Hong Kong Med J 2020 Oct;26(5):413–20 | Epub 17 Sep 2020
Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
REVIEW ARTICLE CME
Labour analgesia: update and literature review
KK Lam, MB, ChB, FHKAM (Anaesthesiology); May KM Leung, MB, BS, FHKAM (Anaesthesiology); Michael G Irwin, MB, ChB, FHKAM (Anaesthesiology)
Department of Anaesthesiology, The University of Hong Kong, Hong Kong
Corresponding author: Dr KK Lam (dr.patricklam.hk@gmail.com)
Abstract
Pain relief is an important component of modern
obstetric care and can be produced by neuraxial,
systemic, or inhalational analgesia or various physical
techniques. We review the most recent evidence on
the efficacy and safety of these techniques. Over the
past decade, the availability of safer local anaesthetics,
ultra-short acting opioids, combined spinal-epidural
needles, patient-controlled analgesic devices, and
ultrasound have revolutionised obstetric regional
analgesia. Recent meta-analyses have supported
epidural analgesia as the most efficacious technique,
as it leads to higher maternal satisfaction and good
maternal and fetal safety profiles. We examine the
controversies and myths concerning the initiation,
maintenance, and discontinuation of epidural analgesia. Recent evidence will also be reviewed
to address concerns about the effects of epidural
analgesia on the rates of instrumental and operative
delivery, lower back pain, and breastfeeding. New
developments in labour analgesia are also discussed.
Introduction
Labour pain is so notoriously painful that opium
and its derivatives have been used in childbirth for
several thousand years, along with numerous folk
medicines and remedies. Nulliparous women suffer
greater sensory pain during the early stage of labour
compared with multiparous women, for whom the
second stage is more intense.1 Labour pain has both
visceral and somatic components.2 The first stage of
labour pain is caused by contraction of the uterus
and gradual dilatation of the cervix. The visceral pain
is carried by small unmyelinated C-fibres through
sympathetic nerves to the T10 to L1 segments of
the dorsal horn of the spinal cord. The pain is often
referred to as located in the front and back of the
lower abdomen and sacrum. Stretching of the
vaginal wall, perineum, and vaginal surface of the
cervix in the later stage of labour causes ischaemic
pain, which is conducted through thick myelinated
A fibres in the pudendal and perineal branches of the
posterior cutaneous nerve in the thigh to the S2 to
S4 nerve roots, Thus, women who are giving birth
feel sharp somatic pain in the perineum.
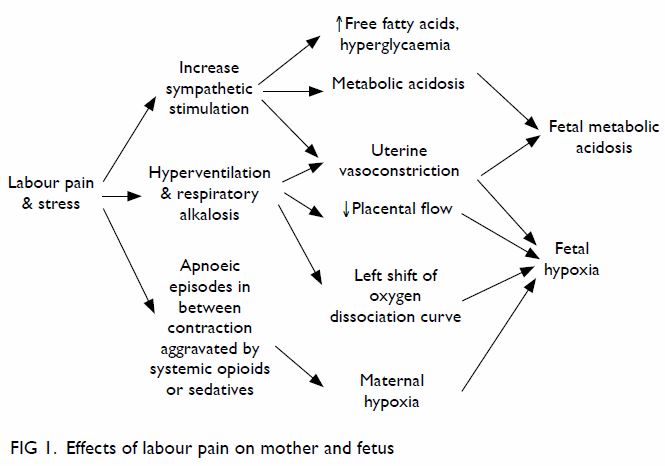
As well as being unpleasant, labour pain may
have harmful effects on the mother and baby,1 3 as pain
stimulates catecholamine release, which constricts
the uterine blood vessels. Pain also causes maternal
hyperventilation, resulting in hypocapnia, which
further constricts the uterine vessels and decreases
the mother’s ventilatory drive between contractions,
thereby causing the left shift of the maternal oxygen
dissociation curve. These factors compromise oxygen
supply to the fetus and can lead to fetal hypoxaemia and fetal metabolic acidosis (Fig 1). Premature
‘bearing down’ can also lead to birth canal trauma
and birth injury. Parenteral opioids can exacerbate
maternal respiratory depression, whereas regional
analgesia can reduce the adverse effects of labour
pain on respiration and the sympathetic nervous
system. Therefore, good labour analgesia should
aim not only to relieve the pain and suffering of the
mother but also to decrease fetal acidosis and make
the delivery process safer for both the mother and
baby. Traditionally, pain relief methods are classified
into non-pharmacological, pharmacological, and
regional techniques. In this article, we examine the
most recent evidence on the efficacy and safety of
the commonly available methods.
Non-pharmacological techniques
Mild labour pain may be reduced by massage,
psychological relaxation techniques, transcutaneous
electrical nerve stimulation, aromatherapy, hypnosis,
sterile water injection, acupuncture, deep breathing,
and hydrotherapy. However, most of the evidence
on non-drug interventions is based on anecdotal
reports from a small number of studies. A Cochrane
systematic review reported that immersion and
relaxation produced good satisfaction, and both
relaxation and acupuncture decreased the use
of forceps and ventouse, with acupuncture also
decreasing the number of Caesarean sections.4
There was insufficient evidence to judge whether or
not hypnosis, biofeedback, sterile water injection,
aromatherapy, and transcutaneous electrical nerve
stimulation are effective.4
Pharmacological techniques
Entonox is a mix of 50% nitrous oxide in oxygen
that has been in use for a long time. It has some
analgesic efficacy, but many women who used it
felt drowsy, nauseous, or were sick.4 Nitrous oxide
has detrimental effects on vitamin B12 metabolism,
and there are valid concerns about occupational
exposure to healthcare professionals in the delivery
suite, although the use of a proper scavenging system
can help. It has the advantage of being easy to use
by self-administration, but around 30% to 40% of
patients found pain relief inadequate with Entonox
alone.5
Sub-anaesthetic doses (0.8% in oxygen) of
sevoflurane have been evaluated as an alternative
to Entonox.6 7 In those studies, despite its lack of
analgesic effects and increased level of sedation,
most women preferred it to Entonox. It also caused
less nausea and vomiting than Entonox. However, there are valid concerns about loss of consciousness,
fetal toxicity, and air pollution; therefore, it is not
popular.
Intramuscular pethidine is widely prescribed.
Pethidine is a potent opioid, making the side-effects
of somnolence, nausea, vomiting, and respiratory
depression common. It is less effective than epidural
analgesia4 and cannot be given near the end of the
first stage or during the second stage of labour
because of its respiratory depressant effects on
the baby. It also has a neuroexcitatory metabolite,
norpethidine.
Remifentanil, an ultra-short acting opioid with
a half-life of about 3 minutes irrespective of the
duration of infusion, is usually given intravenously
using a patient-controlled analgesic pump. In 2001,
we found that the time to first request for rescue
analgesia and maternal satisfaction were higher with
patient-controlled analgesic remifentanil compared
with intramuscular pethidine. There was no sedation,
apnoea, or oxygen desaturation in either group, and
Apgar scores of the groups were similar.8 In 2018, the
RESPITE trial showed that remifentanil halved the
proportion of epidural conversions compared with
intramuscular pethidine.9 The pooled risk ratio for
rescue analgesia of remifentanil relative to pethidine
was 0.54. The study also reported that remifentanil
posed no excessive risk of respiratory depression to
the mothers or babies, thus challenging pethidine’s
routine use as a first-line opioid in the management
of labour pain. Although its analgesia is not superior
to an epidural, remifentanil is an efficacious
alternative for patients who have contra-indications
to epidural administration, including back problems,
coagulopathy, and fixed cardiac output diseases.
Many local and overseas centres have incorporated
this option into their labour pain management
programmes. The RemiPCA SAFE Network has
been established to set standards and monitor
maternal and fetal outcomes when remifentanil is
used for labour analgesia.10
Neuraxial analgesic techniques
Epidural analgesia, introduced in the 1960s, is still
the most effective method of labour pain relief.11 It
involves placing a very fine catheter into the epidural
space for repeat boluses or continuous infusion of
local anaesthetics. This allows for continuous pain
relief throughout labour and ‘top-up’ boluses, if
required, for operative deliveries. New drugs and
technological advancements have improved safety,
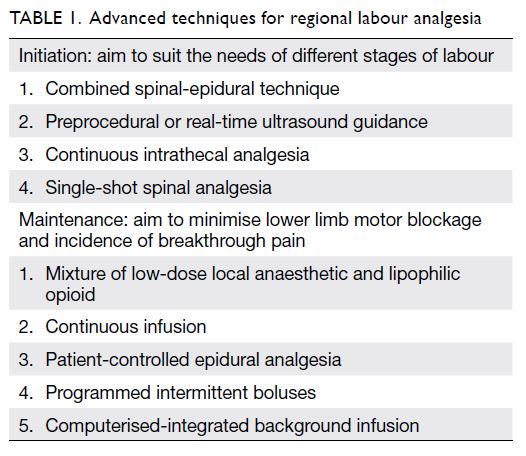
and our understanding of its effects on obstetric
outcomes has been revised (Table 1). Levobupivacaine and
ropivacaine are the newest amide local anaesthetics,
and they are less cardiotoxic than bupivacaine.
Traditionally, a high concentration of local
anaesthetic (eg, 0.2%-0.25% bupivacaine) has been
used to maintain labour epidural analgesia. Over the years, the adoption of a lower concentration of local
anaesthetic (0.0625%-0.1%) and lipophilic opioids
(fentanyl or sufentanil) has lessened side-effects
such as motor blockage and hypotension.12 These
drugs have made it possible for women to walk or
move around more easily in bed and retain a mild
sensation of uterine contraction and urgency of
bearing down, thereby facilitating pushing the baby
out in the second stage of labour. In the Comparative
Obstetric Mobile Epidural Trial study, the use of
low-dose infusion significantly reduced the incidence
of assisted vaginal delivery.13 Meta-analysis showed
that a lower concentration of local anaesthetic
reduces the incidence of assisted vaginal delivery
and urinary retention and shortens the second stage
compared with a higher concentration.14 A 2018
Cochrane review stated that this type of epidural
analgesia has no adverse impact on the proportions
of Caesarean section, long-term backache, or
neonatal outcomes.11
Combined spinal-epidural technique
In the ‘needle-through-needle’ combined spinal-epidural
(CSE) technique, a 25- or 27-G pencil
point spinal needle with a locking device is inserted
through the epidural needle that allows the
deposition of a small dose of local anaesthetic, with
or without opioids, into the cerebrospinal fluid in
the intrathecal space. The onset of analgesia is rapid.
An epidural catheter is then threaded through the
epidural needle after withdrawing the spinal needle.
A review of the complications has concluded that
CSE is equally safe to a conventional epidural.15
The use of CSE has increased relative to that of the
conventional epidural technique, as it has a quicker
onset of analgesia in mothers with severe pain,
those in the advanced stage of labour, and those
who are multiparous. The technique also improves
the success of correct functioning epidural catheter
placement by prior verification of placement in the
subarachnoid space with the spinal needle.16 Despite
the increasingly widespread use of this technique
and numerous published investigations, the
optimal intrathecal drug regimen has not yet been
determined. The disadvantage of CSE is immediate
uncertainty about whether the epidural is working
because of the initial effects of spinal analgesia.
However, a 2016 study refuted this and favoured CSE
earlier detection of failed epidural analgesia.17 The
use of a 27-G spinal needle is preferred, as its small
size is associated with a lower risk of post-dural
puncture headache.18 Although there is faster onset
of analgesia, the effects on maternal satisfaction
are controversial. A systematic review found
no differences in maternal satisfaction, mode of
delivery, or ambulatory ability between CSE and the
conventional epidural technique.19 Subsequently,
the choice between conventional epidural and CSE has often been dictated by the clinical situation,
institutional protocols, available equipment, and
practitioner preference/experience.
Continuous intrathecal technique
In continuous intrathecal labour analgesia, local
anaesthetic with or without opioids is directly
deposited into the intrathecal space using a 23- to
28-G microcatheter. This technique can provide
rapid analgesia or anaesthesia and higher maternal
satisfaction with less use of local anaesthetic, but it
is also associated with more technical difficulties and
catheter failure compared with epidural analgesia. It
is theoretically advantageous in the management of
morbidly obese patients, patients with significant
co-morbidities who cannot tolerate haemodynamic
instability, and patients with potentially difficult
airways who undergo Caesarean section, as it allows
gradual titration and slower onset of subarachnoid
blockage.20 This technique is still uncommonly used
because of various concerns including post-dural
puncture headache and neuraxial infection. Further
studies are required to assess whether it can assist
in the management of patients with conditions that
make neuraxial labour analgesia challenging.
Maintenance of neuraxial analgesia
Once an epidural catheter is placed, analgesia
can be maintained by intermittent top-ups,
continuous infusion, patient-controlled analgesia, or
programmed intermittent epidural boluses (PIEB).
Continuous infusion technique became popular
in the early 1980s. This delivery method reduced
the variability of analgesia during labour, especially
when high concentrations of local anaesthetics were
replaced by low concentrations with the addition of
a lipophilic opioid. Unfortunately, this modality does
not suit all patients despite many combinations of infusion rate, local anaesthetic concentration, and
additives having been investigated. Many patients
still require clinician-initiated top-ups or experience
unacceptable motor blockage.
Patient-controlled epidural analgesia
Patient-controlled epidural analgesia (PCEA) was
first described in 1988.21 Boluses of 4 to 8 mL of
epidural mixture are delivered on patient demand
with a lockout interval of 10 to 20 minutes. As labour
pain has highly variable intensity, and the character
of the pain often changes as it progresses, it makes
sense that patients may be the best managers of
their own pain relief. There is recent evidence that
genetic polymorphism may also affect the patient’s
labour progress and response to labour analgesia.
One example is the Mu opioid receptor gene
single-nucleotide polymorphism (OPRM1, A118G),
which is believed to be present in 30% of women
in labour and may affect the response to neuraxial
opioids.22 23 Administration of PCEA allows for
some self-titration. Over the past 20 years, PCEA
has been widely studied and the technique refined.
High-volume, dilute local anaesthetic solutions
with a continuous background infusion appear
to be the best PCEA regimen.24 The American
Society of Anesthesiologists practice guidelines
for obstetric anaesthesia advise that basal infusion
improves analgesia when provided as part of a PCEA
regimen.25 Studies have also shown that PCEA
requires less anaesthesia intervention, lower doses of
local anaesthetic, and produces less motor blockage
than continuous epidural infusion.26 27 Although
PCEA delivery devices tend to be more expensive
than continuous infusion pumps, the technique may
have important benefits. The optimum method of
administration requires communication with both
the midwife and the patient.
Computer-integrated patient-controlled
epidural analgesia
An alternative approach to determining the
background infusion rate during PCEA is the use of
a computer programme to automatically adjust the
background infusion rate according to the amount of
local anaesthetic used in the previous hour. A laptop
computer is connected to a PCEA pump. In theory,
a system that responds to a patient’s analgesic
requirements should improve efficacy while
minimising the amount of local anaesthetic used
for background infusions. Initial studies with this
system have been encouraging. In a study comparing
demand-only PCEA with computer-integrated
background infusion PCEA (CIPCEA), the CIPCEA
group had similar local anaesthetic consumption
but increased maternal satisfaction.28 Another
study found that CIPCEA reduced the incidence of breakthrough pain without increasing drug
consumption compared with continuous epidural
infusion.29 When CIPCEA was compared with PCEA
using fixed-rate continuous infusion, the CIPCEA
group had higher maternal satisfaction, whereas
local anaesthetic consumption, visual analogue pain
scores, and incidence of breakthrough pain were
similar between the two groups.30 Therefore, an
adjustable background infusion appears to increase
maternal satisfaction and may further reduce the
incidence of breakthrough pain without increasing
local anaesthetic consumption.
Programmed intermittent epidural boluses
Programmed intermittent epidural boluses is a novel
technology in which boluses of epidural mixture
are delivered at predetermined intervals. Improved
analgesia may be offered by PIEB, as the local
anaesthetic is administered in boluses under high
driving pressure, which can disperse the solution
more widely than continuous infusion31 with multi-orifice
catheters.32 A system has been developed
in which a computer delivers both automated and
manual boluses. The authors demonstrated that
this ‘programmed intermittent mandatory epidural
bolus’ with a PCEA regimen provided advantages
over a PCEA plus background infusion regimen:
the former used less local anaesthetic dose, but
resulted in a higher maternal satisfaction and a
longer duration of analgesia. However, there was
no difference in the incidence of breakthrough
pain between the two groups.33 34 In 2012 and 2014,
respectively, Health Canada and the United States
Food and Drug Administration approved PIEB
combined with PCEA (CADD Solis Epidural Pump,
Smiths Medical, St Paul [MN], United States) for
clinical use.35 A 2013 systematic review investigating
PIEB for maintenance of labour analgesia that
included nine randomised controlled trials with
694 patients36 showed that the vast majority of
studies associated PIEB with decreased local
anaesthetic consumption, improved maternal
satisfaction scores, decreased instrumental delivery,
and lessened need for anaesthesia intervention. A
recent trial confirmed that reduced motor blockage
was associated with PIEB,37 although that study
could not identify other significant outcomes.
Ultrasound
Although ultrasound is widely used in the placement
of central venous catheters and peripheral nerve
blockage, it is less commonly used in neuraxial
analgesia for obstetric patients. It can be used either
before the procedure to study the site of needle
entry and the depth of the epidural space or for
real-time needle guidance (Fig 2). Although the
preprocedural use of ultrasound in normal pregnant mothers seems to have limited efficacy among both
experienced clinicians38 and trainees,39 some study
findings have suggested that it is a useful tool40 to
consider in obese patients41 or those with lumbar
spine problems. In 2008, the United Kingdom’s
National Institute for Health and Care Excellence
determined that sufficient evidence had been
published to support the routine use of ‘ultrasound to
facilitate the catheterisation of the epidural space’.42
In March 2016, the American Society of Regional
Anesthesia and Pain Medicine43 published the second
evidence-based medicine assessment of ultrasoundguided
regional anaesthesia to ‘enable practitioners
to make an informed evaluation regarding the role
of ultrasound-guided regional anaesthesia in their
practice’. A high-quality review article by Arzola
outlined the controversies, advantages, and practical
applications of preprocedural ultrasound in obstetric
patients.44
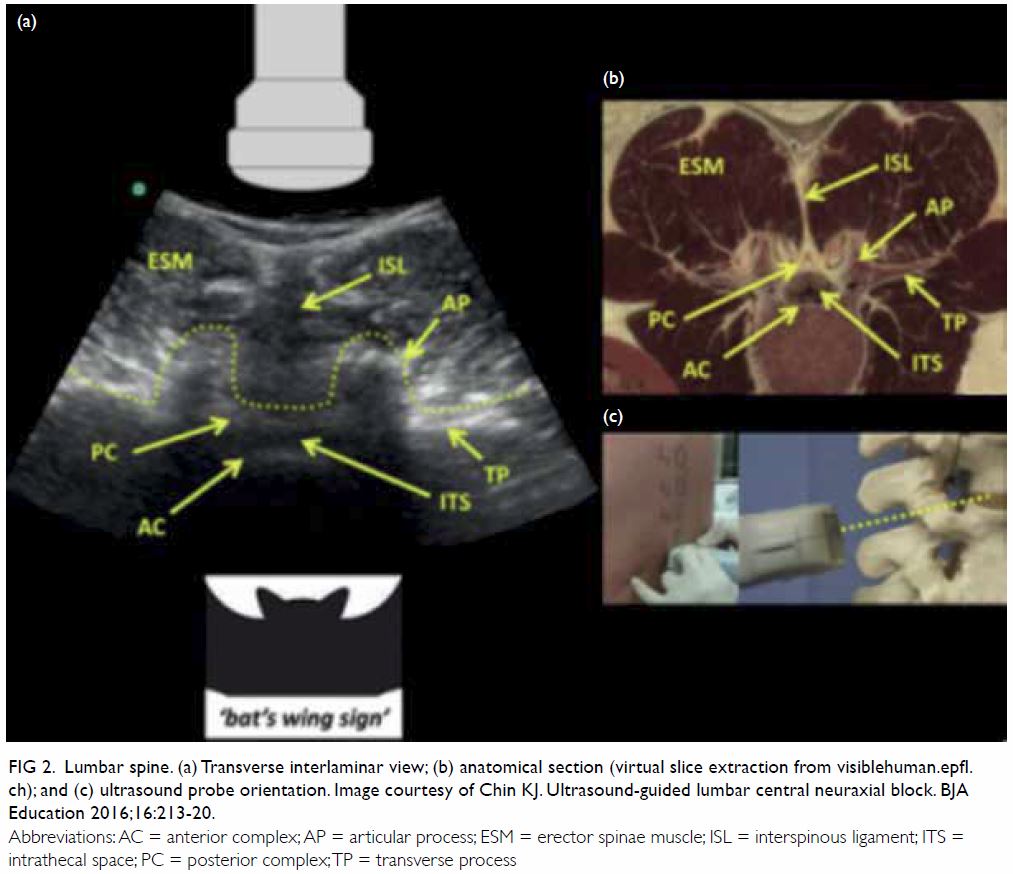
Figure 2. Lumbar spine. (a) Transverse interlaminar view; (b) anatomical section (virtual slice extraction from visiblehuman.epfl. ch); and (c) ultrasound probe orientation. Image courtesy of Chin KJ. Ultrasound-guided lumbar central neuraxial block. BJA Education 2016;16:213-20.
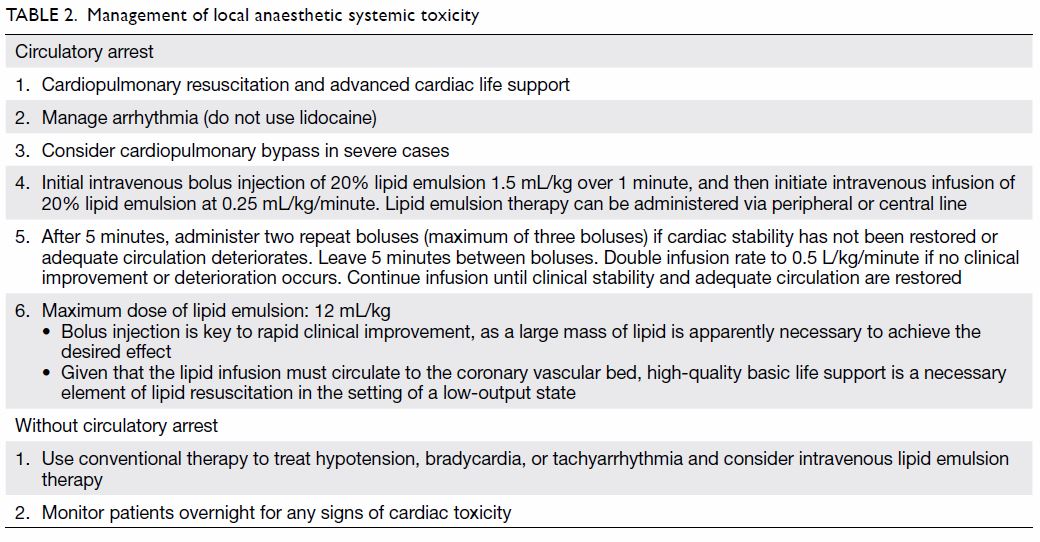
Intralipid infusion
Neuraxial analgesia is now also safer with the availability of intralipid as an antidote for local
anaesthetic toxicity.45 46 Intralipid binds with amide
local anaesthetic molecules in the plasma, thereby
decreasing the free fraction available to bind with
cardiac muscle. It has become widely adopted
as part of the resuscitation protocol for local
anaesthetic-caused systemic toxicity and should be
readily available in all delivery units where neuraxial
analgesia is practised. It is given intravenously by
boluses followed by continuous infusion according
to body weight (Table 2).
When should an epidural catheter
be sited?
Previous concerns that early epidural initiation
(when cervical dilatation <4 cm) would increase the
rate of instrumental delivery and Caesarean section
have been alleviated by more recent research.
Wong et al47 found that neuraxial analgesia in
early labour did not increase the rate of Caesarean
delivery but provided better analgesia and resulted
in a shorter duration of labour than systemic analgesia. The latest Cochrane review indicated that
there is abundant high-quality evidence that early
and late epidural initiation have similar effects on
all measured outcomes.48 The American College of
Obstetricians and Gynaecologists and the American
Society of Anesthesiologists49 have also jointly
emphasised that there is no need to wait until
cervical dilation has reached 4 to 5 cm and stated
that ‘maternal request is a sufficient indication for
pain relief in labour’.50 When delivery is imminent,
the decision to offer regional anaesthesia should
be individualised and depends on various factors
including a woman’s parity, fetal condition, and
whether a prolonged second stage is expected, such
as malposition of the fetus or macrosomia. The Royal
College of Anaesthetists recommends that the time
from epidural request to the anaesthetist attending
should not exceed 30 minutes, after which a second
anaesthetist should be available.51
When should epidural analgesia be
terminated?
There is insufficient evidence to support the
discontinuation of epidural analgesia late in labour
as a means to reduce adverse delivery outcomes.52
Doing so also increases the rate of inadequate pain
relief in the second stage of labour. A meta-analysis
of high-quality studies did not show significant
differences in outcomes with immediate and delayed
pushing in the second stage of labour.53
Other effects
The effects of neuraxial analgesia on successful
breastfeeding have been evaluated in several studies with controversial results. A recent
large, randomised, double-blind, controlled trial
showed that epidural solutions containing fentanyl
concentrations as high as 2 μg/mL did not affect
breastfeeding rates at 6 weeks postpartum.54 The
results correlated with those of another study
investigating women with previous breastfeeding
experience, as both studies showed no difference
in the breastfeeding rates at 6 weeks postpartum
between groups of women who did and did not
receive epidural analgesia.55 Therefore, factors other
than epidural and fentanyl administration can affect
the successful breastfeeding rate.
The association of maternal fever with
epidural analgesia has remained an area of clinical
and research interest.56 A 2016 expert panel defined
maternal fever as maternal temperature of ≥38°C
measured orally for two readings 30 minutes apart.57
Up to one third of mothers may be affected, and
the aetiology and prophylactic prevention are still
not well understood, although the local anaesthetic
used for epidural analgesia is a likely culprit. Sterile
inflammation and activation of inflammasomes
probably play a role,58 and this is an area of ongoing
research.59
Conclusions
Epidural analgesia remains the best method of
relieving pain during labour. Advances in technology
have made it even safer than before. In the absence of
any medical contra-indications, maternal request is
a sufficient indication to initiate epidural analgesia,
and if it is properly conducted, it can be considered
at any stage of labour without affecting the rate
of instrumental or Caesarean delivery. Future improvements may lie in preventing breakthrough
pain via interaction with various closed-loop
feedback drug delivery systems. Remifentanil-based
opioid techniques are becoming a popular alternative
if epidural is contra-indicated.
Author contributions
All authors contributed to the concept of study, drafting of
the manuscript, and critical revision of the manuscript for
important intellectual content. All authors had full access to
the data, contributed to the study, approved the final version
for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
As an editor of the journal, MG Irwin was not involved in the peer review process of the article. The other authors have no
conflicts of interest to disclose.
Acknowledgement
The authors thank Prof Ki-jinn Chin, Associate Professor,
Department of Anesthesia, Toronto Western Hospital,
University of Toronto for permission to use the image in
Figure 2.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
1. Labor S, Maguire S. The pain of labour. Rev Pain 2008;2:15-
9. Crossref
2. Rowlands S, Permezel M. Physiology of pain in labour.
Bailliere Clin Obstet Gynaecol 1998;12:347-62. Crossref
3. Wong CA. Advances in labor analgesia. Int J Womens
Health 2009;1:139-54. Crossref
4. Jones L, Othman M, Dowswell T, et al. Pain management
for women in labour: an overview of systematic reviews.
Cochrane Database Syst Rev 2012;(3):CD009234. Crossref
5. Carstoniu J, Levytam S, Norman P, Daley D, Katz J,
Sandler AN. Nitrous oxide in early labor safety and
analgesic efficacy assessed by a double-blind, placebo-controlled
study. Anesthesiology 1994;80:30-5. Crossref
6. Yeo ST, Holdcroft A, Yentis SM, Stewart A. Analgesia
with sevoflurane during labour: i. Determination of the
optimum concentration. Br J Anaesth 2007;98:105-9. Crossref
7. Yeo ST, Holdcroft A, Yentis SM, Stewart A, Bassett P.
Analgesia with sevoflurane during labour: ii. Sevoflurane
compared with Entonox for labour analgesia. Br J Anaesth
2007;98:110-5. Crossref
8. Ng TK, Cheng BC, Chan WS, Lam KK, Chan MT. A double-blind
randomised comparison of intravenous patient-controlled
remifentanil with intramuscular pethidine for
labour analgesia. Anaesthesia 2011;66:796-801. Crossref
9. Wilson MJ, MacArthur C, Hewitt CA, et al. Intravenous
remifentanil patient-controlled analgesia versus
intramuscular pethidine for pain relief in labour (RESPITE):
an open-label, multicentre, randomised controlled trial.
Lancet 2018;392:662-72. Crossref
10. Melber AA, Jelting Y, Huber M, et al. Remifentanil patient-controlled
analgesia in labour: six-year audit of outcome data of the RemiPCA SAFE Network (2010-2015). Int J
Obstet Anesth 2019;39:12-21.Crossref
11. Anim-Somuah M, Smyth RM, Cyna AM, Cuthbert A.
Epidural versus non-epidural or no analgesia for pain
management in labour. Cochrane Database Syst Rev
2018;(5):CD000331. Crossref
12. Sharma RM, Setlur R, Bhargava AK, Vardhan S. Walking
epidural: an effective method of labour pain relief. Med J
Armed Forces India 2007;63:44-6. Crossref
13. Comparative Obstetric Mobile Epidural Trial (COMET)
Study Group UK. Effect of low-dose mobile versus
traditional epidural techniques on mode of delivery: a
randomised controlled trial. Lancet 2001;358:19-23. Crossref
14. Sultan P, Murphy C, Halpern S, Carvalho B. The effect of
low concentrations versus high concentrations of local
anesthetics for labour analgesia on obstetric and anesthetic
outcomes: a meta-analysis. Can J Anaesth 2013;60:840-54. Crossref
15. Skupski DW, Abramovitz S, Samuels J, Pressimone V,
Kjaer K. Adverse effects of combined spinal-epidural
versus traditional epidural analgesia during labor. Int J
Gynecol Obstet 2009;106:242-5. Crossref
16. Norris MC, Fogel ST, Conway-Long C. Combined spinalepidural
versus epidural labor analgesia. Anesthesiology
2001;95:913-20. Crossref
17. Booth JM, Pan JC, Ross VH, Russell GB, Harris LC, Pan PH.
Combined spinal epidural technique for labor analgesia
does not delay recognition of epidural catheter failures:
a single-center retrospective cohort survival analysis.
Anesthesiology 2016;125:516-24. Crossref
18. Landau R, Ciliberto CF, Goodman SR, Kim-Lo SH, Smiley
RM. Complications with 25-gauge and 27-gauge Whitacre
needles during combined spinal-epidural analgesia in
labor. Int J Obstet Anesth 2001;10:168-71. Crossref
19. Simmons SW, Taghizadeh N, Dennis AT, Hughes D, Cyna AM.
Combined spinal-epidural versus epidural analgesia in
labour. Cochrane Database Syst Rev 2012;(10):CD003401. Crossref
20. Drasner K, Smiley R. Continuous spinal analgesia for labor
and delivery: a born-again technique? Anesthesiology
2008;108:184-6. Crossref
21. Gambling DR, Yu P, Cole C, McMorland GH, Palmer L. A
comparative study of patient controlled epidural analgesia
(PCEA) and continuous infusion epidural analgesia (CIEA)
during labour. Can J Anaesth 1988;35:249-54. Crossref
22. Landau R, Cahana A, Smiley RM, Antonarakis SE, Blouin JL.
Genetic variability of μ-opioid receptor in an obstetric
population. Anesthesiology 2004;100:1030-3. Crossref
23. Sia AT, Lim Y, Lim EC, et al. A118G single nucleotide
polymorphism of human μ-opioid receptor gene influences
pain perception and patient-controlled intravenous
morphine consumption after intrathecal morphine for
postcesarean analgesia. Anesthesiology 2008;109:520-6. Crossref
24. Halpern SH, Carvalho B. Patient-controlled epidural
analgesia for labor. Anesth Analg 2009;108:921-8. Crossref
25. American Society of Anesthesiologists Task Force on
Obstetric Anesthesia. Practice guidelines for obstetric
anesthesia: an updated report by the American Society
of Anesthesiologists Task Force on Obstetric Anesthesia.
Anesthesiology 2007;106:843-63.
26. van der Vyver M, Halpern S, Joseph G. Patient-controlled
epidural analgesia versus continuous infusion for labour
analgesia: a meta-analysis. Br J Anaesth 2002;89:459-65. Crossref
27. D’Angelo R. New techniques for labor analgesia: PCEA and
CSE. Clin Obstet Gynecol 2003;46:623-32. Crossref
28. Lim Y, Sia AT, Ocampo CE. Comparison of computer
integrated patient controlled epidural analgesia vs.
conventional patient controlled epidural analgesia for pain
relief in labour. Anaesthesia 2006;61:339-44. Crossref
29. Sia AT, Lim Y, Ocampo CE. Computer-integrated patientcontrolled
epidural analgesia: a preliminary study on a
novel approach of providing pain relief in labour. Singapore
Med J 2006;47:951-6.
30. Sng BL, Sia AT, Lim Y, Woo D, Ocampo C. Comparison of
computer-integrated patient-controlled epidural analgesia
and patient-controlled epidural analgesia with a basal
infusion for labour and delivery. Anaesth Intensive Care
2009;37:46-53. Crossref
31. Hogan Q. Distribution of solution in the epidural space:
examination by cryomicrotome section. Reg Anesth Pain
Med 2002;27:150-6. Crossref
32. Duncan LA, Fried MJ, Lee A, Wildsmith JA. Comparison of
continuous and intermittent administration of extradural
bupivacaine for analgesia after lower abdominal surgery. Br
J Anaesth 1998;80:7-10. Crossref
33. Sia AT, Lim Y, Ocampo C. A comparison of a basal
infusion with automated mandatory boluses in parturient-controlled
epidural analgesia during labor. Anesth Analg
2007;104:673-8. Crossref
34. Leo S, Sia AT. Maintaining labour epidural analgesia: what
is the best option? Curr Opin Anaesthesiol 2008;21:263-9. Crossref
35. Carvalho B, George RB, Cobb B, McKenzie C, Riley ET.
Implementation of programmed intermittent epidural
bolus for the maintenance of labor analgesia. Anesth Analg
2016;123:965-71. Crossref
36. George RB, Allen TK, Habib AS. Intermittent epidural
bolus compared with continuous epidural infusions for
labor analgesia: a systematic review and meta-analysis.
Anesth Analg 2013;116:133-44. Crossref
37. Ojo OA, Mehdiratta JE, Gamez BH, Hunting J, Habib AS.
Comparison of programmed intermittent epidural boluses
with continuous epidural infusion for the maintenance of
labor analgesia: a randomized, controlled, double-blind
study. Anesth Analg 2020;130:426-35. Crossref
38. Ansari T, Yousef A, El Gamassy A, Fayez M. Ultrasound-guided
spinal anaesthesia in obstetrics: is there an
advantage over the landmark technique in patients with
easily palpable spines? Int J Obstet Anesth 2014;23:213-6. Crossref
39. Arzola C, Mikhael R, Margarido C, Carvalho JC. Spinal
ultrasound versus palpation for epidural catheter insertion
in labour: a randomised controlled trial. Eur J Anaesthesiol
2015;32:499-505. Crossref
40. Perlas A, Chaparro LE, Chin KJ. Lumbar neuraxial
ultrasound for spinal and epidural anesthesia: a systematic
review and meta-analysis. Reg Anesth Pain Med
2016;41:251-60. Crossref
41. Creaney M, Mullane D, Casby C, Tan T. Ultrasound to
identify the lumbar space in women with impalpable bony
landmarks presenting for elective Caesarean delivery
under spinal anaesthesia: a randomised trial. Int J Obstet
Anesth 2016;28:12-6. Crossref
42. National Institute for Health and Care Excellence (NICE).
Interventional procedures guidance (IPG249): Ultrasound-guided
catheterisation of the epidural space. 23 Jan 2008.
43. Neal JM, Brull R, Horn JL, et al. The Second American Society of Regional Anesthesia and Pain Medicine
evidence-based medicine assessment of ultrasound-guided
regional anesthesia: executive summary. Reg Anesth Pain
Med 2016;41:181-94. Crossref
44. Arzola C. Preprocedure ultrasonography before
initiating a neuraxial anesthetic procedure. Anesth Analg
2017;124:712-3. Crossref
45. Kosh MC, Miller AD, Michels JE. Intravenous lipid
emulsion for treatment of local anesthetic toxicity. Ther
Clin Risk Manag 2010;6:449-51. Crossref
46. Weinberg GL. Lipid emulsion infusion: resuscitation for
local anesthetic and other drug overdose. Anesthesiology
2012;117:180-7. Crossref
47. Wong CA, Scavone BM, Peaceman AM, et al. The risk
of Cesarean delivery with neuraxial analgesia given early
versus late in labor. N Engl J Med 2005;352:655-65. Crossref
48. Sng BL, Leong WL, Zeng Y, et al. Early versus late initiation
of epidural analgesia for labour. Cochrane Database Syst
Rev 2014;(10):CD007238. Crossref
49. Lim G, Levine MD, Mascha EJ, Wasan AD. Labor pain,
analgesia, and postpartum depression: are we asking the
right questions? Anesth Analg 2020;130:610-4. Crossref
50. Rendon KL, Wheeler V. Epidural analgesia and risk
of Cesarean delivery. Am Fam Physician 2018 Nov
1;98:Online.
51. Chapter 9: Guidelines for the provision of anaesthesia
services (GPAS). Guidelines for the Provision of
Anaesthesia Services for an Obstetric Population. Royal
College of Anaesthetists; 2020: 9.
52. Torvaldsen S, Roberts CL, Bell JC, Raynes-Greenow CH.
Discontinuation of epidural analgesia late in labour for
reducing the adverse delivery outcomes associated with
epidural analgesia. Cochrane Database Syst Review
2004;(4):CD004457. Crossref
53. Tuuli MG, Frey HA, Odibo AO, Macones GA, Cahill AG.
Immediate compared with delayed pushing in the second
stage of labor: a systematic review and meta-analysis.
Obstet Gynecol 2012;120:660-8. Crossref
54. Lee AI, McCarthy RJ, Toledo P, Jones MJ, White N,
Wong CA. Epidural labor analgesia–fentanyl dose
and breastfeeding success: a randomized clinical trial.
Anesthesiology 2017;127:614-24. Crossref
55. Orbach-Zinger S, Landau R, Davis A, et al. The effect of
labor epidural analgesia on breastfeeding outcomes: a
prospective observational cohort study in a mixed-parity
cohort. Anesth Analg 2019;129:784-91. Crossref
56. Chan JJ, Dabas R, Han RN, Sng BL. Fever during labour
epidural analgesia. Trends in Anaesthesia and Crit Care
2018;20:21-5. Crossref
57. Higgins RD, Saade G, Polin RA, et al. Evaluation and
management of women and newborns with a maternal
diagnosis of chorioamnionitis: summary of a workshop.
Obstet Gynecol 2016;127:426-36. Crossref
58. Sultan P, David AL, Fernando R, Ackland GL. Inflammation
and epidural-related maternal fever: proposed mechanisms.
Anesth Analg 2016;122:1546-53. Crossref
59. Lim G, Facco FL, Nathan N, Waters JH, Wong CA,
Eltzschig HK. A review of the impact of obstetric anesthesia
on maternal and neonatal outcomes. Anesthesiology
2018;129:192-215. Crossref