Hong
Kong Med J 2020 Dec;26(6):486–91 | Epub 4 Dec 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Clinical outcomes of patients with ductal
carcinoma in situ in Hong Kong: 10-year
territory-wide cancer registry study
Michael Co, MB, BS, FRCS1,2; Roger KC Ngan, MB, BS, FRCR3,4,5; Oscar WK Mang, MPH4; Anthony HP Tam, MPH4; KH Wong, MB, ChB, FRCR4,5; Ava Kwong, PhD, FRCS1,2
1Division of Breast Surgery, Department of Surgery, The University of Hong Kong, Hong Kong
2Department of Surgery, Queen Mary Hospital, Hong Kong
3Department of Clinical Oncology, The University of Hong Kong, Hong Kong
4Hong Kong Cancer Registry, Hospital Authority, Hong Kong
5Department of Clinical Oncology, Queen Elizabeth Hospital, Hong Kong
Corresponding author: Prof Ava Kwong (avakwong@hku.hk)
Abstract
Background: Incidence of ductal carcinoma in situ
(DCIS) has increased in recent decades because of
breast cancer screening. This study comprised a
long-term survival analysis of DCIS using 10-year
territory-wide data from the Hong Kong Cancer
Registry.
Methods: This study included all patients diagnosed
with DCIS in Hong Kong from 1997 to 2006. Exclusion
criteria were age <30 years or ≥70 years, lobular
carcinoma in situ, Paget’s disease, and co-existing
invasive carcinoma. Patients were stratified into
those diagnosed from 1997 to 2001 and those
diagnosed from 2002 to 2006. The 5- and 10-year
breast cancer–specific survival rates were evaluated;
standardised mortality ratios were calculated.
Results: Among the 1391 patients in this study,
449 were diagnosed from 1997 to 2001, and 942
were diagnosed from 2002 to 2006. The mean age
at diagnosis was 49.2±9.2 years. Overall, 51.2% of
patients underwent mastectomy and 29.5% received
adjuvant radiotherapy. The median follow-up
interval was 11.6 years; overall breast cancer–specific mortality rates were 0.3% and 0.9% after
5 and 10 years of follow-up, respectively. In total,
109 patients (7.8%) developed invasive breast cancer after a considerable delay. Invasive breast cancer
rates were comparable between patients diagnosed
from 1997 to 2001 (n=37, 8.2%) and those diagnosed
from 2002 to 2006 (n=72, 7.6%).
Conclusion: Despite excellent long-term survival
among patients with DCIS, these patients were
more likely to die of breast cancer, compared with
the general population of women in Hong Kong.
New knowledge added by this study
- The incidence of ductal carcinoma in situ (DCIS) has doubled from the late 1990s to early 2000s.
- Most patients with DCIS in Hong Kong undergo mastectomy.
- Breast cancer–specific mortality rates were 0.3% and 0.9% after 5 and 10 years of follow-up, respectively.
- The overall standardised mortality ratio of patients with DCIS was 5.7, compared with the general population of women in Hong Kong.
- Surgery, with or without radiotherapy, remains the gold-standard treatment modality for patients with DCIS.
- Further investigation is needed regarding the cost-effectiveness of population-wide breast cancer screening implementation.
Introduction
Ductal carcinoma in situ (DCIS) is a premalignant
disease in the breast cancer spectrum, in which
cancer cells are confined within the basement
membrane of the breast ductal system.1 Because
of the enhanced availability of breast imaging and
breast cancer awareness, the incidence of DCIS has
increased over the past two decades.2 Although the
incidence of invasive breast cancer has declined over the past decade, diagnoses of DCIS have continued to rise.3
Although DCIS is the earliest recognised form
of breast cancer, its natural history remains largely
unknown.4 5 Long-term survival studies have found
that mortality of DCIS could be as low as 3% over
10 years of follow-up.6 The current gold standard
treatment for DCIS is surgery, with or without
radiotherapy, according to the type of surgery performed on a particular patient. To the best of our
knowledge, there has been no randomised controlled
trial comparing mastectomy and breast-conserving
surgery in the context of DCIS treatment; however,
a meta-analysis suggested that local recurrence rates
were substantially lower among women treated with
mastectomy.7
In recent decades, the incidence of DCIS
has increased due to the widespread use of breast
imaging screenings, on the basis of enhanced breast
cancer awareness. As in other screening-detected
disorders, there is widespread debate regarding
whether DCIS is overdiagnosed and overtreated.
Some clinicians have advocated a watchful-waiting
strategy for DCIS, with the presumption that not all
DCIS will progress to invasive cancer.8
In contrast to many developed countries,
a population-based breast cancer screening
programme is not available in Hong Kong. A
recent study showed that DCIS is more frequently
detected and treated in the private sector in Hong
Kong, compared with the public health care
system. Notably, DCIS is reportedly detected more
frequently among patients in higher social classes
due to self-initiated breast screening; more than
half of these patients undergo successful treatment
with breast-conserving surgery.9 Here, we present
the results of long-term survival analyses based on a
territory-wide breast cancer registry.
Methods
Data source
This was a retrospective analysis of a territory-wide,
prospectively maintained database from the
Hong Kong Cancer Registry, concerning patients
diagnosed during the period from 1997 to 2006;
data were censored in December 2015 for retrieval
of long-term survival outcomes. The Hong Kong
Cancer Registry is a population-based cancer
registry managed by the Hong Kong Hospital
Authority; this registry has been responsible for
collecting basic demographic data, cancer site
information, and cancer histology results for all
patients diagnosed with cancer in all public and
private medical institutions in Hong Kong since
1963. All raw data were validated by various crosschecking
procedures involving the locally designed
Cancer Case Audit System; they were scrutinised by
multiple quality control processes, commensurate
with the recommendations by the International
Agency for Research on Cancer, a component of the
World Health Organization. Queries and “unusual
cases” were referred to a clinical oncologist for
re-validation.
Cohort selection and statistics
Institutional review board approval was not needed
for this retrospective review of a breast cancer
registry database. This study included all patients
with DCIS who were diagnosed from 1997 to 2006.
Exclusion criteria were age <30 years or ≥70 years,
lobular carcinoma in situ, Paget’s disease, and
co-existing invasive carcinoma (ie, diagnosed within
6 months of DCIS onset). Patients were stratified
into those diagnosed from 1997 to 2001 and those
diagnosed from 2002 to 2006. Five- and 10-year
breast cancer–specific survival rates were evaluated;
standardised mortality ratios were calculated (with
reference to the general population of women in
Hong Kong).
Results
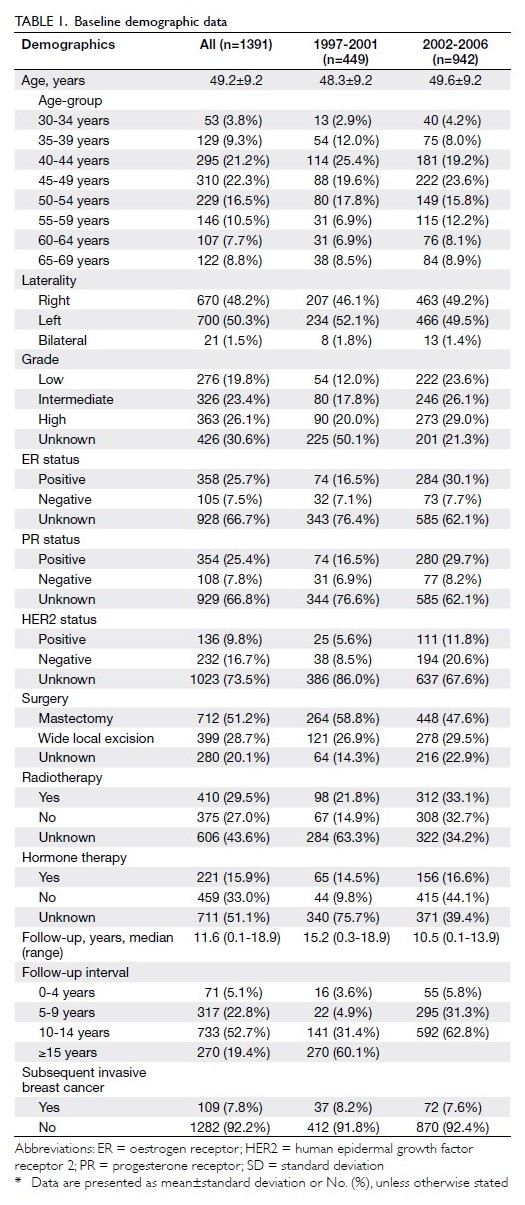
From 1997 to 2006, 1391 patients were diagnosed
with DCIS and included in the Hong Kong Cancer
Registry breast cancer database. In total, 449 patients
were diagnosed from 1997 to 2001, while 942 patients
were diagnosed from 2002 to 2006. The mean age at
diagnosis was 49.2±9.2 years. Most patients (43.5%)
were aged 40 to 49 years (Table 1). More than half of
the patients (n=712, 51.2%) underwent mastectomy,
while 399 (28.7%) underwent breast-conserving
surgery. Overall, 410 patients (29.5%) received
adjuvant radiotherapy. In addition, 221 patients
(15.9%) received risk-reducing hormonal therapy
with tamoxifen (Table 1).
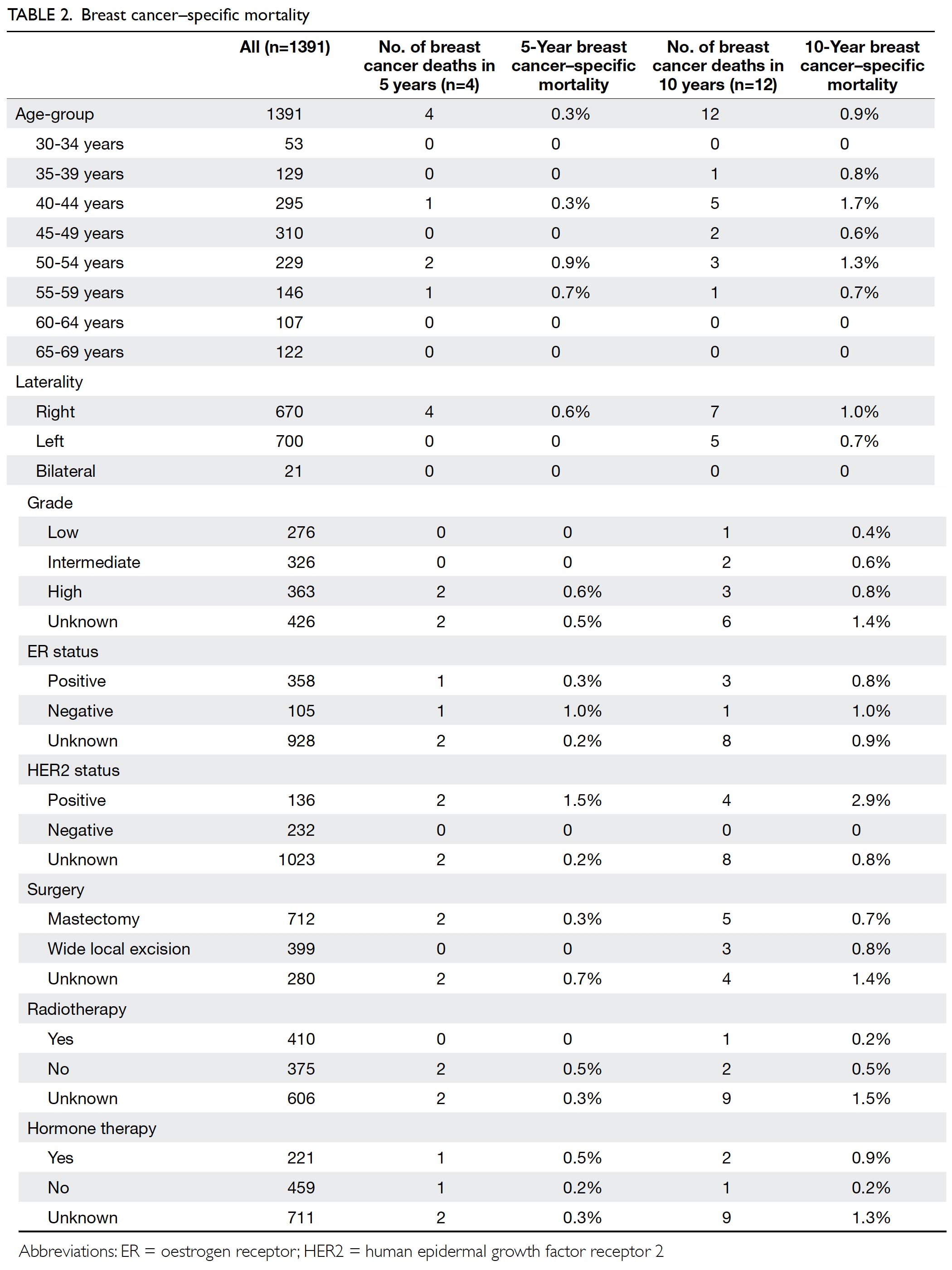
The median follow-up interval was 11.6 years;
overall breast cancer–specific mortality rates were 0.3% and 0.9% after 5 and 10 years of follow-up,
respectively (Table 2). In total, 109 patients (7.8%)
developed invasive breast cancer after a considerable
delay. Invasive breast cancer rates were comparable
between patients diagnosed from 1997 to 2001 and
those diagnosed from 2002 to 2006: 37 (8.2%) and
72 (7.6%), respectively (Table 1).
Subgroup analysis revealed higher breast
cancer–specific mortality in patients with human
epidermal growth factor receptor 2 (HER2)–positive
DCIS after 10 years of follow-up, compared with
patients who exhibited HER2-negative DCIS (2.9%
vs 0%; P=0.0181, Fisher’s exact test). In contrast,
10-year breast cancer–specific mortality rates
were comparable between patients with low-/intermediate-grade DCIS and those with high-grade
DCIS (0.5% vs 0.8%; P=0.6776).
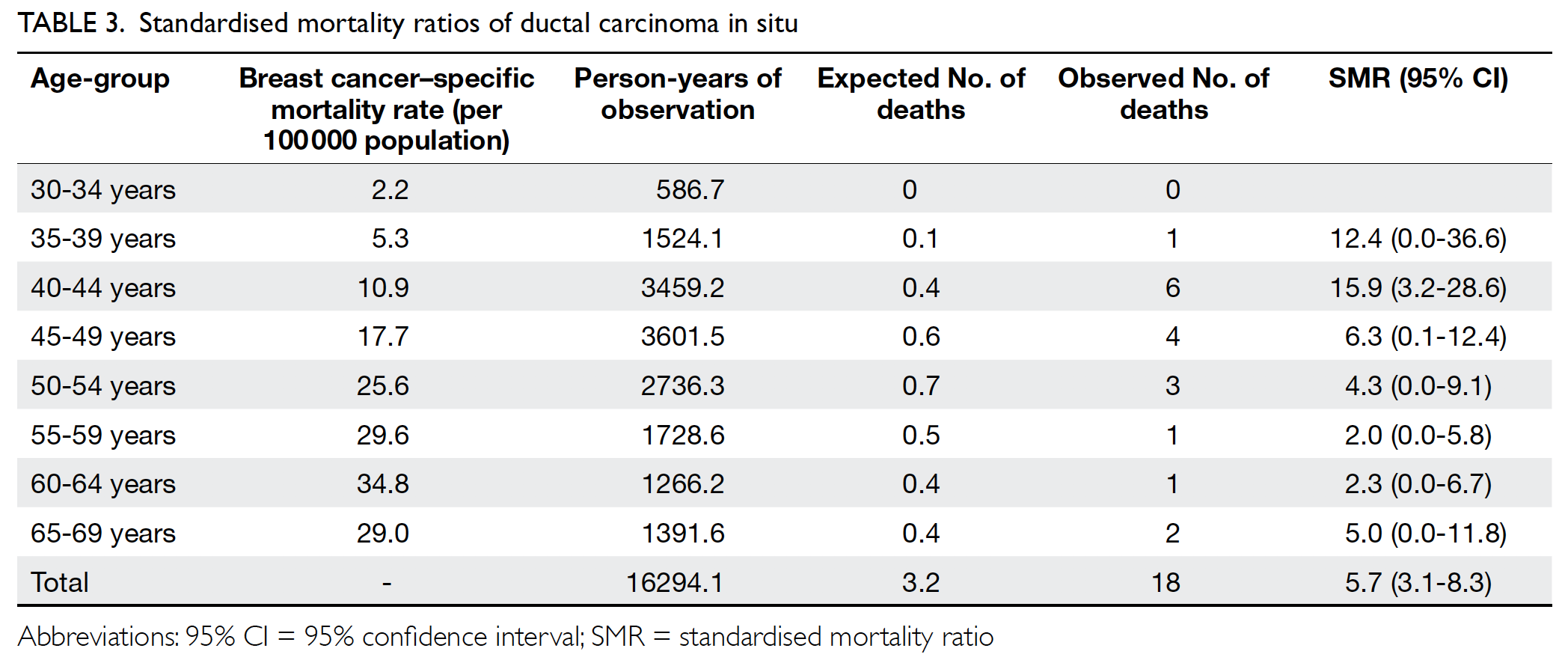
The breast cancer–specific mortality rate (per
100 000) was 2.2 among patients aged 30 to 34 years;
this rate slowly increased and peaked at 34.8 among
patients aged 60 to 64 years (Table 3). Patients
with DCIS were more likely to die of breast cancer,
compared with the general population of women in
Hong Kong (standardised mortality ratio=5.7; 95%
confidence interval=3.1-8.3).
Discussion
Breast cancer is the most common cancer among
women in Hong Kong, such that it constituted 26.1%
of all newly diagnosed cancers among women in
Hong Kong in 2015.10 Notably, a population-wide
breast cancer screening programme is not available
in Hong Kong. However, because of improved
patient-level and population-level education
regarding breast cancer awareness, rates of self-initiated
breast cancer screening by ultrasonography
and mammography have increased over the past
decade.9 This might well explain the doubling of
DCIS incidence from 449 patients (1997-2001) to
942 patients (2002-2006).
The mortality rate of patients with DCIS has
substantially declined over the past few decades in
the United States: the 10-year breast cancer mortality
rate was 3.4% for women who received a diagnosis
from 1978 to 1983, then decreased to 1.9% for women
who received a diagnosis from 1984 to 198911 and
1.1% for women who received a diagnosis from 1988
to 2011.2 The mortality rate of patients with DCIS
in Hong Kong has remained stable during this same
period, despite improved detection through selfinitiated
breast screening. Nevertheless, the 10-year
breast cancer–specific mortality rate of 0.9% in the
current study is comparable with the findings from
Western nations; in particular, the 10-year breast
cancer–specific mortality rate was reportedly 1.8%
in a randomised trial of 1046 Swedish patients with
DCIS, who were diagnosed from 1987 to 1999.12
The underlying reason for such an improvement in survival is beyond the scope of this study, but we
presume that it is multifactorial (eg, earlier detection
and improved surgical oncologic treatment for
DCIS).13 However, reports on the improved survival
of patients with DCIS (including the current cohort) should be interpreted with care, because this improvement may be the result of overdiagnosis of
DCIS.
Overdiagnosis and overtreatment for DCIS
have been a major focus of debate over the past decade.12 Several randomised controlled trials, such
as the COMET (NCT02926911) and LORIS trials,
are currently investigating the feasibility and non-inferiority
of active surveillance with or without
endocrine therapy for management of low-risk DCIS.
Biological markers such as HER2 receptor
and oestrogen receptor statuses have been used for
assessment of prognosis and tumour behaviour in
patients with invasive breast cancers,14 but their roles
in the context of DCIS may have been previously
underestimated. Human epidermal growth factor
receptor 2–positive DCIS is considered the most
unstable precursor among all molecular subtypes,
because of its high invasion rate and frequent
association with a discordant phenotype.15 Our
results may provide clinical validation of this
postulation, because they demonstrated that
the 10-year breast cancer–specific mortality is
significantly worse in patients with HER2-positive
disease. However, because of the relatively small
number of events included in the subgroup analysis,
it may be premature to conclude that positive
HER2 findings are associated with adverse survival
outcome. Oestrogen receptor–positive DCIS
was associated with slightly lower 10-year breast
cancer–specific mortality (Table 2). Indeed, the
use of systemic hormonal therapy with tamoxifen
in patients with oestrogen receptor–positive DCIS
has been shown to reduce the risk of future invasive
cancer.16
We acknowledge that this study was limited by
its retrospective in nature, because all pathological
diagnoses were supplied by the breast cancer database
from The Hong Kong Cancer Registry. A formal
pathology review might have identified patients
whose diagnoses were modified from DCIS (in core
biopsy) to invasive cancers (in final pathology); other
diagnoses might have been modified from invasive
cancers to DCIS. While some researchers reported a
17% exclusion rate after a central pathology review,17 others reported a much lower exclusion rate of 2%
after secondary pathological review of patients with
DCIS.18 19 20 Nevertheless, our analysis was based on
a large territory-wide cancer registry. All data were
maintained and validated in a consistent manner.
In addition, the extended follow-up period enabled
detailed long-term survival analysis for patients with
DCIS.
Conclusion
Data from the Hong Kong Cancer Registry revealed
that the incidence of DCIS doubled from the late
1990s to the early 2000s. The estimated standardised
mortality ratio of patients with DCIS in Hong Kong
was 5.7, compared with the general population of
women in Hong Kong. Our cohort represents one of
the largest DCIS cohorts in the published literature.
For locations where population-wide breast cancer
screening is not available, as in Hong Kong, we
believe that the results of our study support further
investigation of the cost-effectiveness of population-wide
breast cancer screening implementation.
Author contributions
Concept or design: M Co, A Kwong.
Acquisition of data: A Kwong, OWK Mang, RKC Ngan, AHP Tam, KH Wong.
Analysis or interpretation of data: M Co, A Kwong, OWK Mang, AHP Tam.
Drafting of the manuscript: M Co, A Kwong.
Critical revision of the manuscript for important intellectual content: A Kwong, RKC Ngan, KH Wong.
Acquisition of data: A Kwong, OWK Mang, RKC Ngan, AHP Tam, KH Wong.
Analysis or interpretation of data: M Co, A Kwong, OWK Mang, AHP Tam.
Drafting of the manuscript: M Co, A Kwong.
Critical revision of the manuscript for important intellectual content: A Kwong, RKC Ngan, KH Wong.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Acknowledgement
We thank the Dr Ellen Li Charitable Foundation and the Kuok Foundation for their continual support in providing the staff
for collection of the data to make this possible. We also thank members of the Hong Kong Breast Cancer Research group
for their advice on the research during the period of data
collection.
Funding/support
This research was funded by the Dr Ellen Li Charitable Foundation and the Kuok Foundation. The funders had no role
in the design of this study, the analysis and interpretation of
the data, or the decision to submit the results for publication.
Ethics approval
This study was approved by the Hong Kong Hospital Authority West Cluster Research Ethics Committee (Ref UW 09-045). Patient consent was obtained for data collection and analysis.
References
1. Silverstein MJ, Baril NB. In situ carcinoma of the breast. In:
Donegan WL, Spratt JS, editors. Cancer of the Breast. 5th
ed. Saunders: Philadelphia; 2002.
2. Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C.
Incidence of and treatment for ductal carcinoma in situ of
the breast. JAMA 1996;275:913-8. Crossref
3. Surveillance, epidemiology and end results program,
National Cancer Institute. USA government. Previous
version: SEER cancer statistics review, 1975-2009. Available
from: https://seer.cancer.gov/archive/csr/1975_2009_pops09/. Accessed 10 Jan 2019.
4. Sprague BL, Trentham-Dietz A. In situ breast cancer. In:
Li CI, editor. Breast Cancer Epidemiology. New York:
Springer; 2010: 47-72. Crossref
5. Allegra CJ, Aberle DR, Ganschow P, et al. National Institutes
of Health State-of-the-Science Conference Statement:
Diagnosis and Management of Ductal Carcinoma in Situ
September 22-24, 2009. J Natl Cancer Inst 2010;102:161-9. Crossref
6. Co M, Kwong A. Ductal carcinoma in situ of the breast—long term results from a twenty-year cohort. Cancer Treat
Res Commun 2018;14:17-20. Crossref
7. Boyages J, Delaney G, Taylor R. Predictors of local recurrence after treatment of ductal carcinoma in situ: a
meta-analysis. Cancer 1999;85:616-28. Crossref
8. Merrill AL, Esserman L, Morrow M. Clinical decisions. Ductal carcinoma in situ. N Engl J Med 2016;374:390-2. Crossref
9. Yau TK, Chan A, Cheung PS. Ductal carcinoma in situ of breast: detection and treatment pattern in Hong Kong. Hong Kong Med J 2017;23:19-27. Crossref
10. Centre for Health Protection, Department of Health, Hong
Kong SAR Government. Breast Cancer. Available from:
https://www.chp.gov.hk/en/healthtopics/content/25/53.html. Accessed 7 Jul 2018.
11. Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R. Mortality among women with ductal carcinoma
in situ of the breast in the population-based surveillance,
epidemiology and end results program. Arch Intern Med
2000;160:953-8. Crossref
12. Early Breast Cancer Trialists’ Collaborative Group
(EBCTCG), Correa C, McGale P, et al. Overview of the
randomized trials of radiotherapy in ductal carcinoma in
situ of the breast. J Natl Cancer Inst Monogr 2010;2010:162-77. Crossref
13. Co M, Kwong A, Shek T. Factors affecting the underdiagnosis
of atypical ductal hyperplasia diagnosed by core
needle biopsies—a 10-year retrospective study and review
of the literature. Intl J Surg 2018;49:27-31. Crossref
14. Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of
human breast tumours. Nature 2000;406:747-52. Crossref
15. Harada S, Mick R, Roses RE, et al. The significance of
HER-2/neu receptor positivity and immunophenotype in
ductal carcinoma in situ with early invasive disease. J Surg
Oncol 2011;104:458-65. Crossref
16. Boughey JC, Gonzalez RJ, Bonner E, Kuerer HM. Current
treatment and clinical trial developments for ductal
carcinoma in situ of the breast. Oncologist 2007;12:1276-87. Crossref
17. Collins LC, Achacoso N, Haque R, et al. Risk factors for
non-invasive and invasive local recurrence in patients
with ductal carcinoma in situ. Breast Cancer Res Treat
2013;139:453-60. Crossref
18. Bijker N, Peterse JL, Duchateau L, et al. Risk factors
for recurrence and metastasis after breast-conserving
therapy for ductal carcinoma-in-situ: analysis of European
Organization for Research and Treatment of Cancer Trial
10853. J Clin Oncol 2001;19:2263-71. Crossref
19. Fisher ER, Costantino J, Fisher B, et al. Pathologic findings
from the National Surgical Adjuvant Breast Project
(NSABP) Protocol B-17. Five-year observations concerning
lobular carcinoma in situ. Cancer 1996;78:1403-16. Crossref
20. Rakovitch E, Mihai A, Pignol JP, et al. Is expert breast
pathology assessment necessary for the management
of ductal carcinoma in situ? Breast Cancer Res Treat
2004;87:265-72. Crossref