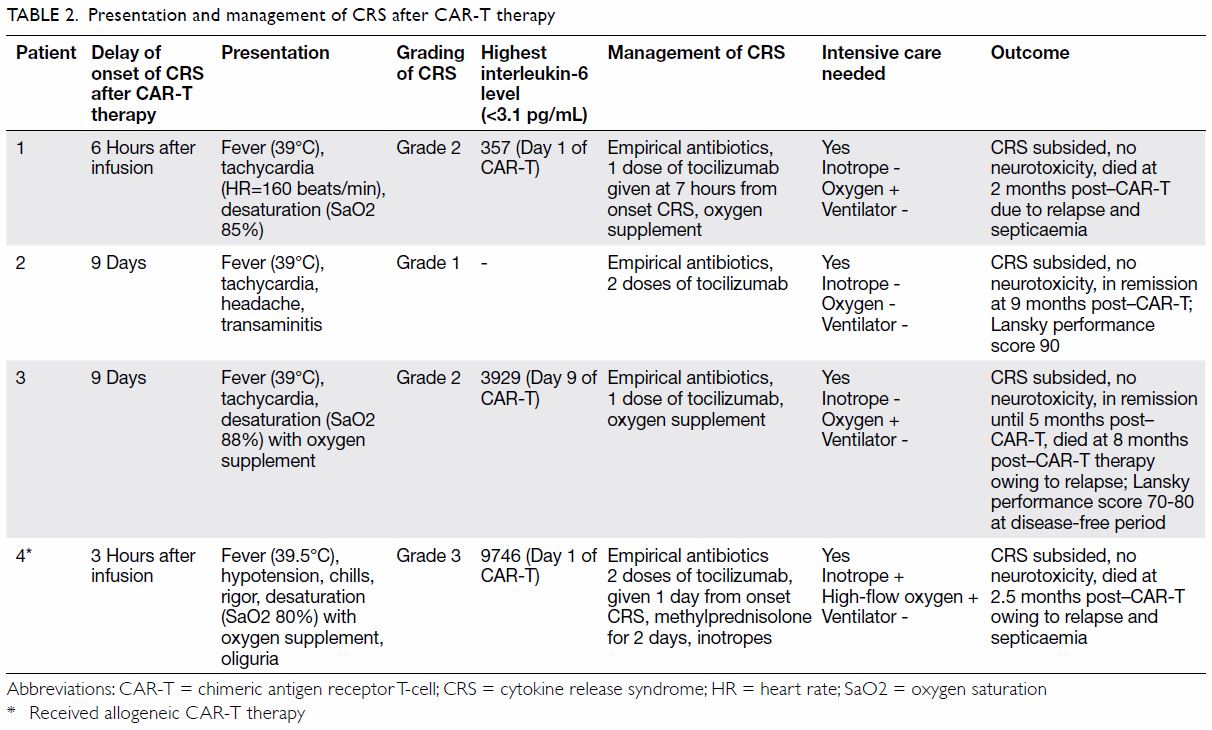
and outcome are shown in Tables 1 and 2.
All patients experienced bone marrow
relapses shortly following haematopoietic stem cell
transplantation or had refractory leukaemic disease
before receiving CAR-T therapy (Table 1). Three
patients had a high leukaemic disease burden (>75%
blast in bone marrow) prior to CAR-T therapy. For
presentation of CRS, the
first 10 days was the peak onset time, from 6 hours
to 9 days following CAR-T cell infusion. The most
common presenting symptoms were high fever with
temperature >39°C, tachycardia, hypotension, and desaturation. As clinical differentiation from sepsis
was difficult, all patients received empirical broad-spectrum
antibiotics. Patients were managed with
oxygen supplementation via a nasal cannula or high-flow
oxygen when there was desaturation. Inotropic
support in the intensive care unit was provided in the
presence of hypotension. No child required invasive
ventilatory support. Systemic steroid was prescribed
only to patient 4 who had grade 3 CRS. No patient
developed neurotoxicity and all were discharged
from the intensive care unit.
Three patients (patients 1, 3, and 4) succumbed
to disease relapse 2 to 8 months after CAR-T therapy.
One patient (patient 2) remained disease-free for
9 months after CAR-T therapy with satisfactory
Lansky performance score.
Discussion
Chimeric antigen receptor T-cell therapy is a
promising novel therapeutic option for relapsed and
refractory CD19+ B-cell ALL in children and young
adults.2 Cytokine release syndrome and neurotoxicity
are the two most severe complications of CAR-T
therapy. This has been reported to occur any time in
the first 2 weeks after infusion of CAR-T cells. Up to
45% to 91% of patients develop CRS including serious
CRS in 8.3% to 43% of cases.3 The American
Society for Blood and Marrow Transplantation
consensus grading system for CRS is based on the
assessment of three vital signs: temperature, blood
pressure, and oxygen saturation. Patients with fever
(temperature >38°C) alone constitute grade 1 CRS;
patients with fever and hypotension without the need
for a vasopressor are considered grade 2; patients
with fever, hypotension requiring vasopressor and/or hypoxia requiring oxygen supplementation are
grade 3. In grade 4 CRS, patients have fever with hypotension requiring multiple vasopressors and
positive pressure ventilation.4
In recent years many centres in Western
countries and mainland China have started to
provide CAR-T therapy, either as part of a clinical trial or as standard treatment using commercial
CAR-T cell products. The treatment will soon be
introduced in Hong Kong so local experience of
managing CRS will be of interest to our readers.
Cytokine release syndrome is a systemic inflammatory response that can be triggered by
a variety of factors such as infection and certain
drugs. The term “cytokine release syndrome” was
first used in the early 1990s when the anti–T-cell
antibody muromonab-CD3 (OKT3) was introduced
as an immunosuppressive treatment for solid
organ transplantation. Recently, with the success
of the newer T-cell-engaging immunotherapy,
namely blinatumomab, there has been a refocus on
CRS since it represents one of the most frequent
serious complications.5 The clinical features of CRS
sometimes overlap with those of haemophagocytic
lymphohistiocytosis or macrophage activation
syndrome.6 In our cohort, the peak onset was
observed in the first 10 days following CAR-T therapy.
A high disease burden prior to CAR-T therapy may
be associated with severe CRS. Close monitoring
and early intervention are key for successful control
of CRS. Remaining alert for this condition and
timely institution of monoclonal antibody against
interleukin-6 receptor (tocilizumab 8 mg/kg;
12 mg/kg if body weight <30 kg), or adding systemic
steroid in severe cases together with intensive
cardiorespiratory support is the recommended
treatment for CRS.3 4 Institutes are advised to have
tocilizumab readily available prior to commencement
of CAR-T therapy since timely control of CRS by
this agent is vital to prevent progression of cytokine
storm. The mortality of CRS has now been much
reduced with clinicians acquiring more experience
in managing complications. Vigorous respiratory
and circulatory support in an intensive care unit
is also essential.7 Gardner et al3 reported that early
intervention with tocilizumab and/or systemic
steroid in patients with early signs of CRS did not
negatively impact the anti-tumour potency of CD19
CAR-T therapy.
Our treatment outcome seems inferior to that
reported in the literature in which 3-month remission
rate was 81%; 73% at 6 months and 50% at 12 months.2
In our cohort, two patients (50%) remained in disease
remission at 3 months whereas only one (25%) was in
remission 9 months post–CAR-T therapy. However,
the case number is small and comprised of patients
with multiple relapses, three of whom developed
relapse after haematopoietic stem cell transplantation
and one who had very refractory disease. These
patients are well known to be a group with one of the
worst prognoses and most individuals do not survive
long-term.
Some centres utilise CAR-T therapy as a bridge
before transplantation as consolidative therapy
for relapsed or refractory ALL. Unfortunately, in
our four patients, three were at a very early post-transplant
stage and would be unable to tolerate a
second transplant. In other case scenarios, namely
those with chemorefractory ALL, CAR-T therapy
may play a role in bridging prior to haematopoietic stem cell transplantation. Recent clinical trials have
adopted alternative CAR-T therapy strategies such
as bispecific or sequential CAR-T therapy that may
have a more potent anti-leukaemic effect.8
In conclusion, early recognition of CRS and
early intervention with vigorous cardiopulmonary
support and timely initiation of anti–interleukin-6
receptor therapy can achieve good control of CRS.
Chimeric antigen receptor T-cell therapy is now
offered as a new salvage therapy for patients with
relapsed/refractory CD19+ B-cell ALL.
Author contribution
Concept or design: BS Li, CK Li.
Acquisition of data: FWT Cheng, GKS Lam, G Joynt, KL Hon.
Analysis or interpretation of data: FWT Cheng.
Drafting of the manuscript: FWT Cheng.
Critical revision of the manuscript for important intellectual content: CK Li.
Acquisition of data: FWT Cheng, GKS Lam, G Joynt, KL Hon.
Analysis or interpretation of data: FWT Cheng.
Drafting of the manuscript: FWT Cheng.
Critical revision of the manuscript for important intellectual content: CK Li.
Conflicts of interest
As an Editor of the Journal, KL Hon was not involved in the peer review process. The other authors have disclosed no
conflicts of interest.
Funding/support
This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the tenets of
the Declaration of Helsinki. The patient provided written
informed consent for all treatments and procedures.
References
1. Cheng FW, Lam GK, Cheuk DK, et al. Overview of
treatment of childhood acute lymphoblastic leukemia in
Hong Kong. HK J Paediatrics (New Series) 2019;24:184-91.
2. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel
in children and young adults with B-cell lymphoblastic
leukemia. N Engl J Med 2018;378:439-48. Crossref
3. Gardner RA, Ceppi F, Rivers J, et al. Preemptive mitigation
of CD19 CAR T-cell cytokine release syndrome without
attenuation of anti-leukemic efficacy. Blood 2019;134:2149-58. Crossref
4. Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol 2019;37:48-52. Crossref
5. Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab
versus chemotherapy for advanced acute lymphoblastic
leukemia. N Engl J Med 2017;376:836-47. Crossref
6. Crayne C, Cron RQ. Pediatric macrophage activation
syndrome, recognizing the tip of the iceberg. Eur J
Rheumatol 2019:1-8. Crossref
7. Hon KL, Luk MP, Fung WM, et al. Mortality, length of stay,
bloodstream and respiratory viral infections in a paediatric
intensive care unit. J Crit Care 2017;38:57-61. Crossref
8. Wang N, Hu X, Cao W, et al. Efficacy and safety of CAR19/22
T-cell cocktail therapy in patients with refractory/relapsed
B-cell malignancies. Blood 2020;135:17-27. Crossref