Hong Kong Med J 2019 Oct;25(5):349–55 | Epub 11 Oct 2019
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Emergency attendances and hospitalisations for
complications after transrectal ultrasound-guided prostate biopsies: a
five-year retrospective multicentre study
KC Cheng, FHKAM (Surgery), FCSHK1; WC Lam, MB, ChB1;HC Chan, FHKAM (Surgery), FCSHK1;
CC NgoFHKAM (Surgery), FCSHK2; MH Cheung, FHKAM (Surgery), FCSHK2; HS So, FHKAM (Surgery), FCSHK1; KM Lam, FHKAM (Surgery), FCSHK3
1 Department of Surgery, United
Christian Hospital, Kwun Tong, Hong Kong
2 Department of Surgery, Tseung Kwan O
Hospital, Tseung Kwan O, Hong Kong
3 Private Practice, Chiron Medical,
Central, Hong Kong
Corresponding author: Dr KC Cheng (bryan.ckc@gmail.com)
Abstract
Introduction: Transrectal
ultrasound-guided (TRUS) prostate biopsy is an established procedure for
diagnosis of prostate cancer. Complications after TRUS biopsy are not well
reported in Hong Kong. This study evaluated the 5-year incidences of
TRUS biopsy complications and potential risk factors for those complications.
Methods: This was a
retrospective review of biopsies performed from 2013 to 2017 in two
local hospitals, using data retrieved from electronic medical records.
The primary outcome was the occurrence of complications requiring either
emergency attendances or hospitalisations within 30 days after biopsy.
Potential risk factors were examined using multiple logistic regression
analysis.
Results: In total, 1699 men were
included (mean age ± standard deviation: 67 ± 7 years; median
prostate-specific antigen level: 7.9 μg/L [interquartile range, 5.5-12.6
μg/L]); 4.3% had pre-biopsy bacteriuria. Overall, 5.7% and 3.8% of
post-biopsy complications required emergency attendances and
hospitalisations, respectively. Gross haematuria and rectal bleeding
requiring emergency attendances developed in 2.1% and 0.4% of men; 0.8%
and 0.4% required hospitalisations. Furthermore, 1.5% of men developed
acute urinary retention requiring hospitalisations; 1.9% and 1.2% had
post-biopsy infections requiring emergency attendances and
hospitalisations, respectively, and 0.9% had urosepsis requiring
hospitalisations. Prostate volume >48 cc was associated with an
increased risk of post-biopsy retention (odds ratio 2.75, 95% confidence
interval: 1.23-4.17).
Conclusions: The rate of overall
complications after TRUS biopsy was low. The most common complications
requiring emergency attendances and hospitalisations were gross
haematuria and acute urinary retention, respectively. Prostate volume
>48 cc increased the risk of post-biopsy urinary retention.
New knowledge added by this study
- Complications requiring emergency attendances or hospitalisations after transrectal ultrasound-guided (TRUS) prostate biopsies are uncommon.
- The most common complications requiring emergency attendances and hospitalisations are gross haematuria and acute urinary retention, respectively.
- The presence of a large prostate (volume >48 cc) increases the risk of acute urinary retention after TRUS biopsy. However, no specific factors are associated with increased risk of post-biopsy infections.
- Patients with large prostate should be counselled for the increased risk of urinary retention after TRUS biopsy.
- Despite the presence of antibiotic-resistant bacteria in urine and blood cultures, patients who develop sepsis after TRUS biopsy are likely to recover after a brief period of hospitalisation.
Introduction
Transrectal ultrasound-guided (TRUS) prostate biopsy, introduced in 1989,1 is an
established and longstanding procedure for detection of prostate cancer.
Because it can be learned rapidly and comprises a simple, office-based
procedure, TRUS biopsy remains the most commonly performed procedure for
diagnosis of prostate cancer.2 3 However, TRUS biopsy is associated with
significant risks. Instances of bleeding are common, including haematuria,
rectal bleeding, and haemospermia; however, these are generally mild and
self-limiting.4 The most worrisome
complication is post-biopsy infection, which occurs in 0% to 6.3% of men
after TRUS biopsy.4 The risk is low, but
the consequences are serious in affected patients. There is recent
evidence to suggest that increasing numbers of quinolone-resistant
organisms are contributing to the development of post-biopsy sepsis.4
In Hong Kong, there have been few reports of TRUS biopsy
complications. Some studies have focused on infective complications in
relatively small numbers of patients.5
6 Therefore, we reviewed TRUS biopsies
performed over a 5-year period in two local hospitals to evaluate the
incidences and types of complications, as well as their associated risk
factors. This could provide an important insight into the overall TRUS biopsy
complications, including infective and non-infective complications in the
local population.
Methods
Patients and study design
This retrospective cohort analysis included men who
underwent TRUS biopsy procedures during the period from 2013 to 2017 in United
Christian Hospital, Hong Kong and Tseung Kwan O Hospital, Hong Kong. All
patients who underwent TRUS biopsy procedures were included in the analysis.
Indications for biopsy included elevated prostate-specific antigen (PSA)
level, suspicious digital rectal examination of the prostate, restaging
biopsies in incidental prostate cancer detected in transurethral
prostatectomy or in patients under active surveillance of prostate cancer,
and previous suspicion of prostate cancer (eg, high-grade prostate
intraepithelial neoplasia or atypical small acinar proliferation).
Pre-biopsy blood tests were performed to determine complete blood count,
clotting profile, and PSA level. Mid-stream urine was collected 3 to 4
weeks prior to biopsy for bacterial culture analysis. A course of
antibiotic treatment was administered if pre-biopsy bacteriuria was
detected, based on the sensitivity profile of the involved bacteria.
Anticoagulant medications and clopidogrel were discontinued prior to
biopsy; the duration of cessation and any requirement for heparin coverage
were determined by physicians. The use of low-dose aspirin was continued
during biopsy. Oral bisacodyl tablets were used for rectal preparation on
the morning of the biopsy procedure. Quinolone antibiotic prophylaxis with
oral levofloxacin 500 mg was prescribed 1 hour prior to biopsy, then
continued for 2 days after biopsy. This report was compiled in accordance
with the STROBE guidelines.7 The
principles outlined in the Declaration of Helsinki were followed.
Biopsy procedure
All biopsies were performed as day procedures. A
7.5-MHz biplanar transrectal ultrasound probe and 18-gauge needles with
side-firing needle-guides were used for biopsy. Each patient was
positioned in the left lateral posture with both hips and knees flexed.
Prostate size measurement was calculated using the ellipsoidal formula.
Topical lidocaine jelly and local anaesthetic injection with 10 mL of 1%
plain lidocaine were used routinely in one hospital; these were injected
into the area between the prostatic base and seminal vesicles. The other
hospital used topical lidocaine alone. Six-core to 12-core systemic
biopsies were performed depending on the hospital involved and the time
frame of the biopsy procedure, as the two centres have changed the
practice in performing more number of cores with time. Each patient was
discharged on the same day after completion of the procedure. Clinical
follow-up was performed at 4 weeks post-biopsy in an out-patient clinic to
review the pathology findings.
Follow-up assessment
Patients who were admitted for biopsies were
identified using the Clinical Data Analysis and Reporting System. Clinical
records (ie, discharge summary, emergency case notes, clinic consultation
notes, laboratory results, and ultrasound findings) were retrieved using
the hospital-based Clinical Management System and the territory-wide
Electronic Patient Record, which comprises a centralised medical records
system shared by all public hospitals. Thus, men who had been admitted to
another public hospital for complications could be identified. The
patients’ records were examined and the occurrence of complications was
determined using a standardised form. During post-biopsy follow-up
examinations, clinical records from the Clinical Management System were
examined to identify any potential attendances or admissions to private
sector hospitals owing to complications. The primary outcome in this study
was the occurrence of complications within 30 days after biopsy.
Complications were defined as events requiring either emergency
attendances or hospitalisations; these events were analysed separately.
Post-biopsy urinary tract infections (PBI) were defined as the presence of
urinary tract infection symptoms (dysuria, with or without frequency,
urgency, or suprapubic pain) after biopsy, with or without sepsis. Based
on the Sepsis-3 criteria, sepsis was defined as an acute increase in the
Sequential Organ Failure Assessment score of ≥2.8
Acute urinary retention (AUR) was defined as acute painful retention of
urine requiring catheterisation. Any lower urinary tract symptoms (LUTS)
that occurred or worsened after biopsy, which required emergency
attendances, were also recorded.
Statistical analysis
Statistical calculations were computed with the
SPSS (Windows version 22.0; IBM Corp, Armonk [NY], United States). For
examination of potential risk factors, continuous variables, such as PSA
level and prostate size, were categorised based on the median values. The
Chi squared test was used to compare complications between the two
hospitals. Multiple logistic regression models were used to investigate
potential risk factors for complications.
Results
In total, 1710 men were admitted to either of the
two hospitals for TRUS biopsy procedures during the study period. Eleven men were
excluded because they refused to undergo TRUS biopsy after admission; therefore,
1699 men were included in the study. The mean age (± standard deviation)
of the men was 67 ± 7 years and median PSA level was 7.9 μg/L
(interquartile range, 5.5-12.6 μg/L). Of the 1699 men in the study, 310
(18.2%) had a suspicious digital rectal examination of the prostate; the
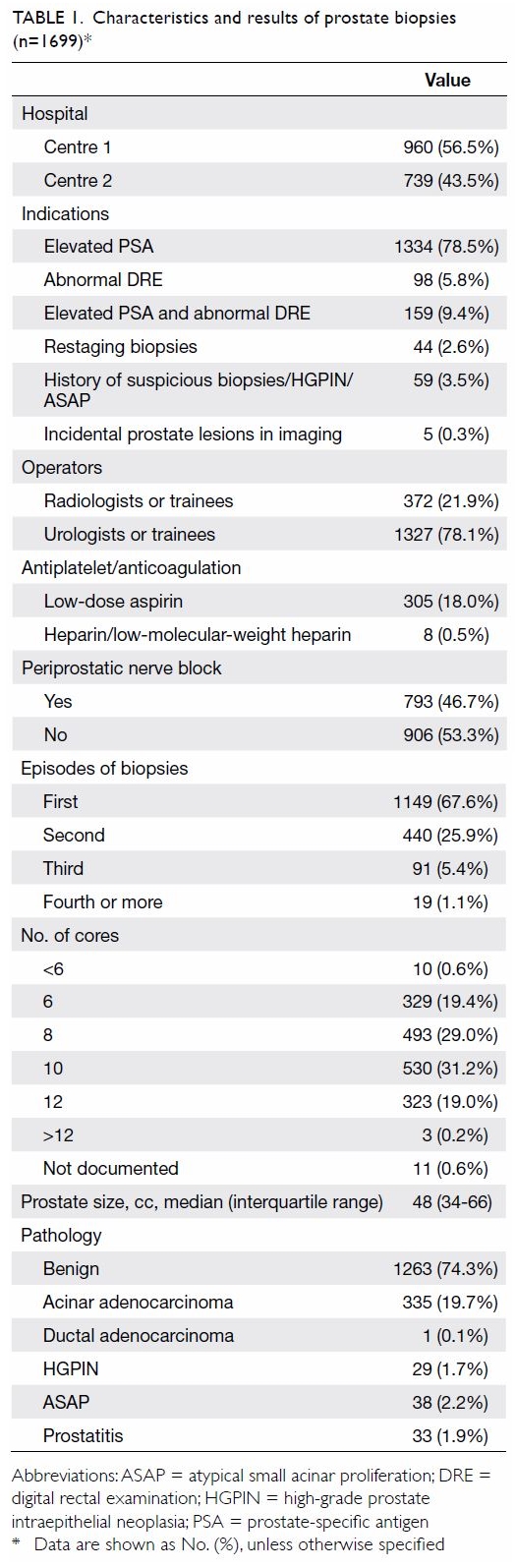
overall cancer detection rate was 19.8%. Characteristics and results of
the biopsies are shown in Table 1. Overall, 5.7% and 3.8% of post-biopsy
complications required emergency attendances and hospitalisations,
respectively (Table 2). There were no occurrences of mortality in
the entire cohort.
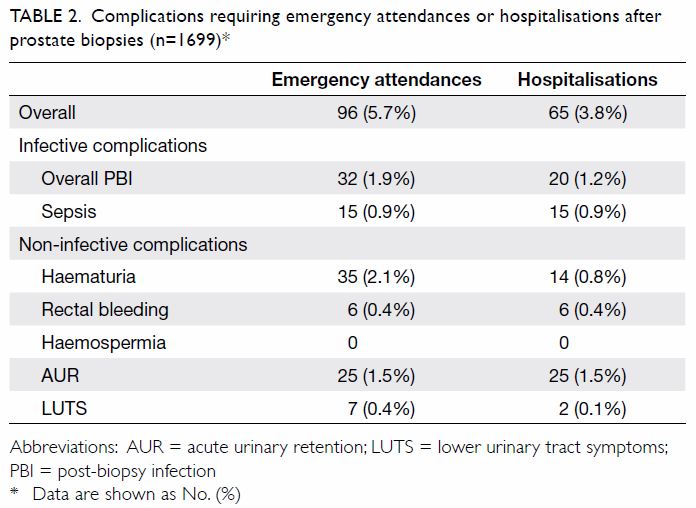
Table 2. Complications requiring emergency attendances or hospitalisations after prostate biopsies (n=1699)
Bleeding complications
Overall, 2.1% of men in the study developed gross
haematuria requiring emergency attendances, and 0.8% were hospitalised for
further management. Haematuria subsided with conservative treatment in all
affected men; no transfusions or emergency surgical interventions were
needed. Rectal bleeding occurred in 0.4% of men; all required
hospitalisations. Rectal bleeding resolved spontaneously in all affected
men, except two who required rectal packing with adrenaline gauze for
haemostasis. There were no cases of haemospermia requiring emergency
attendances. No risk factors could be identified for emergency attendances
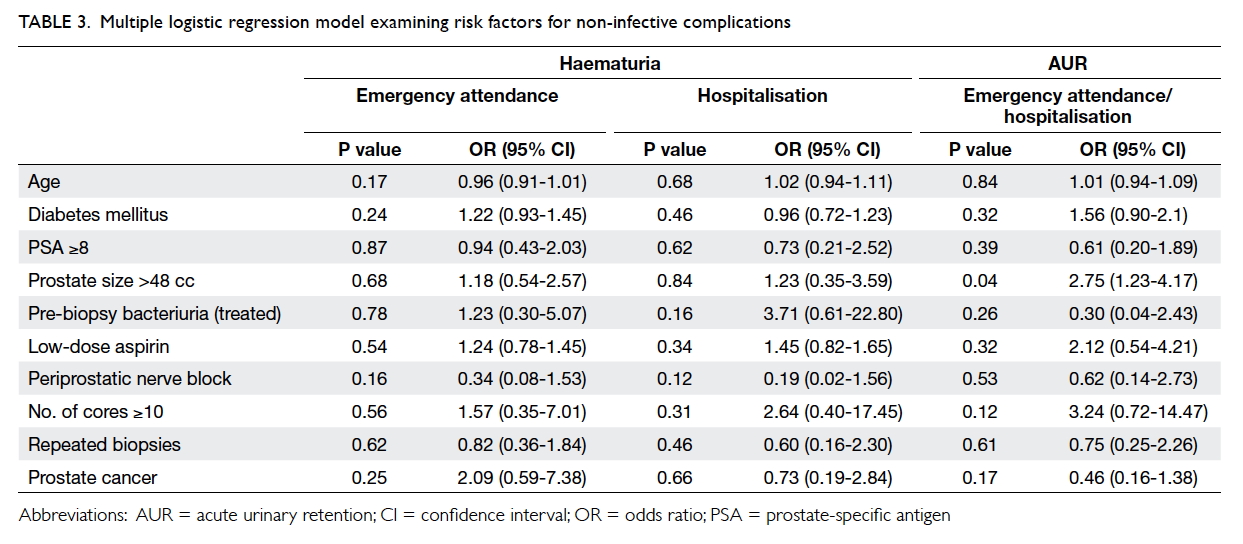
or hospitalisations related to any bleeding complications (Table
3). Importantly, the continuation of low-dose aspirin was not
associated with an increased rate of bleeding complications.
Retention of urine and lower urinary tract symptoms
In all, 1.5% of men in the study developed AUR; all
required hospitalisations. During these hospitalisations, the men were
assessed by voiding trials; all were able to void spontaneously within 2
to 3 days. Acute-onset LUTS was present in 0.4% of men who had emergency
attendances, and 0.1% of the men required hospitalisation. Prostate size
>48 cc was associated with a nearly 3-fold increase in the risk of
post-biopsy retention (odds ratio=2.75, 95% confidence interval:
1.23-4.17; Table 3). No risk factors were identified with
respect to the occurrence of LUTS.
Post-biopsy infection
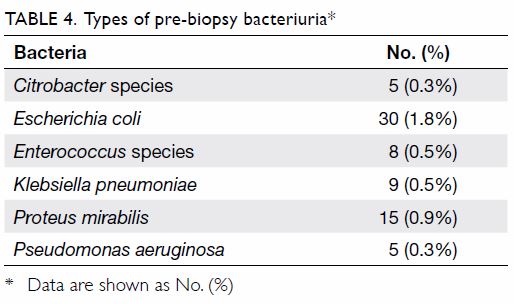
Pre-biopsy bacteriuria was present in 4.3% of men
in this study. The most common causative bacterial species was Escherichia
coli (1.8%) [Table 4]. Emergency attendances and hospitalisation
rates for PBI were 1.9% and 1.2%, respectively. Sepsis occurred in 0.9% of
men in this study, all of whom required hospitalisations (Table
2). Among patients who developed sepsis, none had a positive
pre-biopsy urine culture. Post-sepsis urine cultures were positive in
46.7% (7/15) of the men who developed sepsis; all of these positive
cultures showed growth of E coli, and 57% (4/7) of the cultures
demonstrated quinolone resistance. Blood cultures were positive in 40%
(6/15) of the men who developed sepsis; all of these positive cultures
showed growth of E coli, and 83% (5/6) of the cultures
demonstrated quinolone resistance. None of the men required intensive care
and none developed prostate abscesses. The median hospital stay for men
with sepsis was 6 days (interquartile range, 4-10 days).
Treatment for bacteriuria and the presence of
diabetes mellitus both showed no associations with overall infection or
urosepsis. No other factors tested including age and prostate size were
associated with infective complications. There were no differences in the
rates of overall complications requiring either emergency attendances
(6.5% vs 4.6%, P=0.10) or hospitalisations (3.9% vs 3.8%, P=0.95) between
the two hospitals. Moreover, there were no differences in the rates of
overall post-biopsy infection or sepsis (0.8% vs 1.6%, P=0.13 and 0.5% vs
1.4%, P=0.19).
Discussion
Non-infective complications
Non-infective complications after TRUS biopsy were common
in this study; fortunately, most comprised minor complications that did
not require additional treatment. Using questionnaires and telephone for
follow-up of patients who underwent TRUS biopsy, the ProtecT Study group found
that haematuria occurred in 65.8%, rectal bleeding occurred in 36.8%, and
haemospermia occurred in 92.6%, within 35 days after biopsy.9 A recent systematic review of TRUS biopsy complications
reported wider ranges of complication rates: haematuria in 27.9% to 64.5%
of patients, haemospermia in 6% to 90.1% of patients, and rectal bleeding
in 11.5% to 40% of patients.4 These
wide ranges of complication rates were largely dependent on the methods by
which the complications were registered. In our study, the reported
bleeding rate was lower, as we only included patients with complications
requiring emergency attendances. The differences in our findings suggest
that post-biopsy bleeding might generally be mild; thus, it does not
require medical consultation.
Prostate size is reportedly associated with the
risk of haematuria after biopsies, as is the number of cores, although
this particular point remains controversial.10
11 12
However, our study did not find evidence to support these relationships.
The post-biopsy retention rate in our study was comparable with that in
the literature (0.2%-1.7%).4 All
men had successful voiding trials in our cohort and did not require
surgical intervention. Importantly, we found that prostate size was a risk
factor for post-biopsy retention, consistent with the results of two other
studies.10 11
Infective complications
Infective complications requiring hospitalisation
have been reported in 0% to 6.3% of patients after TRUS biopsy.4 The Global Prevalence Study of Infections in Urology
2013 revealed post-biopsy infection in 5.2% of patients; of them, 3%
required hospitalisation.3 A recently published population-based study
showed an increasing trend in infective complications, comprising a
four-fold increase in overall hospitalisations over 10 years.13 In the present study, we could not perform any
temporal analyses of complications because the length of the study was
insufficient; to the best of our knowledge, there have been no such
temporal analyses in Hong Kong. The infection rate in our cohort was
comparatively lower than that of most international studies,4 and similar to that in prior studies elsewhere in Asia14 15
(0% and 0.5% of PBI), as well as in Hong Kong5
6 (0.5% and 3.9%). Reasons for the
apparent lower infection rate in people of Asian ethnicity compared with
those of other ethnicities are unclear. Tsu et al6
reported that patients who underwent TRUS biopsy exhibited a high prevalence
(53.6%) of antibiotic-resistant flora in the rectum, although the PBI rate
remained low among these patients (2.4%). Numerous risk factors have been
associated with the development of PBI.4
However, in the present study, we did not identify any factors that could
predict the risk of PBI.
A positive urine culture was not a mandatory
requirement to define PBI in this study, as a significant proportion of
men who had urinary tract infection symptoms without systemic inflammatory
response syndrome were treated and discharged directly from the emergency
department, and most did not provide urine cultures. Thus, the emergency
case notes were reviewed to determine whether PBI had occurred. In
contrast, for men who had been hospitalised with sepsis, urine and blood
cultures were available for analysis.
There were no reports of mortality in our cohort.
In general, death directly related to biopsy is exceedingly rare and most
patients die because of other factors. The reported mortality rates after
TRUS biopsy are 0.09% to 1.3%, depending on the length of the post-biopsy
follow-up period.4 Data from a
prostate cancer screening trial showed a mortality rate of 0.095% in
biopsy patients, which was comparable to that of the control group.
Notably, the mortality rate in biopsy patients was lower than that in
patients who had no biopsies; none of the deaths in the study were related
to the biopsy procedure.16
Transperineal or transrectal approaches
There has been a recent surge of interest, both in
Hong Kong and internationally, in performing transperineal prostate
biopsies. Transperineal biopsies are advantageous in that they have an
extremely low risk of sepsis and enable improved sampling of tumours in
the anterior prostate.17 In
transperineal biopsy, the needle is passed through clean and prepared
skin, rather than faeces or bowel; this method is presumed to eliminate
post-biopsy infection. In 2013, a large systematic review of transperineal
biopsy showed no instances of sepsis, with only a few reported cases of
PBI (0%-1.6%).4 Transrectal biopsy
exhibits difficulty in sampling the anterior prostate. Indeed,
transperineal biopsy reportedly exhibits a superior cancer detection rate,
especially in terms of tumours in the anterior prostate.18 19
Despite these advantages in the rate of post-biopsy
sepsis and sampling of anterior tumours, the transperineal approach has
limitations. These include longer operating time, greater
procedure-related pain, and increased post-biopsy retention, particularly
in relation to the use of template mapping protocols.20 21 A
systematic review and meta-analysis conducted in 2012, which compared the
outcomes of transperineal and transrectal biopsies, did not show any
differences in rates of complications between the two approaches.22 In our opinion, additional studies are needed to
compare the two approaches in terms of cancer detection rate,
complications, cost-effectiveness, and patient-reported outcomes before
wide adoption of the transperineal approach is recommended.
In early 2018, we began exploratory use of
transperineal prostate biopsy; thus far, we have used it for assessment of
71 patients. None of the patients have shown signs of sepsis or urinary
tract infections; two patients were readmitted after biopsy for urethral
bleeding and three patients were readmitted for urinary retention. The
number of biopsies performed thus far is insufficient for a meaningful
comparison with existing data from transrectal biopsies.
Limitations and future studies
To the best of our knowledge, this is the first
study in Hong Kong to provide data regarding non-infective complications
of TRUS biopsy. It provides valuable information for patients and can be used by
clinicians during treatment counselling. Special precautions and education
are needed for patients with a large prostate, as they exhibit an
increased risk of post-biopsy retention. Nonetheless, the value of this
study was limited by its retrospective nature.
The complications recorded were based solely on
emergency attendances and hospitalisations in all public hospitals;
importantly, attendances to private sector hospitals might have been
missed. However, because approximately 90% of in-patient care in Hong Kong
is provided by public hospitals, we presume that our approach enabled us
to retrieve data regarding the vast majority of post-biopsy complications
that required hospitalisations.23
In addition, patients who had attended private hospitals for
complications, then attended public out-patient clinics for follow-up,
could be identified and recorded unless they also selected private clinic
follow-up.
Milder complications which did not require
emergency attendances or hospitalisations, as well as sexual dysfunction
and post-biopsy pain, could not be assessed in this study. Because of its
retrospective design, we also could not report on prior antibiotics
exposure and travel history among the patients, which limits analyses of
risk factors. The number of cores taken could have affected the rate of
complications.4 Approximately 20%
of men in the cohort had sextant biopsies. The use of this lower number of
cores might have led to underestimation of the rate of complications,
compared with current standards for biopsy, in which 10 to 12 cores are
taken.
Finally, a locoregional prospective multicentre
study with other Asian nations would provide valuable insights into
complications after prostate biopsies in the Asian population; it would
also aid in assessments of differences in complications compared with
Western nations.
Conclusions
Complications requiring emergency attendances or
hospitalisations after transrectal prostate biopsy were uncommon; the most
common complications requiring emergency attendances and hospitalisations
were gross haematuria and AUR, respectively. Prostate volume >48 cc was
a risk factor for post-biopsy urinary retention, but no specific risk
factors were identified for post-biopsy infections. Patients with large
prostate should be counselled for the increased risk of urinary retention
after TRUS biopsy.
Author contributions
All authors had full access to the data,
contributed to the study, approved the final version for publication, and
take responsibility for its accuracy and integrity.
Concept or design: KC Cheng, KM Lam.
Acquisition of data: KC Cheng, WC Lam, KM Lam.
Analysis or interpretation of data: KC Cheng.
Drafting of the article: KC Cheng.
Critical revision for important intellectual content: HC Chan, CC Ngo, MH Cheung, HS So.
Acquisition of data: KC Cheng, WC Lam, KM Lam.
Analysis or interpretation of data: KC Cheng.
Drafting of the article: KC Cheng.
Critical revision for important intellectual content: HC Chan, CC Ngo, MH Cheung, HS So.
Declaration
This research has been presented in part at the
15th Urological Association of Asia Congress 2017, 4-6 August 2017, Hong
Kong.
Conflicts of interest
All authors have disclosed no conflicts of
interest.
Acknowledgement
We acknowledge and express our gratitude to Dr YS
Chan and Dr Alvin Chan for the data entry.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Kowloon
Central/Kowloon East Research Ethics Committee (Ref KC/KE-19-0182/ER-1).
References
1. Hodge KK, McNeal JE, Stamey TA.
Ultrasound guided transrectal core biopsies of the palpably abnormal
prostate. J Urol 1989;142:66-70. Crossref
2. Prostate Cancer: Diagnosis and
Treatment. Cardiff, UK: National Collaborating Centre for Cancer; 2014.
3. Wagenlehner FM, van Oostrum E, Tenke P,
et al. Infective complications after prostate biopsy: outcome of the
Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a
prospective multinational multicentre prostate biopsy study. Eur Urol
2013;63:521-7. Crossref
4. Loeb S, Vellekoop A, Ahmed HU, et al.
Systematic review of complications of prostate biopsy. Eur Urol
2013;64:876-92. Crossref
5. Chan ES, Lo KL, Ng CF, Hou SM, Yip SK.
Randomized controlled trial of antibiotic prophylaxis regimens for
transrectal ultrasound-guided prostate biopsy. Chin Med J (Engl)
2012;125:2432-5.
6. Tsu JH, Ma WK, Chan WK, et al.
Prevalence and predictive factors of harboring fluoroquinolone-resistant
and extended-spectrum beta-lactamase-producing rectal flora in Hong Kong
Chinese men undergoing transrectal ultrasound-guided prostate biopsy.
Urology 2015;85:15-21. Crossref
7. von Elm E, Altman DG, Egger M, Pocock
SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening
the Reporting of Observational Studies in Epidemiology (STROBE) statement:
guidelines for reporting observational studies. J Clin Epidemiol
2008;61:344-9. Crossref
8. Singer M, Deutschman CS, Seymour CW, et
al. The Third International Consensus Definitions for Sepsis and Septic
Shock (Sepsis-3). JAMA 2016;315:801-10. Crossref
9. Rosario DJ, Lane JA, Metcalfe C, et al.
Short term outcomes of prostate biopsy in men tested for cancer by
prostate specific antigen: prospective evaluation within ProtecT study.
BMJ 2012;344:d7894. Crossref
10. Raaijmakers R, Kirkels WJ, Roobol MJ,
Wildhagen MF, Schrder FH. Complication rates and risk factors of 5802
transrectal ultrasound-guided sextant biopsies of the prostate within a
population-based screening program. Urology 2002;60:826-30. Crossref
11. Zaytoun OM, Anil T, Moussa AS, Jianbo
L, Fareed K, Jones JS. Morbidity of prostate biopsy after simplified
versus complex preparation protocols: assessment of risk factors. Urology
2011;77:910-4. Crossref
12. Ghani KR, Dundas D, Patel U. Bleeding
after transrectal ultrasonography-guided prostate biopsy: a study of 7-day
morbidity after a six-, eight- and 12-core biopsy protocol. BJU Int
2004;94:1014-20. Crossref
13. Nam RK, Saskin R, Lee Y, et al.
Increasing hospital admission rates for urological complications after
transrectal ultrasound guided prostate biopsy. J Urol 2010;183:963-8. Crossref
14. Raheem OA, Casey RG, Galvin DJ, et al.
Discontinuation of anticoagulant or antiplatelet therapy for transrectal
ultrasound-guided prostate biopsies: A single-center experience. Korean J
Urol 2012;53:234-9. Crossref
15. Shigemura K, Matsumoto M, Tanaka K,
Yamashita M, Arakawa S, Fujisawa M. Efficacy of combination use of
beta-lactamase inhibitor with penicillin and fluoroquinolones for
antibiotic prophylaxis in transrectal prostate biopsy. Korean J Urol
2011;52:289-92. Crossref
16. Pinsky PF, Parnes HL, Andriole G.
Mortality and complications after prostate biopsy in the Prostate, Lung,
Colorectal and Ovarian Cancer Screening (PLCO) trial. BJU Int
2014;113:254-9. Crossref
17. Pepe P, Garufi A, Priolo G, Pennisi M.
Transperineal versus transrectal MRI/TRUS fusion targeted biopsy:
detection rate of clinically significant prostate cancer. Clin Genitourin
Cancer 2017;15:e33-6. Crossref
18. Ong WL, Weerakoon M, Huang S, et al.
Transperineal biopsy prostate cancer detection in first biopsy and repeat
biopsy after negative transrectal ultrasound-guided biopsy: the Victorian
Transperineal Biopsy Collaboration experience. BJU Int 2015;116:568-76. Crossref
19. Huang S, Reeves F, Preece J, Satasivam
P, Royce P, Grummet JP. Significant impact of transperineal template
biopsy of the prostate at a single tertiary institution. Urol Ann
2015;7:428-32. Crossref
20. Guo LH, Wu R, Xu HX, et al. Comparison
between ultrasound guided transperineal and transrectal prostate biopsy: a
prospective, randomized, and controlled trial. Sci Rep 2015;5:16089. Crossref
21. Skouteris VM, Crawford ED, Mouraviev
V, et al. Transrectal ultrasound-guided versus transperineal mapping
prostate biopsy: complication comparison. Rev Urol 2018;20:19-25.
22. Shen PF, Zhu YC, Wei WR, et al. The
results of transperineal versus transrectal prostate biopsy: a systematic
review and meta-analysis. Asian J Androl 2012;14:310-5. Crossref
23. Kong X, Yang Y, Gao J, et al. Overview
of the health care system in Hong Kong and its referential significance to
mainland China. J Chin Med Assoc 2015;78:569-73. Crossref