Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORTS
Familial dysalbuminaemic hyperthyroxinaemia
with discordant thyroid function test results: two case reports
Nike KC Lau, MA(Cantab), MBChB (CUHK)1 †; Teresa KC Tsui, FHKCPath, FHKAM (Pathology)1;
Jeffrey SS Kwok, FHKCPath, FHKAM (Pathology)1; Kitty KT Cheung, MRCP, FHKAM (Medicine)2; CC Chow, FHKCP, FHKAM (Medicine)2; CK Yeung, FHKCP, FHKAM (Medicine)3; YP Yuen, FHKCPath, FHKAM (Pathology)1 ‡
1 Department of Chemical Pathology, Prince of Wales Hospital, Hong Kong
2 Department of Medicine and Therapeutics, Prince of Wales Hospital, Hong Kong
3 Department of Medicine, Tseung Kwan O Hospital, Hong Kong
† Currently affiliated with Chemical Pathology Laboratory, Department of Pathology, Princess Margaret Hospital, Hong Kong
‡ Currently affiliated with Department of Pathology, Hong Kong Children's Hospital, Kowloon Bay, Hong Kong
Corresponding author: Dr Nike KC Lau (lkc416@ha.org.hk)
Case report
Discordant thyroid function test results are a
commonly encountered diagnostic challenge. When
the inverse log-linear relationship between thyroid
stimulating hormone (TSH) and free thyroxine (fT4)
is absent, a wide range of differential diagnoses should
be considered. This requires the investigatory efforts
of endocrinologists and chemical pathologists.
We report the diagnostic journey of two Chinese
patients with discordant thyroid function test due
to familial dysalbuminaemic hyperthyroxinaemia
(FDH) [OMIM #615999].
Patient 1 was a woman with no known family
history of thyroid disorder. In 2003, she presented
at age 49 years with palpitations and weight loss
and was diagnosed with thyrotoxicosis (laboratory
test results not available). She was prescribed an
antithyroid drug for 18 months and her disease
was in apparent remission. She presented again in
December 2009, at age 55 years, with weight loss
and anxiety. Total thyroxine tested in the private
sector was elevated at 186.4 nmol/L (reference
interval 58-155 nmol/L; platform not specified).
She was commenced on carbimazole in view of her
“relapse” and referred to endocrinology clinic for
further management in March 2010. Her first paired
TSH and fT4 (Roche Elecsys) results in April 2011
during carbimazole therapy revealed a discordant
pattern with a normal TSH of 1.90 mIU/L and an
elevated fT4 at 23.5 pmol/L (Table 1). Due to the
apparent “persistent thyrotoxicosis”, she underwent
radioactive iodine (RAI) thyroid ablation in May
2011, and carbimazole was stopped after her fT4 was
“normalised”. Monitoring with fT4 only, her disease
appeared to be in remission. Nonetheless her next
paired thyroid function test in May 2013 showed
elevated TSH 41.8 mIU/L and low fT4 11.9 pmol/L,
consistent with frank post-RAI hypothyroidism. As a result, she was prescribed thyroxine replacement
and her TSH was normalised (3.28 mIU/L) by June
2014. She was subsequently followed up in the
general outpatient clinic. She was referred again to
the endocrine clinic in June 2016 because both TSH
and fT4 were elevated despite good compliance
with thyroxine treatment. Withholding thyroxine
replacement led to elevation of TSH (19.8 mIU/L)
while fT4 (15.0 pmol/L) and free triiodothyronine
(3.71 pmol/L) were within their respective reference
intervals. Chemical pathologists were consulted
for discordant thyroid function test results. This
specimen was also tested using the Abbott Architect
platform and showed similar thyroid function test
results. Considering her TSH levels were never
suppressed, TSH-dependent thyrotoxicosis was
suspected although her serum alpha-subunit level
was normal. She was then referred for genetic
testing.
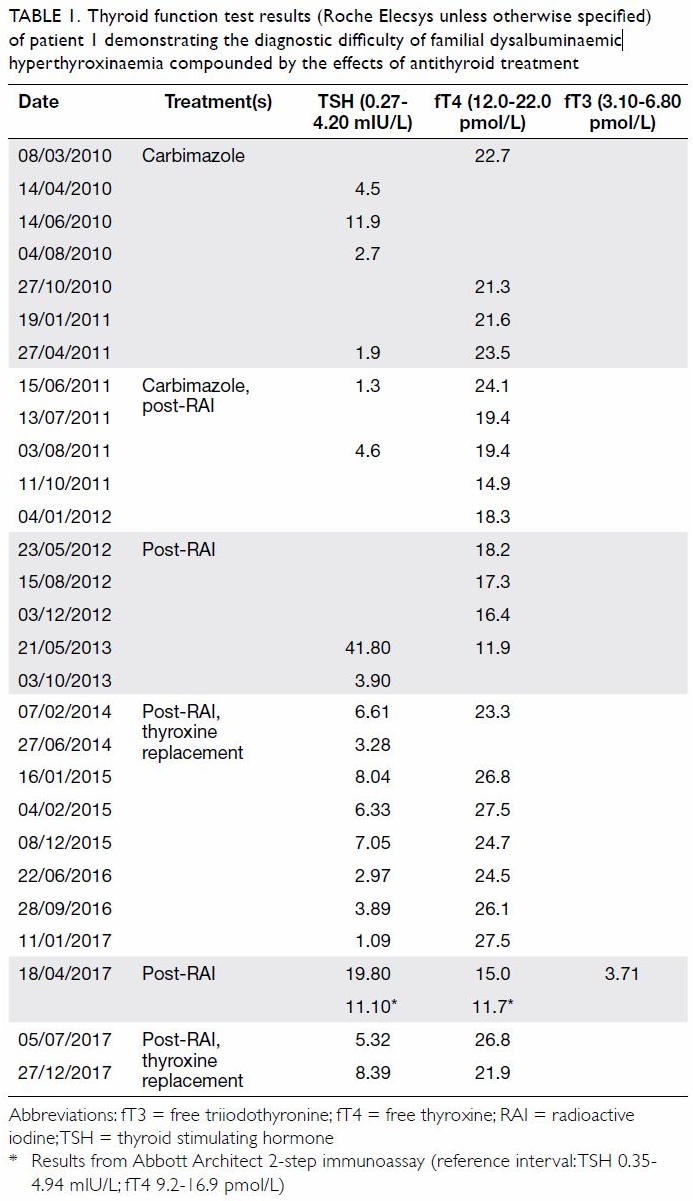
Table 1. Thyroid function test results (Roche Elecsys unless otherwise specified) of patient 1 demonstrating the diagnostic difficulty of familial dysalbuminaemic hyperthyroxinaemia compounded by the effects of antithyroid treatment
Patient 2 was a 34-year-old man who, in
February 2004, presented with unintentional weight
loss. His thyroid function test revealed normal
TSH but elevated fT4 (laboratory test results not
available). At follow-up examination, the patient’s
TSH was re-checked (1.58 mIU/L; Roche Elecsys)
but not his fT4, and test results for antithyroglobulin
and antimicrosomal antibodies were negative. In
view of such normal results, no further follow-up
was arranged until the patient had another
thyroid function test on Beckman Coulter Access.
This was tested during a preoperative assessment
in February 2017 and revealed an elevated fT4
(36.3 pmol/L) with a non-suppressed TSH
(1.29 mIU/L). He was referred to the endocrinology
clinic for further assessment. The patient reported
a family history of thyroid disease: his mother had
thyrotoxicosis treated with RAI, a maternal uncle
had an unspecified thyroid illness, and an elder brother demonstrated a similar thyroid function test
pattern. After discussion with chemical pathologists,
subsequent samples were tested on other platforms
and showed variable elevation of fT4 (Table 2).
Among the four platforms, the degree of elevation in
fT4 was greatest on Beckman Coulter Access (209%-224% of upper reference limit), followed by Roche
Elecsys (155%). Siemens ADVIA Centaur (101%),
and Abbott Architect (105%). The results from all
four platforms were above the upper reference limit.
Free triiodothyronine was also elevated on Beckman
Coulter Access (108%), Abbott Architect (107%) and
Siemens ADVIA Centaur (103%) platforms, although
to a lesser degree. In view of the non-suppressed
TSH level, the possibility of a TSH-secreting tumour
was considered. Magnetic resonance imaging
showed a 2-mm hypo-enhancing anterior pituitary
lesion. Biochemical investigations showed normal
serum alpha-subunit level and thyrotropin releasing
hormone stimulation test. The patient was referred
for genetic testing.

Table 2. Thyroid function test results of patient 2 on various platforms, without interfering effects of antithyroid treatment
Genetic testing for the THRB gene (OMIM
*190160) was performed in both patients, in view
of possible resistance to thyroid hormone syndrome
(OMIM #188570). No pathogenic variant was
detected by polymerase chain reaction and Sanger
sequencing. Further testing in both patients for FDH
targeting exon 7 of the ALB gene (OMIM *103600;
Refseq NG_009291.1/NM_000477.6/NP_000468.1)
showed heterozygous c.725G>A p.(Arg242His), a
reported pathogenic variant in Chinese.1
Discussion
Familial dysalbuminaemic hyperthyroxinaemia is an
autosomal dominant condition caused by variants of
albumin, the gene product of ALB.2 The prevalence
of FDH has been estimated to be 0.01% in Caucasian
populations but much higher (1.0%-1.8%) in Hispanic
populations.3 The prevalence is uncertain in East
Asian populations. The variant found in our patients,
c.725G>A, has an allele frequency of 0.005437% (1
in 18 392) in East Asian populations, according to
the Genome Aggregation Database. However, FDH is less commonly observed than expected in Hong
Kong. The under-recognition of FDH may be due
to its asymptomatic nature, and the use of TSH
without fT4 for screening of thyroid dysfunction.
Our patients were identified only because of their
apparent thyrotoxic symptoms and testing of fT4
on an affected platform. Although no treatment is
required for FDH, it remains an important entity
to recognise due to the potentially devastating
complications of inappropriate treatment such
as iatrogenic hypothyroidism as in patient 1, and
adverse effects of antithyroid medications such
as agranulocytosis. Family cascade screening
and counselling should be considered to prevent
undesirable misinterpretation and unnecessary
treatment of other affected family members. It is
strongly discouraged to treat either TSH or fT4
blindly, as is sometimes observed in clinical practice.
Once these patients are treated with antithyroid
drugs, often with only fT4 used for monitoring,
there is an added risk to the development of frank
hypothyroidism as the actual fT4 level is lower than
the assayed value.
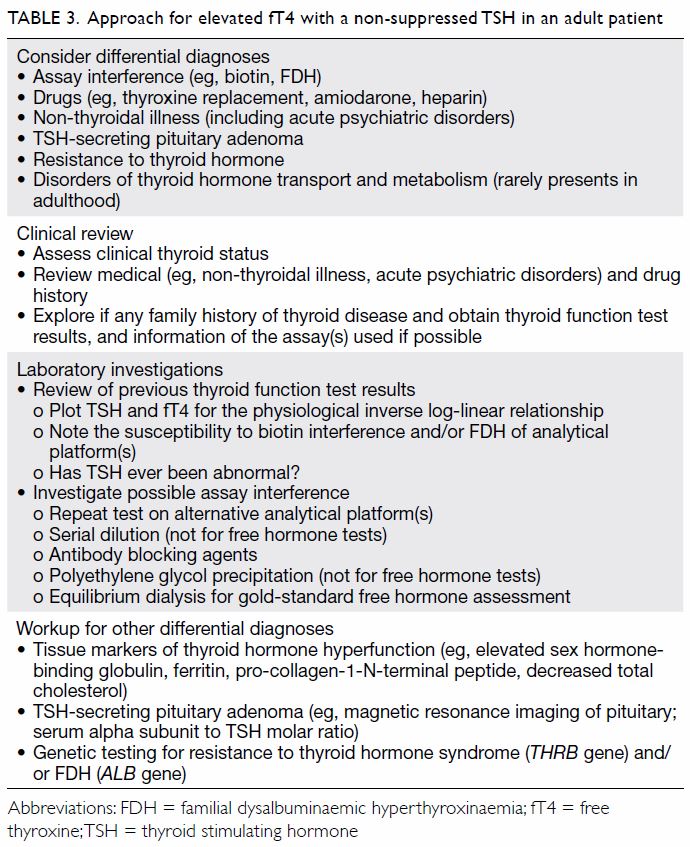
Here we discuss the approach to an elevated
fT4 with a non-suppressed TSH in adults, which
often requires the concerted efforts of a chemical
pathologist and endocrinologist (Table 3). For a
more comprehensive review of the general approach
to discordant thyroid function test, the reader is
advised to consult the excellent review by Koulouri
et al.3 Possible differential diagnoses of an elevated
fT4 with a non-suppressed TSH in an adult patient
include assay interference (including FDH), drugs
(such as thyroxine replacement, amiodarone,
heparin), non-thyroidal illness (including acute
psychiatric disorders), TSH-secreting pituitary
adenoma, and resistance to thyroid hormone.3
First, clinical correlation of thyroid status with
other clinical signs is essential in the interpretation
of such thyroid function pattern. It would be sensible
to exclude conditions that can be examined by a
clinical or drug history, such as the effects of drug
therapy, acute psychiatric episodes or non-thyroidal
illness. A family history of thyroid disorder should
be explored, because it may suggest an inherited
condition, such as in patient 2.
Second, any previous thyroid function results
should be reviewed. In patients with thyroid
function test results incompatible with clinical
features, at least one paired TSH and fT4 and/or free triiodothyronine should be performed to
better detect discordant results. Patients who have
previously shown a normal thyroid function pattern
are less likely to have inherited conditions such as
resistance to thyroid hormone or FDH. For FDH,
it is important to note the assay platform used
(hinted by the reference intervals provided), as
a change of assay from one that is less affected by FDH to one that is prone to interference in FDH
may give the clinician a false sense of an acquired
condition. Moreover, the TSH level in patients with
FDH should be normal unless due to thyroid-related
treatment, or genuine pituitary or thyroid disease. It
is often useful to plot the TSH and fT4 values and
try to observe the reciprocal relationship between
them. In FDH patients, such a relationship should be
largely preserved even after antithyroid treatment,
despite an upward shift in fT4 (Fig). This suggests
the presence of a functional negative feedback
system with the hypothalamus-pituitary-thyroid
axis and is less likely to be a case of interference.
Thyroxine replacement may further complicate the
thyroid function test pattern if thyroxine is taken
shortly before blood taking, although the usual
thyroid function test requested for patients receiving
thyroxine replacement is only TSH.
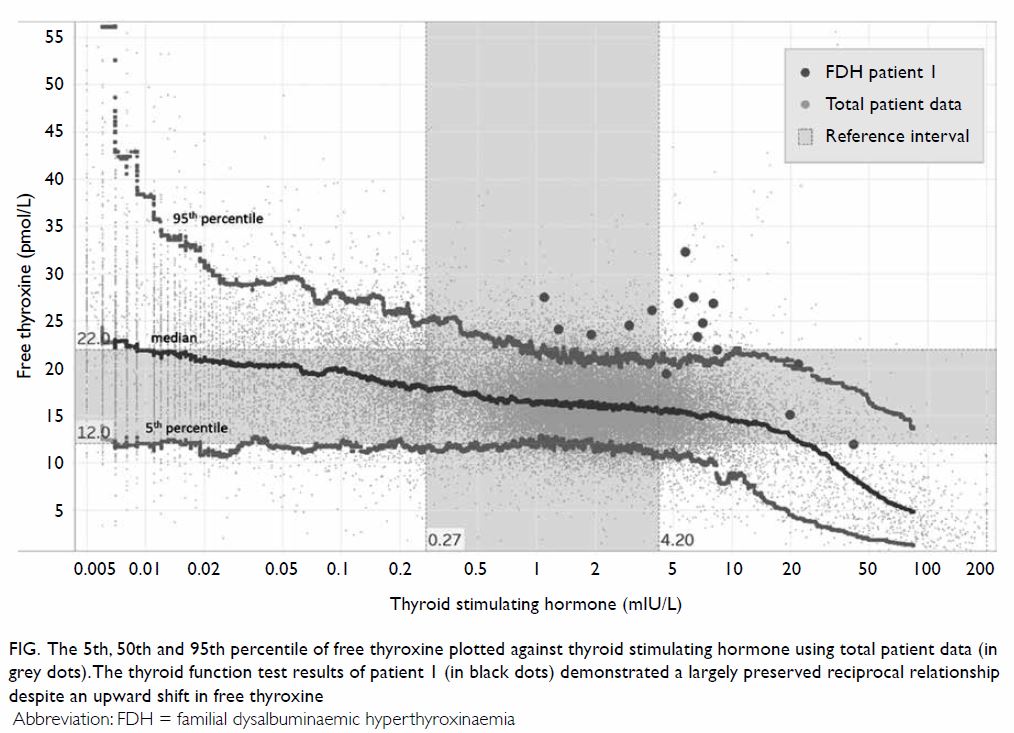
Figure. The 5th, 50th and 95th percentile of free thyroxine plotted against thyroid stimulating hormone using total patient data (in grey dots). The thyroid function test results of patient 1 (in black dots) demonstrated a largely preserved reciprocal relationship despite an upward shift in free thyroxine
Third, further investigations for possible assay
interference should be discussed with chemical
pathologists. In most clinical laboratories, thyroid
function tests are performed using automated
immunoassays that are prone to interference, for example by macro-TSH, biotin, anti-streptavidin
antibodies, anti-ruthenium antibodies, thyroid
hormone autoantibodies or heterophilic antibodies.
Further testing such as assay comparison, serial
dilution, antibody blocking agents, and polyethylene
glycol precipitation may be considered to provide
useful clues for possible interference.4 Often either
the TSH or fT4 assay is affected. Nonetheless, in
some cases such as biotin interference, both TSH
and fT4 can be affected if they both use the biotin-streptavidin-based separation method in the assay.
In patients with FDH, due to an increased binding
affinity of thyroxine to albumin, total thyroxine is
elevated, and to a lesser extent total triiodothyronine,
although fT4 measured using equilibrium dialysis,
which only minimally disrupts the equilibrium
between the free and the bound portion of thyroxine,
should be normal.4 Nevertheless, the presence
of this albumin variant has been shown to lead to
overestimation of fT4 levels in Roche Elecsys and
Siemens Immulite (competitive one-step assays),
whereas Abbott Architect (two-step assay) is less
affected, because the thyroxine analogue does
not come into contact with the variant albumin.5
Beckman Coulter Access, a two-step assay, is an
exception as it also shows significant overestimation
of fT4. The practical implication is that when FDH is suspected, one should examine the type of fT4 assay
used, and repeat testing on a less affected platform.
Apart from the usual investigations for TSH-secreting
pituitary adenoma such as magnetic
resonance imaging of the pituitary and serum alpha
subunit to TSH molar ratio, or genetic testing for
resistance to thyroid hormone, further biochemical
testing of the thyroid status of the patient should
be considered. Tissue markers of thyroid hormone
hyperfunction such as increased sex hormone-binding
globulin, ferritin, pro-collagen-1-N-terminal
peptide, and decreased cholesterol may
provide additional evidence of a thyrotoxic state.6
The absence of these may suggest resistance to
thyroid hormone, or assay interference. Further
investigations such as serum thyroglobulin, thyroid
antibodies (including anti-TSH receptor antibodies),
thyroid scan, and ultrasonography may provide
additional clues to the thyroid status in difficult
cases. However, their use should be discussed with
endocrinologists or relevant specialists to ensure
optimal test utilisation and interpretation.
In summary, although both our patients
ultimately received the correct diagnosis of FDH
after collaboration between endocrinologists and
chemical pathologists, the complications associated
with misinterpretation and inappropriate treatment illustrate the importance of a good understanding
of the analytical pitfalls of thyroid function test
assays and proper follow-up investigations and
management of discordant thyroid function test.
Clinicians should be aware of the approach to
discordant thyroid function, especially those who
treat thyroid conditions.
Author contributions
Concept or design: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: NKC Lau, TKC Tsui, JSS Kwok.
Drafting of the article: NKC Lau, TKC Tsui, JSS Kwok.
Critical revision for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: NKC Lau, TKC Tsui, JSS Kwok.
Drafting of the article: NKC Lau, TKC Tsui, JSS Kwok.
Critical revision for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
We would like to thank all laboratory and clinical staff who assisted in the cross-platform testing and management of the
patients.
Funding/support
This case report received no specific grant from any funding
agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Informed consent was obtained from all patients.
References
1. Tiu SC, Choi KL, Shek CC, Lau TC. A Chinese family
with familial dysalbuminaemic hyperthyroxinaemia. Hong
Kong Med J 2003;9:464-7.
2. Kragh-Hansen U, Galliano M, Minchiotti L. Clinical, genetic,
and protein structural aspects of familial dysalbuminemic
hyperthyroxinemia and hypertriiodothyroninemia. Front
Endocrinol (Lausanne) 2017;8:297. Crossref
3. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M.
Pitfalls in the measurement and interpretation of thyroid
function tests. Best Pract Res Clin Endocrinol Metab
2013;27:745-62. Crossref
4. Favresse J, Burlacu MC, Maiter D, Gruson D. Interferences
with thyroid function immunoassays: clinical implications
and detection algorithm. Endocr Rev 2018;39:830-50. Crossref
5. Cartwright D, O’Shea P, Rajanayagam O, et al. Familial
dysalbuminemic hyperthyroxinemia: a persistent
diagnostic challenge. Clin Chem 2009;55:1044-6. Crossref
6. Singh BK, Yen PM. A clinician’s guide to understanding
resistance to thyroid hormone due to receptor mutations
in the TRα and TRβ isoforms. Clin Diabetes Endocrinol
2017;3:8. Crossref