Hong Kong Med J 2020 Feb;26(1):27–34 | Epub 22 Jan 2020
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Association between beta-blocker use and obesity in
Hong Kong Chinese elders: a post-hoc analysis
KL Leung, BPharm, BSc1; Winnie Fong,
BPharm1; Ben Freedman, MB, BS, PhD2; Beata Bajorek,
PhD, BPharm3; Vivian WY Lee, PharmD, BCPS4
1 School of Pharmacy,
Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong
Kong
2 Heart Research Institute, Charles
Perkins Centre, University of Sydney, Sydney, Australia
3 Graduate School of Health, University
of Technology Sydney, Sydney, Australia
4 Centre for Learning Enhancement And
Research, The Chinese University of Hong Kong, Shatin, Hong Kong
Corresponding author: Prof Vivian WY Lee (vivianlee@cuhk.edu.hk)
Abstract
Introduction: Studies of
Caucasian populations have shown that beta-blockers may exacerbate
weight gain, a risk factor for many chronic diseases. Still,
beta-blockers are the most prescribed antihypertensives in the Chinese
population in Hong Kong. We aimed to explore the association between
beta-blocker use, hypertension, and weight status of this population.
Methods: A post-hoc analysis regarding body mass index (BMI) and the use of beta-blockers was performed based on the medication profile of community-dwelling older adults. Participants’ BMI, hypertension diagnosis, name, dose, frequency, route of administration of beta-blockers, and other drugs that may alter body weight were recorded.
Results: Of 1053 Chinese individuals aged ≥65 years (mean age 76.9±7.2 years, 80% female) from 32 elderly centres in Hong Kong, 18% (185/1053) of them consumed beta-blockers. That group also had a significantly larger proportion of obese individuals (45.9% vs 32.1%, P=0.002). After adjusting for other weight-altering drugs, beta-blockers remained a significant predictor of overweight and obesity (P=0.001). As the hypertensive population had significantly higher BMI than the normotensive population (24.3±3.6 vs 22.9±3.5, P<0.001), a sub-analysis on those with hypertension diagnosis confirmed that only the hypertensive population taking atenolol had a significantly larger population
of obese individuals (BMI ≥25) compared with those who took metoprolol (58.9% vs 38.5%, P=0.03) and those who did not take any beta-blockers (58.9% vs 38.4%, P=0.007).
Conclusions: Our findings taken together with other guideline reservations cast doubt on whether beta-blockers, particularly atenolol, should be the major drug prescribed to older adults with hypertension.
New knowledge added by this study
- Beta-blocker consumption is associated with obesity in Chinese older adults.
- Hypertensive population taking atenolol had the largest portion of obesity.
- Strong and unique association of obesity and atenolol usage.
- Healthcare professionals should be more vigilant concerning initiation of therapy for hypertension and ongoing surveillance of weight, such as carefully assessing baseline characteristics (including both body mass index and blood pressure status) before prescribing a beta-blocker, and regular monitoring of both parameters in hypertension treatment, particularly for patients with obesity and those who have not yet become obese if beta-blockers are prescribed.
Introduction
Hypertension is highly prevalent and a key risk
factor for cardiovascular disease.1
Less well recognised by patients and health professionals alike is that
some of the pharmacotherapies used to treat hypertension may adversely
impact other cardiovascular risk factors by causing weight gain. More
specifically, the weight gain effects of beta-adrenergic antagonists
(beta-blockers) have been highlighted by many studies of Caucasian
patients.2 3 4 5 Many guidelines no longer list beta-blockers as
first-line antihypertensives.6 7 8
However, the 2018 hypertension guidelines published by the European
Society of Cardiology and the European Society of Hypertension,9 the joint statement published in 2012 by the European
Society of Hypertension and the European Association for the Study of
Obesity,10 and a position paper of
The Obesity Society and the American Society of Hypertension11 still advocate the use of beta-blockers in patients
with both hypertension and obesity because beta-blockade is more effective
in lowering blood pressure (BP) in patients with obesity than in patients
who are thinner.12 In Hong Kong,
two recent large database studies found that beta-blockers are still the
most commonly prescribed antihypertensives, although they are used
relatively less in younger patients aged <55 years.13 14
Furthermore, only 4% of Hong Kong patients have their antihypertensive
treatment upgraded by changing from beta-blockers to firstline agents.15
The generally increasing prevalence of obesity
among older adults is an important factor in drug-induced weight gain.16 Studies have shown that obesity
in older people is associated with functional impairment and co-morbidity,
including hypertension, type 2 diabetes, coronary heart disease, heart
failure, and dementia.17 18 19 These
chronic conditions, for which being overweight is a risk factor, are also
worryingly increasing in prevalence.20
21 22
This renders weight management in older persons an important health issue.
To date, there has been a lack of research to
confirm the weight gain effects of beta-blockers in non-Caucasian
populations, and this is particularly germane because so many older Chinese
patients with hypertension are still receiving beta-blocker therapy.
The objective of this study was to explore the
association between beta-blocker use, hypertension, and overweight/obesity
in a cohort of older Chinese people. The specific objectives were to:
(1) identify the proportion of patients prescribed beta-blockers; (2)
compare the body mass index (BMI) of beta-blocker users with non-users;
and (3) compare the effects of different beta-blockers on BMI.
Methods
Study design
A post-hoc analysis was undertaken using an
existing dataset comprising the medication profiles of a cohort of
community-dwelling older adults in Hong Kong. The data were originally collected
(July to August in 2016) for a primary study seeking to explore the
relationship between diet and the prevalence of atrial fibrillation.23 The study was approved by the Survey and Behavioural
Research Ethics Committee of The Chinese University of Hong Kong and was
conducted in accordance with the Declaration of Helsinki. Written informed
consent was obtained from all study participants. Confidentiality
agreement forms were signed by all data collectors. STROBE reporting
guidelines were implemented in this manuscript.
Study population
The original study cohort comprised 1665 people
attending one of 32 neighbourhood elderly recreational community centres
in Hong Kong. The inclusion criteria included those who were Hong Kong
Chinese residents, aged ≥65 years, living in the community, and able to
speak and understand Cantonese. The exclusion criteria included those with
terminal health conditions and/or significant cognitive impairment that
would preclude participation because of communication barriers (eg, severe
mental illness, dementia).
Data collection
All primary study data were collected during a
summer community outreach programme—a territory-wide medical outreach
service in Hong Kong provided by volunteer students from the Faculty of
Medicine, The Chinese University of Hong Kong. The data were originally
collected via face-to-face interviews with the participants in Cantonese
and recorded on paper-based questionnaires by trained volunteer students.
At each outreach visit, participants’ body weight,
height, and BP were measured, BMI calculated, medication profile
extracted, and demographic data recorded. Calculated BMIs were compared
with the Asian BMI classification set by the World Health Organization, in
which BMI ≥23 and ≥25 are considered as overweight and obese,
respectively.24 A self-reported
diagnosis of hypertension and diabetes was recorded and verified against
the patient’s current medication profile. The self-reported diagnosis of
hypertension was the only evidence to determine whether the patient had
hypertension or not. As part of the logistics and service provided by our
outreach, BP was also measured using an Omron HEM-7011TM
electronic blood pressure monitor (Omron Healthcare, Kyoto Japan), which
has an “A/A” performance classification under British Hypertension Society
criteria (ie, indicating that at least 80% and 95% of readings are within
an absolute difference of 5 to 10 mm Hg from each other, respectively25). The readings were compared against 2017 American
Heart Association guidelines,6 but
such readings were not used to diagnose hypertension. To ensure accuracy,
for each patient, the BP measurements were repeated after 10 minutes of
rest when the first reading was found to be elevated (ie, BP >120/80
mm Hg). When a beta-blocker had been prescribed, the name, dose, frequency,
and route of administration of the agent were additionally recorded. The
consumption of other drugs known to alter body weight was also recorded
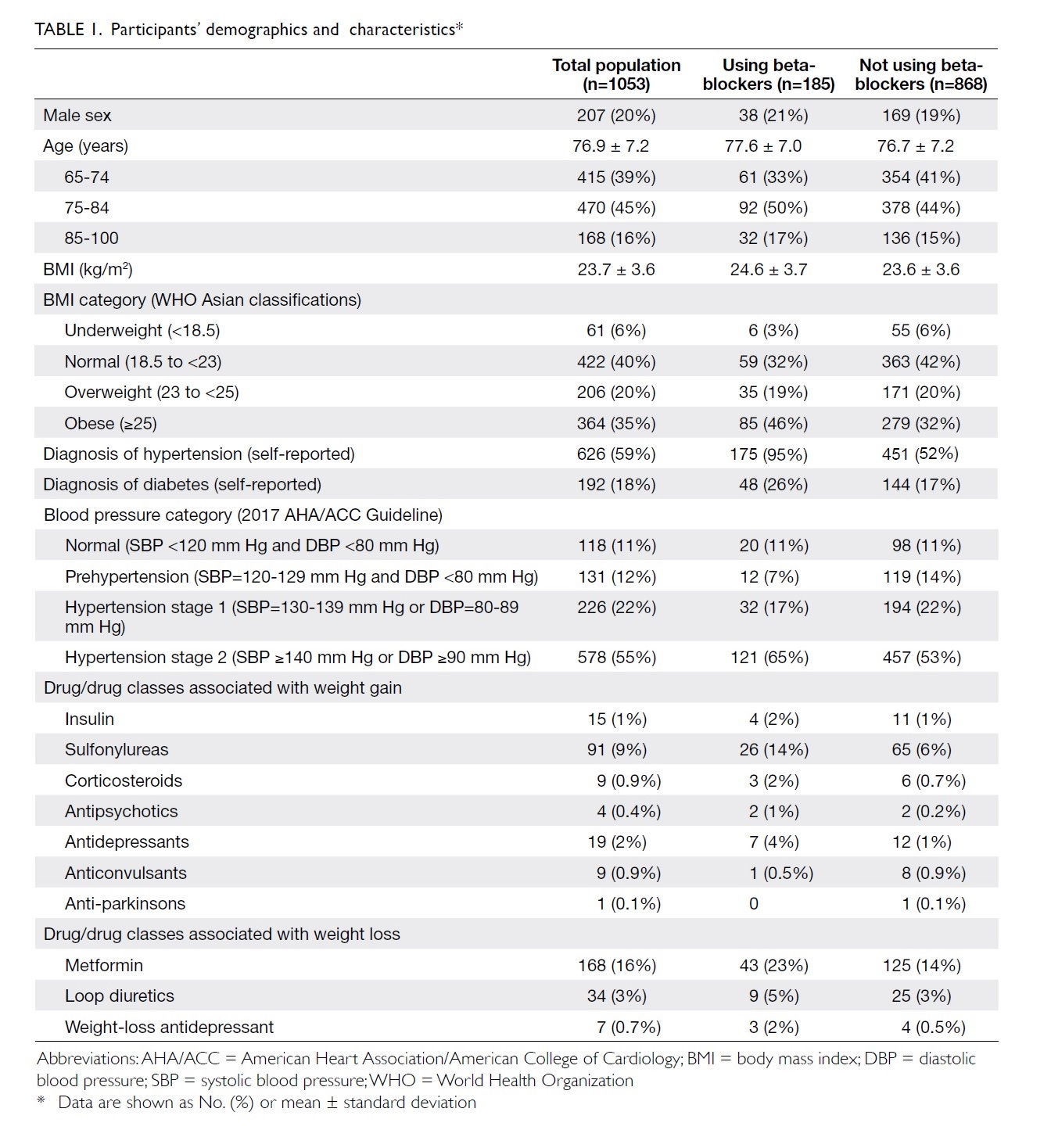
and listed in Table 1.
Statistical analysis
Data handling (data entry, verification, and
analysis) was computerised using SPSS (Windows version 23.0; IBM Corp,
Armonk [NY], United States). Only the patients with complete drug profiles
and all variables recorded were included. Any incomplete drug profile or
missing variables (eg, BMI) were considered missing data and were not
included in the analysis. Independent samples t tests were used to
compare BMI values between people taking different beta-blockers. Binary
logistic regression was used to verify the contributions of various drug
classes and co-morbidities on a stratified binary BMI parameter (normal
and underweight vs overweight and obesity). Chi squared tests were used to
detect any significant differences in the proportion of patients with high
BMI across different types of beta-blockers. A P value of <0.05 was
considered statistically significant.
Results
Participant characteristics
Among the 1665 individuals participating in the
summer community outreach programme, data pertaining to 1053 were included
in the analysis after screening against the inclusion and exclusion
criteria. The participants’ demographics and characteristics are shown in
Table 1. People taking beta-blockers were generally
heavier (mean BMI 24.6±3.7 vs 23.6±3.6, P<0.001). From the perspective
of BP measurement readings, around 80% of the participants (804/1053) had
elevated BP measurements during the outreach visit; of these, one third
(286/804) had no history of hypertension and were not on any medications
likely to be used for treating hypertension. The remaining two thirds
(518/804) self-reported having hypertension. From the perspective of
self-reported hypertension, 81.9% of those with self-reported hypertension
(513/626) had hypertensive readings, and only 6% (40/626) had normotensive
readings; the remaining ones had borderline hypertensive readings (12%,
73/626).
Overall, 185 (18%) of the 1053 participants were
using one beta-blocker. Among the range of beta-blockers used, atenolol
and metoprolol were the most frequently prescribed (40% and 53%,
respectively), followed by propranolol (5%) and bisoprolol (2%). Among the
beta-blocker users, the majority (95%, 175/185) had a self-reported
history of hypertension. In those participants with self-reported
hypertension who were not using beta-blockers (n=451), the main
antihypertensive agents prescribed were calcium channel blockers (71.3%),
angiotensin-converting enzyme inhibitors (22.3%), angiotensin receptor
blockers (12.1%), alpha-blockers (7.9%), methyldopa (4.2%), hydralazine
(1.0%), and reserpine (0.2%).
Body mass index and beta-blocker use
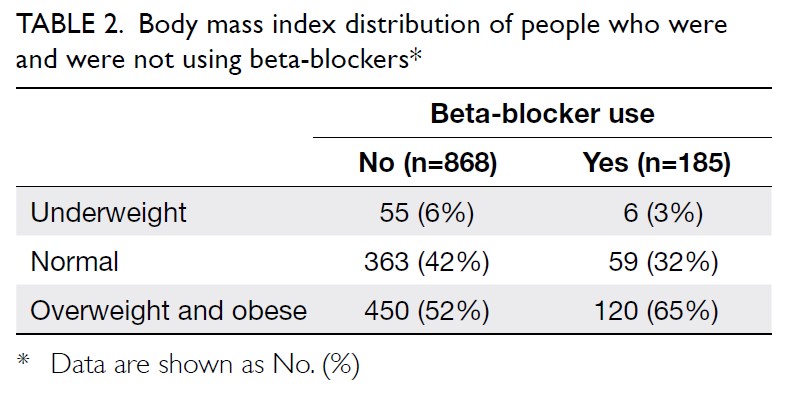
Overall, 54.1% (570/1053) of the participants
were overweight or obese. The summary of patients’ BMI with or without
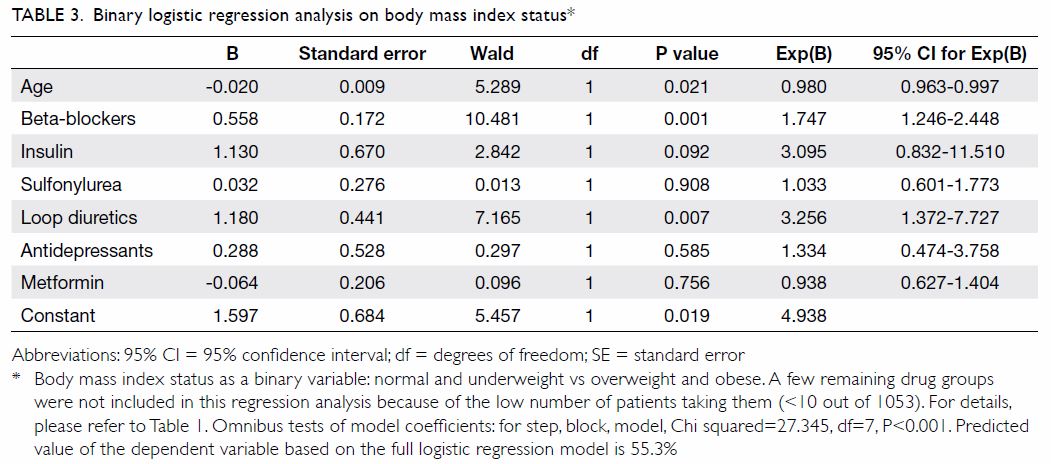
beta-blockers is summarised in Table 2. After adjusting for consumption of various
weight-altering drug classes, binary logistic regression showed that
beta-blockers were the only drug class that made a significant
contribution to stratified BMI status (ie, classification as overweight or
obese) [Table 3]. Among those prescribed beta-blockers
(n=185), a significantly higher proportion was either overweight or obese
compared with those who were not taking beta-blockers (n=868) [64.8% vs
51.8%, P=0.002; Table 2]. This difference is most evident when
comparing the proportion of patients with obesity across beta-blocker
users and non-users (45.9% vs 32.1%, respectively; P=0.002).
Participants deemed to have hypertension (based
solely on self-reported diagnosis of hypertension plus verification
against medication profile, but not on BP measurement during the outreach
service) had a significantly higher BMI than those who were normotensive
(mean BMIs: 24.3±3.6 vs 22.9±3.5, respectively; P<0.001). Although this
difference may not be clinically significant, it triggered further
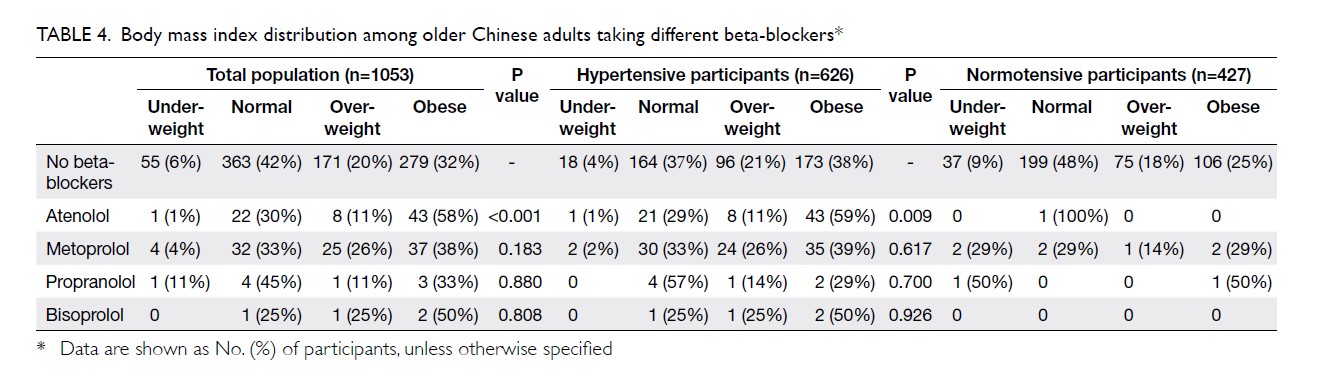
sub-analysis on participants with hypertension diagnosis. A sub-analysis on
the patients with self-reported diagnosis of hypertension was performed to
confirm the association between the use of beta-blockers and BMI (Table
4). Among these participants with self-reported diagnosis of
hypertension (n=626), a significantly higher proportion of patients with
obesity (BMI >25) was observed in those using atenolol compared with
those taking metoprolol (58.9% vs. 38.5%, P=0.031) or those who did not
use any beta-blockers (58.9% vs 38.4%, P=0.007). Those using atenolol had
a significantly higher BMI than those who did not use beta-blockers (mean
BMIs: 25.3±3.5 vs 24.1±3.6, P=0.01).
Binary logistic regression analysis found that loop
diuretics were associated with BMI reduction. However, concerning
mechanism and indication, loop diuretics function by enhancing salt and
water excretion and are clinically used to maintain euvolaemia or prevent
volume expansion.26 In other
words, it makes no significant contribution to alteration of dry body
weight, which is used for BMI determination and obesity evaluation.
Therefore, despite the above findings, loop diuretics were excluded from
our further analysis.
Discussion
Our study presents preliminary findings regarding
the potential real-world impact of beta-blockers on weight, noting the
high proportion of community-dwelling older adults using these agents.
We found a significant association between obesity/overweight with the use
of beta-blockers in older Chinese adults. The difference was largely driven by
the strong association between obesity and atenolol (rather than other
beta-blockers). This may have important ramifications on therapeutic
choice if the association is causal.
The potential mechanisms by which beta-blockers may
induce weight gain include reduction of total energy expenditure (by
5%-10%), which may involve (1) decreased resting energy expenditure; (2)
increased feelings of tiredness, with subsequent reduction of
non-exercise-associated thermogenesis; (3) inhibition of lipolysis; and
(4) enhancement of insulin resistance.27
Many studies of Caucasian populations have reported
the weight gain effects of beta-blockers, being associated with a mean
weight gain of 1.2 kg (range, -0.4 kg to 3.5 kg),27
which could explain our findings of 2.6 kg higher mean body weight in
people taking beta-blockers (mean body weight of people who did vs did not
take beta-blockers: 57.8±9.7 kg vs 55.2±9.8 kg, respectively; P=0.002
respectively), but we do not have longitudinal data to make pre-versus
post-drug commencement comparisons. Several studies have compared impact
on weight between selected beta-blockers and alternative antihypertensive
medications, ie, atenolol versus captopril,2
metoprolol versus thiazide diuretics,3
atenolol versus chlorthalidone,4
and atenolol versus nifedipine,5
with all reporting weight gain (or reduced weight loss) in the
beta-blocker treatment group. A long-term follow-up study also reported
sustained weight gain in a propranolol treatment group compared with a
placebo group.28
Despite the findings of previous studies, to date,
there have been no intraclass head-to-head studies in humans regarding
beta-blocker-induced weight gain. The two beta-blockers of note, atenolol
and metoprolol, both being β1-selective beta-blockers, seemingly have no
plausible cause that could account for such differences between them. Our
study suggests that atenolol and metoprolol (the two most commonly used
beta-blockers in Hong Kong13 14) may have different effects on weight. We found a
significantly higher BMI and a higher proportion of patients with obesity
in those taking atenolol compared with those who did not, especially among
those defined as being hypertensive. This difference in obesity was also
significant for the comparison with metoprolol. Patients taking metoprolol
did not show any significant difference from patients who were not on any
beta-blockers.
In our study, the mean difference in BMI between
those using atenolol and no beta-blockers was about 1.2. Converting the
mean BMI difference in a 60-kg woman reveals a mean body weight difference
of 3.1 kg. Whether such a degree of weight gain is clinically significant
in light of age-related weight gain is worthwhile to discuss. Indeed,
age-related weight gain is an important factor of concern. According to a
study of older Chinese adults, the median weight change from 20 years old
to baseline was 11.8 kg and 11.5 kg for men and women, respectively.29 Another 10-year follow-up study also reported that a
modest weight gain (2.5-5 kg) was not associated with an increase in
mortality.30 Therefore, a gain of
3.1 kg alone may not be clinically significant. Yet, such a modest weight
gain can be additive to physiological age-related weight gain and
contribute to obesity. While age-related weight gain may not be a
modifiable factor, the selection of pharmacotherapy is definitely one.
Particularly, the use of beta-blockers may not actually be the best
therapeutic option for hypertension management. Switching to other
first-line agents that have no weight gain effects would reduce the
possibilities to become obese.
Given our findings, it is important to note the
variable recommendations around the use of beta-blockers in hypertension
management. Although a number of international guidelines advocate the use
of beta-blockers in patients with both obesity and hypertension,9 10 11 the 2017 American Heart Association guideline
criticised atenolol for its inferior efficacy in treatment of
hypertension.6 Moreover, a
meta-analysis of atenolol versus other antihypertensive treatments also
reported higher overall and cardiovascular mortality and more frequent
strokes with atenolol treatment.31
Given these reservations about atenolol and our findings of higher
prevalence of obesity in those taking atenolol, it is uncertain whether
atenolol should continue to be the most-used drug for hypertension. This
is relevant to many parts of the world, including Hong Kong, where
atenolol appears to be the first-line therapy for hypertension in older
patients and may be associated with an adverse effect on weight and BMI.
To the best of our knowledge, this is the first
study to evaluate the association between BMI and beta-blocker use in a
Chinese population. Additionally, this is the first study highlighting the
intraclass differences between beta-blockers in terms of possible weight
gain effects (as illustrated by the proportion of obesity), specifically
in the hypertensive Chinese population. Therefore, our findings have
implications for local clinical practice given the high rate of use of
beta-blockers, particularly atenolol, in the older adults in Hong
Kong, despite guideline recommendations.
In considering this study’s findings, it is
important to acknowledge some of its limitations. First, given the
cross-sectional nature of the study, no temporal or causal relationships
can be fully assessed or confirmed. We only found an association between
beta-blocker usage and obesity in hypertensive patients. We did not assess
weight gain, as the term weight gain has a temporal element that should be
validated with duration of drug therapy and weight changes throughout a
certain period. Second, there is an issue with ‘confounding by
indication’: it is possible that some study patients were obese when their
antihypertensive therapy was initiated and, consequently, their physicians
elected to use beta-blockers, given the recommendation by the few
international consensuses and guidelines.9-12 However, since this was a
post-hoc analysis based on a cross-sectional study, it is difficult to
figure out the temporal sequence—whether beta-blockers were initiated
because the patients were obese, or the patients became obese after taking
beta-blockers. Third, the results were subject to selection bias because
(a) study participation was voluntary, likely representing those who were
more physically and/or socially active, and (b) those who were home-bound
or had limited access to outdoor environments were not available for
inclusion. Third, although the selection criteria were not designed in
favour of women, the greater participation of women in our study is common
in community-based investigations in Hong Kong,32
33 34
35 36
37 probably reflecting their
greater participation in community-based and health-related activities.32 33
Although the self-reported diagnoses of hypertension were verified against
the medication profiles, it is possible that beta-blockers were prescribed
for an alternative indication, such as ischaemic heart disease.
Conclusions
Our study has reported a high proportion of
beta-blocker use among Hong Kong older adults with hypertension.
Beta-blocker users, and more specifically atenolol users, have a
significantly higher BMI, as well as a higher propensity towards obesity
compared with non-users. Our results can remind clinicians of the
possibility that beta-blockers, particularly atenolol, may worsen weight
control in hypertensive patients with obesity or cause significant weight
gain in those who are not yet obese. Our findings taken together with
other guideline reservations cast doubt on whether beta-blockers,
particularly atenolol, should be the major drug prescribed to older adults with hypertension.
Author contributions
All authors contributed to the concept of study,
acquisition and analysis of data, wrote the article, and had critical
revision for important intellectual content. All authors had full access
to the data, contributed to the study, approved the final version for
publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study was approved by the Survey and
Behavioural Research Ethics Committee of The Chinese University of Hong
Kong (Ref 14610518) and was conducted in accordance with the Declaration
of Helsinki. Written informed consent was obtained from all study
participants. Confidentiality agreement forms were signed by all data
collectors.
References
1. World Health Organization. A global
brief on hypertension: silent killer, global public health crisis.
Available from:
http://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en.
Accessed 5 Mar 2019.
2. UK Prospective Diabetes Study Group.
Efficacy of atenolol and captopril in reducing risk of macrovascular and
microvascular complications in type 2 diabetes: UKPDS 39. BMJ
1998;317:713-20. Crossref
3. Wikstrand J, Warnold I, Olsson G,
Tuomilehto J, Elmfeldt D, Berglund G. Primary prevention with metoprolol
in patients with hypertension. Mortality results from the MAPHY study.
JAMA 1988;259:1976-82. Crossref
4. Davis BR, Oberman A, Blaufox MD, et al.
Effect of antihypertensive therapy on weight loss. The Trial of
Antihypertensive Interventions and Management Research Group. Hypertension
1992;19:393-9. Crossref
5. Houston MC, Olafsson L, Burger MC.
Effects of nifedipine GITS and atenolol monotherapy on serum lipids, blood
pressure, heart rate, and weight in mild to moderate hypertension.
Angiology 1991;42:681-90. Crossref
6. Whelton PK, Carey RM, Aronow WS, et al.
2017 ACC/ AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/ NMA/PCNA guideline for the
prevention, detection, evaluation, and management of high blood pressure
in adults: a report of the American College of Cardiology/ American Heart
Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol
2018;71:e127-248. Crossref
7. National Institute of Health and Care
Excellence. Hypertension in adults: diagnosis and management (Clinical
Guideline 127). Available from: https://www.nice.org.uk/guidance/cg127.
Accessed 5 Mar 2019.
8. National Heart Foundation of Australia.
Guideline for diagnosis and management of hypertension in adults—2016.
Melbourne: National Heart Foundation of Australia; 2016.Crossref
9. Williams B, Mancia G, Spiering W, et al.
2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur
Heart J 2018;39:3021-104. Crossref
10. Jordan J, Yumuk V, Schlaich M, et al.
Joint statement of the European Association for the Study of Obesity and
the European Society of Hypertension: obesity and difficult to treat
arterial hypertension. J Hypertens 2012;30:1047-55. Crossref
11. Landsberg L, Aronne AJ, Beilin LJ, et
al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and
treatment: a position paper of The Obesity Society and the American
Society of Hypertension. J Clin Hypertens (Greenwich) 2013;15:14-33. Crossref
12. Dentali F, Sharma AM, Douketis JD.
Management of hypertension in overweight and obese patients: a practical
guide for clinicians. Curr Hypertens Rep 2005;7:330-6. Crossref
13. Wong MC, Tam WW, Cheung CS, et al.
Antihypertensive prescriptions over a 10-year period in a large Chinese
population. Am J Hypertens 2013;26:931-8. Crossref
14. Wong MC, Tam WW, Wang HH, et al.
Predictors of the incidence of all-cause mortality and deaths due to
diabetes and renal diseases among patients newly prescribed
antihypertensive agents: a cohort study. Int J Cardiol 2013;168:4705-10. Crossref
15. Wong MC, Tam WW, Cheung CS, et al.
Initial antihypertensive prescription and switching: a 5 year cohort study
from 250,851 patients. PLoS One 2013;8:e53625. Crossref
16. Mathus-Vliegen EM. Obesity and the
elderly. J Clin Gastroenterol 2012;46:533-44. Crossref
17. Han TS, Tajar A, Lean ME. Obesity and
weight management in the elderly. Br Med Bull 2011;97:169-96. Crossref
18. Zamboni M, Mazzali G, Zoico E, et al.
Health consequences of obesity in the elderly: a review of four unresolved
questions. Int J Obes (Lond) 2005;29:1011-29. Crossref
19. Bui AL, Horwich TB, Fonarow GC.
Epidemiology and risk profile of heart failure. Nat Rev Cardiol
2011;8:30-41. Crossref
20. Klonoff DC. The increasing incidence
of diabetes in the 21st century. J Diabetes Sci Technol 2009;3:1-2. Crossref
21. Hajjar I, Kotchen JM, Kotchen TA.
Hypertension: trends in prevalence, incidence, and control. Annu Rev
Public Health 2006;27:465-90. Crossref
22. Savarese G, Lund LH. Global public
health burden of heart failure. Card Fail Rev 2017;3:7-11. Crossref
23. Leung K, Fong W, Lau PS, et al. Impact
of dining out and food intake pattern on atrial fibrillation prevalence in
Hong Kong Chinese elders. Value Health 2018;21:S57. Crossref
24. WHO Expert Consultation. Appropriate
body-mass index for Asian populations and its implications for policy and
intervention strategies. Lancet 2004;363:157-63. Crossref
25. Coleman A, Freeman P, Steel S, Shennan
A. Validation of the Omron 705IT (HEM-759-E) oscillometric blood pressure
monitoring device according to the British Hypertension Society protocol.
Blood Press Monit 2006;11:27-32. Crossref
26. Casu G, Merella P. Diuretic therapy in
heart failure—current approaches. Eur Cardiol 2015;10:42-7. Crossref
27. Pischon T, Sharma AM. Use of
beta-blockers in obesity hypertension: potential role of weight gain. Obes
Rev 2001;2:275-80. Crossref
28. Rössner S, Taylor CL, Byington RP,
Furberg CD. Long term propranolol treatment and changes in body weight
after myocardial infarction. BMJ 1990;300:902-3. Crossref
29. Zhu J, Xiang YB, Cai H, et al.
Associations of obesity and weight change with physical and mental
impairments in elderly Chinese people. Maturitas 2018;108:77-83. Crossref
30. Park SY, Wilkens LR, Maskarinec G,
Haiman CA, Kolonel LN, Marchand LL. Weight change in older adults and
mortality: the Multiethnic Cohort Study. Int J Obes (Lond) 2018;42:205-12.
Crossref
31. Carlberg B, Samuelsson O, Lindholm LH.
Atenolol in hypertension: is it a wise choice? Lancet 2004;364:1684-9. Crossref
32. Leung GT, Leung KF, Lam LC.
Classification of late-life leisure activities among elderly Chinese in
Hong Kong. East Asian Arch Psychiatry 2011;21:123-7.
33. Woo J, Mak B, Yeung F. Age-friendly
primary health care: an assessment of current service provision for older
adults in Hong Kong. Health Serv Insights 2013;6:69-77. Crossref
34. Su EX, Lin YQ, Zhang SL, Leung GT, Lam
LC, Chiu HF. Physical activity and cognitive function of community Chinese
elderly in Hong Kong (HK) and Guangzhou (GZ). Int Psychogeriatr
2015;27:959-66. Crossref
35. Kwok T, Wong A, Chan G, et al.
Effectiveness of cognitive training for Chinese elderly in Hong Kong. Clin
Interv Aging 2013;8:213-9. Crossref
36. Shen C, Schooling CM, Chan WM, Lee SY,
Leung GM, Lam TH. Self-reported diabetes and mortality in a prospective
Chinese elderly cohort study in Hong Kong. Prev Med 2014;64:20-6. Crossref
37. Cheung MC, Ting W, Chan LY, et al.
Leisure participation and health-related quality of life of community
dwelling elders in Hong Kong. Asian J Gerontology Geriatr 2009;4:15-23.