© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
COMMENTARY
Management of bilateral rhino-orbital cerebral
mucormycosis
Stacey C Lam, MB, ChB, MRCSEd1,2;
Hunter KL Yuen, MRCS, FCOphthHK1,2
1 Department of Ophthalmology and Visual
Sciences, Hong Kong Eye Hospital, Hong Kong
2 Department of Ophthalmology and Visual
Sciences, The Chinese University of Hong Kong, Shatin, Hong Kong
Corresponding author: Dr Stacey C Lam (staceylam@gmail.com)
Introduction
Mucormycosis is a group of invasive infections
caused by filamentous Mucorales fungi, most commonly Rhizopus
species.1 Although rare, Mucorales
is the third most common cause of fungal infection after Candida
and Aspergillus species.2
Mucorales are opportunistic fungi that can be found in soil, as well as
the mouth, nasal tract, and faeces of healthy individuals. Contact with
Mucorales occurs through spore inhalation; the organism can then invade
the paranasal sinus mucosa and erode through the bony walls of the orbit
and skull base into the brain, causing orbital and cerebral infections,
respectively. Fungal growth is enhanced in high-glucose, high-iron, and
acidic environments.1 Moreover,
elevated glucose and iron levels up-regulate glucose-regulated protein 78
(GRP78) and promote endothelial cell invasion.3
The hallmark of mucormycosis is angioinvasion, causing arteritis, vessel
thrombosis, tissue ischaemia, and necrosis with bony destruction.1 Angioinvasion also allows the organism to disseminate
to other organs, and the ischaemic necrosis impedes the delivery of
antifungal agents to target sites.
Rhino-orbital cerebral mucormycosis (ROCM) is a
rare but life-threatening fungal infection that occurs in
immunocompromised or diabetic patients. Bilateral involvement in ROCM has
been reported in only a few cases.1
4 5
Exemplar case
Rhino-orbital cerebral mucormycosis is usually
unilateral, with the infection spreading from the nasal mucosa to the
sinuses, orbit and brain. We experienced a severe case of bilateral ROCM.
A 70-year-old obese woman presented with poor appetite and right eye pain
for 10 days. She had a history of well-controlled type 2 diabetes mellitus
(haemoglobin A1c 6.5%, 10 months before presentation). On admission, she
was febrile, with right upper lid and facial swelling, right proptosis and
ophthalmoplegia. She had elevated blood glucose (21.8 mmol/L), elevated
haemoglobin A1c (17.9%), and urine test revealed ketone bodies and
leukocytes. Her preliminary diagnosis was right orbital cellulitis with
sinusitis, diabetic ketoacidosis, and urinary tract infection. Despite
insulin infusion and hydration, aggressive antibiotics with ceftriaxone,
metronidazole, and amphotericin B, the patient developed septic shock,
acute coronary syndrome, frontal lobe stroke, and acute kidney injury
requiring dialysis.
At 3 days after admission, the patient developed
signs of right endophthalmitis and right forehead phlebitis. Urgent
aqueous and vitreous tap with injection of intravitreal vancomycin and
ceftazidime was performed; both cultures were negative. Nasal biopsy
yielded Rhizopus oryzae and mucormycosis, and she was started on
systemic liposomal amphotericin B, posaconazole and anidulafungin.
Infected tissue was urgently debrided by orbital exenteration with
functional endoscopic sinus surgery. Intra-operative endoscopy showed
nasal hyphae (Fig). After surgery, the wound was irrigated daily
with amphotericin B. Despite local and systemic antifungal treatment, the
infection was not controlled and 1 week later, spread to the contralateral
sinus, orbit, and eye with endophthalmitis. The patient succumbed 4 weeks
after admission.
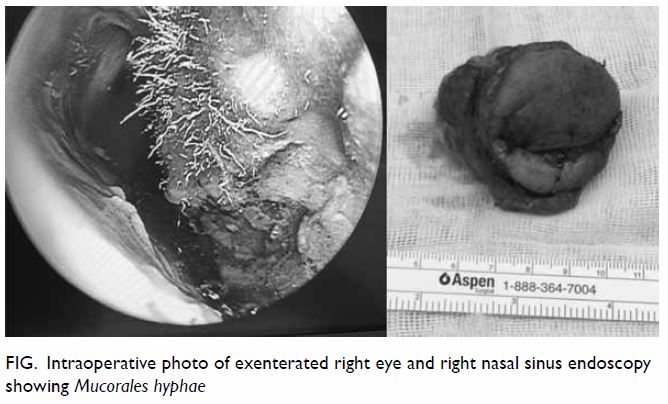
Figure. Intraoperative photo of exenterated right eye and right nasal sinus endoscopy showing Mucorales hyphae
Discussion
Effective treatment of ROCM includes (1) early
diagnosis; (2) reversal of underlying risk factors; (3) surgical
debridement where applicable; and (4) prompt antifungal therapy.2
First, early diagnosis depends on a high index of
clinical suspicion. Onset of mucormycosis can be nonspecific with malaise
and fever. With orbital involvement, there can be orbital cellulitis,
orbital apex syndrome, and cavernous sinus thrombosis. Occurrence of
mental state changes, hemiparesis, or seizures suggest intracranial
extension.2 Endoscopy and
radiography appearance lag behind clinical progression, so in suspicious
cases, blind biopsies of sinus mucosa or thickened extraocular muscles are
warranted.2 Computed tomography
findings are nonspecific, with sinusitis or thickening of extraocular
muscles, but may be useful in delineating the extent of the infection and
in guiding surgical debridement.2
Magnetic resonance imaging is more sensitive than computed tomography for
detecting orbital and cerebral involvement, and may demonstrate focal lack
of enhancement with devitalised sinus mucosa.6
Definitive diagnosis is made by demonstration of fungal hyphae in tissue
specimens. Fungal invasion may be patchy, so multiple biopsies may be
required for definitive diagnosis.2
Second, it is critical to reverse any underlying
immunocompromised state. This includes aggressive management to restore
euglycaemia and normal acid base in diabetic ketoacidotic patients,
stopping immunosuppressive agents, and avoiding iron and blood
transfusions.2 Other therapies
include iron chelating agents, hyperbaric oxygen, and adjunctive cytokine
therapy.2 5
Third, surgical debridement is critical, as blood
vessel thrombosis and resulting tissue necrosis impedes delivery of
necessary antifungal agents to the site of infection. Orbital exenteration
has been found to make a significant difference in survival only in
patients with fever.6 Involved
tissues rarely bleed, so surgeons should debride until well-perfused
bleeding tissue is encountered. Daily repeated debridement may be needed,
and subsequent surgeries may be needed for reconstruction when the
infection subsides. Bilateral exenteration for bilateral mucormycosis is
uncommon with poor prognosis, but survival from bilateral exenteration has
been reported.5 For our case,
bilateral exenteration was not attempted as the patient already had poor
prognosis and the surgery would be extremely disfiguring if the patient
survived.
Fourth, first-line antifungal treatment includes
lipid-based amphotericin B, which destroys the cell wall of the fungus.
This is given systemically, locally irrigated, and packed in affected
areas. Fluconazole, voriconazole, and itraconazole do not have reliable
activity against mucormycosis.2
Posaconazole, a trizole antifungal, has been suggested as a salvage
therapy for those who are refractory or intolerant to polyene.
Poor survival is associated with delayed diagnosis
and treatment (61% if commenced within first 12 days of presentation
compared to 33% if after 13 days), cerebral involvement (hemiparesis or
hemiplegia), bilateral sinus involvement, renal disease, and possibly
facial necrosis.7
Owing to the aggressive nature of mucormycosis, the
mortality rate is high. Although an infrequent diagnosis, there should be
a high index of suspicion for mucormycosis in patients with predisposing
factors and orbital symptoms, in order to prevent treatment delay.
Author contributions
HKL Yuen contributed to the concept of study,
acquisition and analysis of data, and critical revision for important
intellectual content. SC Lam wrote the article.
Conflicts of interest
As an epidemiology advisor of the Editorial Board,
HKL Yuen was not involved in the peer review process of the article. The
other author has disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study adhered to the tenets of the Declaration
of Helsinki.
References
1. Jiang N, Zhao G, Yang S, et al. A
retrospective analysis of eleven cases of invasive rhino-orbito-cerebral
mucormycosis presented with orbital apex syndrome initially. BMC
Ophthalmol 2016;16:10. Crossref
2. Spellberg B, Edwards J Jr, Ibrahim A.
Novel perspectives on mucormycosis: pathophysiology, presentation, and
management. Clin Microbiol Rev 2005;18:556-69. Crossref
3. Liu M, Spellberg B, Phan QT, et al. The
endothelial cell receptor GRP78 is required for mucormycosis pathogenesis
in diabetic mice. J Clin Invest 2010;120:1914-24. Crossref
4. Oladeji S, Amusa Y, Olabanji J, Adisa A.
Rhinocerebral mucormycosis in a diabetic case report. J West African Coll
Surg 2013;3:93-102.
5. De La Paz MA, Patrinely JR, Marines HM,
Appling WD. Adjunctive hyperbaric oxygen in the treatment of bilateral
cerebro-rhino-orbital mucormycosis. Am J Ophthalmol 1992;114:208-11. Crossref
6. Hargrove RN, Wesley RE, Klippenstein KA,
Fleming JC, Haik BG. Indications for orbital exenteration in mucormycosis.
Ophthalmic Plast Reconstr Surg 2006;22:286-91. Crossref
7. Ferry AP, Abedi S. Diagnosis and
management of rhino-orbitocerebral mucormycosis (phycomycosis). A report
of 16 personally observed cases. Ophthalmology 1983;90:1096-104. Crossref

