Hong Kong Med J 2024;30:Epub 13 Aug 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
MEDICAL PRACTICE
Management of chronic kidney disease: a Hong Kong consensus recommendation
Sydney CW Tang, MD, PhD1; Kelvin KL Ho, FRCP (Edinburgh), FHKAM (Medicine)2; Welchie WK Ko, FHKAM (Family Medicine)3; Albert Lee, MD, FHKAM (Family Medicine)4; CB Leung, FRCP, FHKAM (Medicine)5; WK Lo, MD, FHKCP6; Ronald CW Ma, FRCP, FHKCP7; SL Pang, MB, BS, FHKAM (Family Medicine)6; Kathryn CB Tan, MBBCh, MD8; MW Tsang, FRCP9; Martin CS Wong, MD, FHKAM (Family Medicine)4; William CW Wong, MPH, MD10; Francis KM Wong, FRCP6; CC Szeto, MD, FRCP11
1 Division of Nephrology, Department of Medicine, School of Clinical Medicine, The University of Hong Kong, Queen Mary Hospital, Hong Kong SAR, China
2 Department of Nephrology, Virtus Medical Group, Hong Kong SAR, China
3 Department of Family Medicine and Primary Healthcare, Queen Mary Hospital, Hong Kong SAR, China
4 The Jockey Club School of Public Health and Primary Care, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
5 Central Administration Office, Hong Kong Baptist Hospital, Hong Kong SAR, China
6 Private Practice, Hong Kong SAR, China
7 Division of Endocrinology and Diabetes, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong SAR, China
8 Division of Endocrinology and Metabolism, Department of Medicine, School of Clinical Medicine, The University of Hong Kong, Queen Mary Hospital, Hong Kong SAR, China
9 United Medical Practice, Hong Kong SAR, China
10 Department of Family Medicine and Primary Care, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
11 Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong SAR, China
Corresponding author: Prof Sydney CW Tang (scwtang@hku.hk)
Abstract
Chronic kidney disease (CKD) imposes a significant
burden on healthcare systems worldwide, and
diabetes is a major risk factor for CKD. There is
currently no consensus in Hong Kong regarding the
prioritisation of early identification and intervention
for CKD. A comprehensive and Hong Kong–specific
diabetes and CKD treatment guideline is also lacking.
A multidisciplinary group of experts discussed
issues surrounding the current management of
CKD and reviewed evidence in the context of local
experience to support recommendations. The
experts used a modified Delphi approach to finalise
recommendations. Consensus was regarded as ≥75%
acceptability among all expert panel members. The
panel members finalised 14 CKD-focused consensus
statements addressing disease definition, screening,
disease monitoring, lifestyle management, and
treatment strategies. The recommendations provided
are relevant to the Hong Kong healthcare setting and
can be used as a guide by physicians across various
specialties to facilitate the appropriate management
of CKD.
Introduction
Chronic kidney disease (CKD) is a leading cause
of mortality that affects >800 million people
worldwide, and its burden is the greatest among
individuals with a lower socio-economic status.1 In
Hong Kong, survey data from 2020 to 2022 showed
that 0.7% of the general population aged ≥15 years
had a confirmed diagnosis of renal impairment.2
Chronic kidney disease is classified into five
stages; stages 4 and 5 have a considerably increased
risk of death or risk of cardiovascular events.3 Early detection of CKD in adults can prevent progression
to kidney failure, while early identification by
screening provides an opportunity to stratify patients
according to risk, thereby enabling treatment that
can modify the disease course.3 Diabetes is a leading
risk factor for CKD; >40% of people with diabetes
will develop CKD, and many of these people will
require dialysis and transplantation.4 Considering the increasing prevalence of diabetes,4 it is important to develop comprehensive guidelines for the treatment of diabetes and CKD.
In Hong Kong, there has been no consensus
on prioritising early identification and intervention
for CKD. Guidelines for early CKD evaluation and
management have not been universally adopted, due
to a lack of incentives.5 This article documents the
findings of an expert panel established to formulate
the first consensus recommendations for CKD
screening and management in Hong Kong, with the
intention of providing practical guidance to local
healthcare practitioners based on evidence and
expert opinion.
Methods
Literature search
A search of PubMed was conducted to identify peer-reviewed
articles regarding CKD screening and
treatment. Local (ie, Hong Kong or Chinese study
populations) English-language publications from
January 2017 to September 2022 were retrieved.
Publication types were limited to clinical trials (ie,
randomised controlled or controlled clinical trials),
practice guidelines, and meta-analyses or systematic
reviews.
Consensus method
In accordance with published international
guidelines4 6 7 8 and literature search results, consensus
development leaders (first author and last author)
drafted a set of preliminary statements concerning
the definition, screening, and management of
CKD. Twelve Hong Kong experts (nephrologists,
endocrinologists, and family medicine specialists
from public hospitals and private clinics) were
invited to join the development leaders to form a
14-member consensus expert panel. All panel
members were tasked with reviewing the draft
statements in the context of current local practice and available evidence, and then discussed those
statements during two expert meetings held in
October and November 2022.
The consensus statements were developed
through a modified Delphi process. Panellists
evaluated each draft statement using a 5-point Likert
scale (A: accept completely; B: accept with some
reservations; C: accept with major reservations; D:
reject with reservations; E: reject completely). When
necessary, statements were modified, and a second
vote was conducted. A consensus was recorded if
≥75% of the group accepted a statement completely
or with reservations. When applicable, the level of
evidence was evaluated using the Oxford Centre for
Evidence-Based Medicine 2011 Levels of Evidence.9 10
Consensus statements
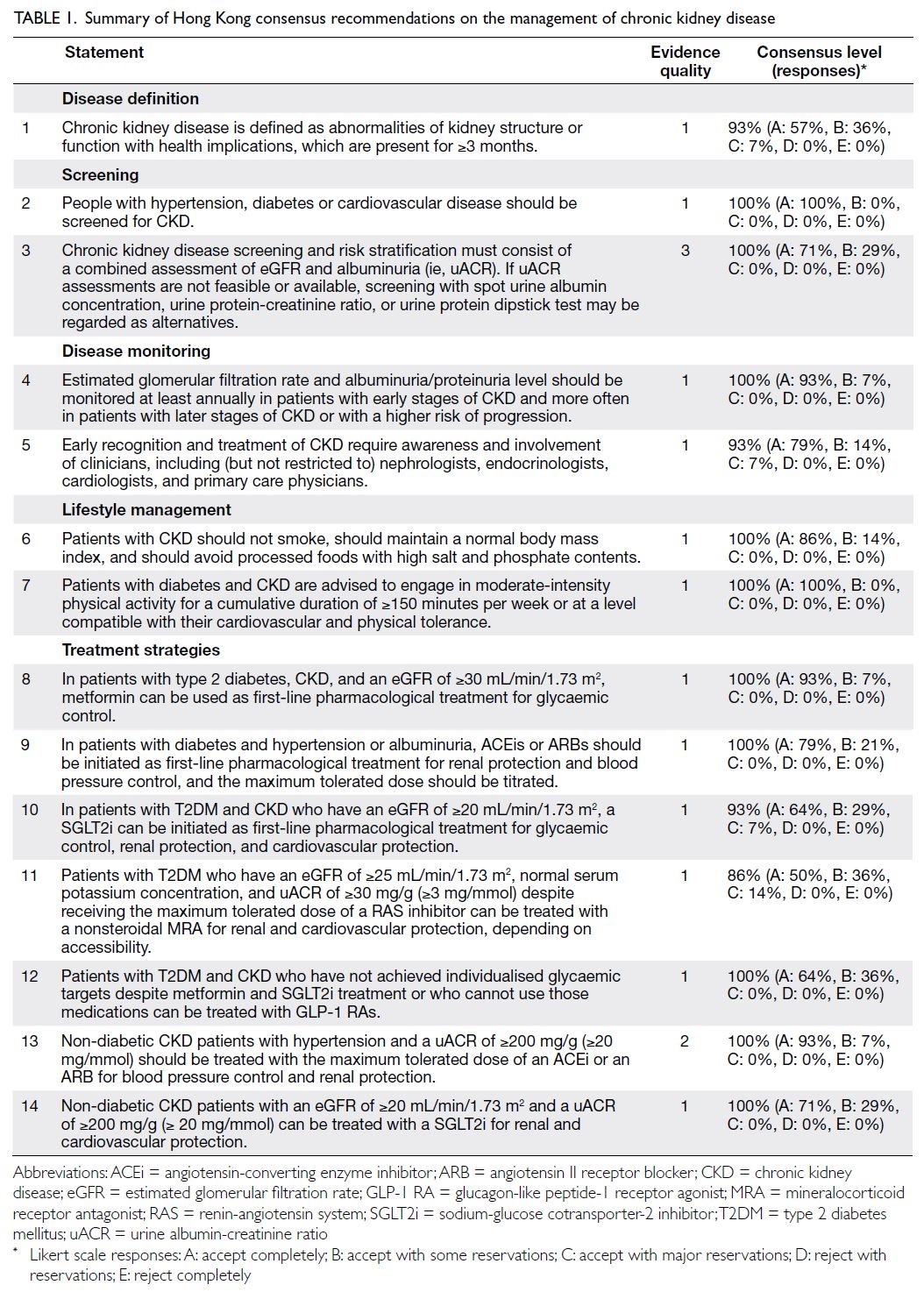
In total, 14 statements met the threshold for consensus; these are summarised in Table 1.
Disease definition
Statement 1: Chronic kidney disease is defined as abnormalities of kidney structure or function
with health implications, which are present for ≥3 months.
Chronic kidney disease stages 1 and 2 are
characterised by structural abnormalities and
persistent proteinuria, albuminuria, or haematuria.
Patients present with a normal to mildly decreased
estimated glomerular filtration rate (eGFR) of
≥60 mL/min/1.73 m2, as well as other markers of
kidney disease. Stage 3 is characterised by impaired
kidney function, defined as an eGFR between 30
and 59 mL/min/1.73 m2 on at least two occasions ≥3
months apart, irrespective of other markers of kidney
disease. Stage 4 is defined as a severely reduced eGFR
(15-29 mL/min/1.73 m2), and stage 5 is considered
kidney failure (eGFR <15 mL/min/1.73 m2).3
Most patients with early CKD are asymptomatic
and unaware of their disease. Diagnosis is often
based on incidental findings during routine medical
examinations. The detection of CKD in its early
stages could lead to timely interventions, avoid
inappropriate exposure to nephrotoxic agents, and
delay CKD progression.3
Screening
Statement 2: People with hypertension, diabetes
or cardiovascular disease should be screened for
chronic kidney disease.
The screening of patients with higher CKD
risk provides an opportunity to modify the disease
course. Hypertension, diabetes, and cardiovascular
diseases each has a single intermingled cause-and-effect
relationship with CKD.1 Hypertension is a
common cause of CKD, particularly in older adults,
as well as a risk factor for faster progression of kidney disease.3 The Hong Kong Renal Registry lists
hypertension as the third most common cause of
renal replacement therapy in Hong Kong.11
In Hong Kong, diabetes is the most common
primary aetiology leading to renal replacement
therapy (49.6%).11 It has also been identified as a risk factor for CKD; most patients with stages 1
and 2 CKD are asymptomatic (36.0% and 47.1%,
respectively).12 This recommendation is consistent
with the 2019 Hong Kong College of Physicians
Clinical Practice Guidelines for the Provision of
Renal Services in Hong Kong.13
Statement 3: Chronic kidney disease screening
and risk stratification must consist of a combined
assessment of estimated glomerular filtration rate
and albuminuria (ie, urine albumin-creatinine
ratio). If urine albumin-creatinine ratio assessments
are not feasible or available, screening with spot
urine albumin concentration, urine protein-creatinine
ratio, or urine protein dipstick test may be
regarded as alternatives.
Measurements of renal function are complex,
and no single method provides an accurate overall
assessment of renal function. Combined evaluation
of GFR and albuminuria is the gold standard for CKD
screening.14 Glomerular filtration rate screening
detects existing kidney damage, whereas albuminuria
screening detects kidney damage occurring before
substantial loss of nephron mass.14 A change in
albuminuria level also serves as a surrogate endpoint
for CKD progression.15
In clinical practice, eGFR is used.14 The
Kidney Disease: Improving Global Outcomes
(KDIGO) guideline defines CKD as an eGFR of
<60 mL/min/1.73 m2 or the detection of markers
associated with kidney damage, or both, that persists
for ≥3 months, regardless of the underlying cause.14
Estimated glomerular filtration rate–based CKD
detection can accurately assess kidney function.16 In
clinical practice, the eGFR is often derived from the serum creatinine concentration using the Chronic
Kidney Disease Epidemiology Collaboration
equation or the Modification of Diet in Renal
Disease Study equation. Recent studies suggest
that the Chronic Kidney Disease Epidemiology
Collaboration equation predicts prognosis more
accurately than the Modification of Diet in Renal
Disease Study equation.3 17 18
Although urinary albumin excretion is an
important prognostic biomarker for CKD, various
methodologies are currently used to measure urinary
albumin concentrations; these methodologies are not
standardised in clinical practice.14 The gold standard
for urinary albumin measurement is the urine
albumin-creatinine ratio (uACR). The normal range
of uACR is <30 mg/g (<3 mg/mmol); values above
this range indicate kidney damage. The KDIGO 2012
guideline provided the reference ranges for eGFR
and uACR categories (Table 2; see Disclaimer at the
end of this article).3
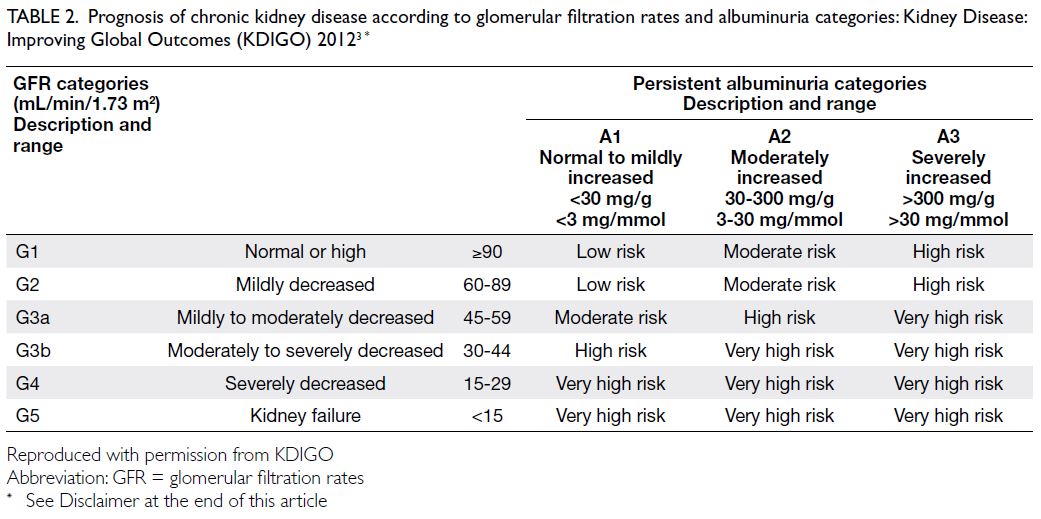
Table 2. Prognosis of chronic kidney disease according to glomerular filtration rates and albuminuria categories: Kidney Disease: Improving Global Outcomes (KDIGO) 20123
The expert panel recognised that uACR testing
is not available to all clinicians. Therefore, spot
screening for urinary albumin concentration, urine
protein-creatinine ratio (uPCR), or a urine protein
dipstick test may be regarded as alternatives. A
cohort study of Indo-Asian patients showed that
spot screening for urinary albumin concentration
and the uACR could be considered comparable to
screening for albuminuria.19 Another study has also
shown that the uPCR is positively correlated with
the uACR.20 A uPCR of >200 mg/g (>20 mg/mmol) indicates a high risk of kidney damage21; a dipstick protein reading of ≥1+ also indicates kidney damage. The expert panel noted that a diagnosis should be
confirmed by repeated testing.
Disease monitoring
Statement 4: Estimated glomerular filtration rate
and albuminuria/proteinuria level should be
monitored at least annually in patients with early
stages of chronic kidney disease and more often in
patients with later stages of chronic kidney disease
or with a higher risk of progression.
Patients with CKD are often asymptomatic,
especially in the early stages of disease.22 Risk
factors contributing to CKD progression include the
underlying cause of CKD, reduced eGFR, albuminuria
level, age, sex, race/ethnicity, elevated blood
pressure, hyperglycaemia, dyslipidaemia, smoking,
obesity, and history of cardiovascular disease.3
The panel agreed that frequent (at least annual)
monitoring of eGFR and albuminuria or proteinuria
levels is important to ensure early detection of disease
progression and prevent worsening. Nonetheless,
uACR/proteinuria measurements may not be
meaningful for patients with advanced CKD, kidney
failure, or nephrotic syndrome.
Statement 5: Early recognition and treatment of
chronic kidney disease require awareness and
involvement of clinicians, including (but not
restricted to) nephrologists, endocrinologists,
cardiologists, and primary care physicians.
A CKD management programme should
integrate CKD screening, patient risk stratification,
and treatment within existing health services
and processes.23 Many international guidelines
recommend a multidisciplinary approach for CKD
screening and management.4 6 7 8
Patient risk stratification enabling appropriate
referral to speciality care and increased follow-up
frequency (when needed) can improve treatment
efficiency.24 A survey of primary care physicians
in the United States showed that a robust
multidisciplinary care team composed of dietitians,
case managers, pharmacists, and health educators
is desirable for enhancing patient education and
facilitating self-management of risk factors for
CKD progression.25 Primary care physicians with
access to multidisciplinary care teams agreed that
this approach was extremely helpful.25 Strategies
to improve patient awareness of CKD, adherence
to treatment, and achievement of CKD care goals
should include greater access to effective self-management
support within primary care.25
Lifestyle management
Statement 6: Patients with chronic kidney disease
should not smoke, should maintain a normal body
mass index, and should avoid processed foods with
high salt and phosphate contents.
Smoking has been identified as a risk factor for CKD in Chinese and other populations,26 27 and a cohort study from Taiwan revealed that obesity
was associated with an increased risk of kidney
failure, consistent with international data.28 This
recommendation is aligned with the Asian Pacific
Society of Nephrology recommendation that people
with diabetic kidney disease undergo smoking
cessation interventions, maintain a healthy body
mass index, and consume a diet rich in plant-based
proteins and free of processed meats with high salt
and phosphate contents.8
Statement 7: Patients with diabetes and chronic
kidney disease are advised to engage in moderate-intensity
physical activity for a cumulative duration
of ≥150 minutes per week or at a level compatible
with their cardiovascular and physical tolerance.
Improvements in physical activity levels
offer cardiometabolic, kidney, and musculoskeletal
benefits to the general population, including
patients with diabetes.29 A systematic review
identified exercise training as a potential strategy to
improve eGFR and body mass index while reducing
conventional blood pressure (as measured by
auscultation or oscillometric methods) in patients
with CKD.30 The expert panel agreed that clinicians
should encourage patients with CKD to engage in
moderate-intensity activities such as brisk walking,
water aerobics, cycling, tennis, ballroom dancing, or
general gardening.29
Treatment strategies
Statement 8: In patients with type 2 diabetes
mellitus, chronic kidney disease, and an estimated
glomerular filtration rate of ≥30 mL/min/1.73 m2,
metformin can be used as first-line pharmacological
treatment for glycaemic control.
An evaluation of the effectiveness of common
medications used to treat type 2 diabetes mellitus
(T2DM) showed that metformin is superior to
dipeptidyl peptidase-4 inhibitors and comparable
to thiazolidinediones and sulfonylureas in terms of
reducing glycated haemoglobin levels (with pooled
mean differences in glycated haemoglobin levels of
-0.37%, -0.07%, and 0.07%, respectively).31 A meta-analysis
concluded that metformin is superior to
sulfonylureas in reducing the risk of hypoglycaemia
among patients with normal kidney function (odds
ratio [OR]=0.11; 95% confidence interval [CI]=0.06-0.20) and among patients with impaired kidney
function (OR=0.17; 95% CI=0.11-0.26).4 This
recommendation is aligned with KDIGO guidance
for patients with mild to moderate loss of kidney
function and an eGFR of ≥30 mL/min/1.73 m2.4
Statement 9: In patients with diabetes and
hypertension or albuminuria, angiotensin-converting enzyme inhibitors or angiotensin II
receptor blockers should be initiated as first-line
pharmacological treatment for renal protection and
blood pressure control, and the maximum tolerated
dose should be titrated.
A review by Strippoli et al32 demonstrated the
impacts of angiotensin-converting enzyme inhibitors
(ACEis) and angiotensin II receptor blockers (ARBs)
in terms of preventing kidney disease progression;
both classes of medications significantly reduced
the risk of progression to end-stage kidney disease
compared with placebo or no treatment (relative
risk [RR]=0.60; 95% CI=0.39-0.93 and RR=0.78;
95% CI=0.67-0.91, respectively). Angiotensin-converting
enzyme inhibitors and ARBs also
significantly reduced the risk of progression from
microalbuminuria to macroalbuminuria (RR=0.45;
95% CI=0.29-0.69 and RR=0.49; 95% CI=0.32-0.75, respectively) and reduced the risk of serum
creatinine doubling (RR=0.68; 95% CI=0.47-1.00
and RR=0.79; 95% CI=0.67-0.93, respectively).32
Evaluation of ACEi or ARB efficacy in a Chinese
population revealed that patients taking ACEis or
ARBs had a lower mortality risk compared with
untreated patients (OR=0.77; 95% CI=0.58-0.90).33
Although combination therapy with an ACEi
and an ARB is superior to either medication as
monotherapy in terms of reducing proteinuria and
blood pressure,34 such combination therapy can
lead to higher incidences of hyperkalaemia and
hypotension, especially in patients with advanced
CKD.35 Individualised patient management involving
potassium binders may expand the applications of
combined therapy34; however, the panel noted that
some hypotensive patients may not tolerate ACEis
or ARBs, and this approach is less popular because
of emerging treatment options for CKD. Combined
therapy should only be considered by experienced
clinicians after careful assessment and discussion
with the patient.
Statement 10: In patients with type 2 diabetes
mellitus and chronic kidney disease who have an
estimated glomerular filtration rate of ≥20 mL/min/1.73 m2, a sodium-glucose cotransporter-2 inhibitor can be initiated as first-line
pharmacological treatment for glycaemic control,
renal protection, and cardiovascular protection.
Sodium-glucose cotransporter-2 inhibitors
(SGLT2is) deliver glycaemic control while conferring
cardiovascular36 37 38 39 and renal40 41 42 43 44 45 benefits to patients
with T2DM and CKD who have an eGFR between
25 and 90 mL/min/1.73 m2. In patients with T2DM
and various levels of cardiovascular and renal risk,
SGLT2i lowered all-cause mortality (OR=0.85; 95%
CI=0.79-0.92), cardiovascular mortality (OR=0.84;
95% CI=0.76-0.92), non-fatal myocardial infarction (OR=0.87; 95% CI=0.79-0.97), and kidney failure
(OR=0.71; 95% CI=0.57-0.89) compared with
placebo.46 The inhibitors are expected to reduce the
incidence of kidney failure per 1000 patients over 5
years for patients with very low (1 case), low (3 cases),
moderate (6 cases), high (25 cases), and very high (38
cases) baseline risk.46 The effectiveness of SGLT2is
in terms of glycaemic control is attenuated among
patients with an eGFR of <45 mL/min/1.73 m2.47
Thus, additional therapy for glycaemic control may
be needed in this population.
Statement 11: Patients with type 2 diabetes mellitus
who have an estimated glomerular filtration rate
of ≥25 mL/min/1.73 m2, normal serum potassium
concentration, and urine albumin-creatinine ratio
of ≥30 mg/g (≥3 mg/mmol) despite receiving the
maximum tolerated dose of a renin-angiotensin
system inhibitor can be treated with a nonsteroidal
mineralocorticoid receptor antagonist for renal
and cardiovascular protection, depending on
accessibility.
Multiple studies have demonstrated the
renal and cardiovascular protective effects of
mineralocorticoid receptor antagonists in patients
with diabetes and CKD.48 49 50 In the FIDELIO-DKD
study (Finerenone in Reducing Kidney Failure
and Disease Progression in Diabetic Kidney
Disease), a lower incidence (18% vs 21%; P=0.001)
compared with placebo was observed for the
primary composite outcome of kidney failure, a
sustained 40% decline in eGFR, or death from renal
causes among T2DM patients with an eGFR of
≥25 mL/min/1.73 m2 who received finerenone.48 The
trial showed that finerenone reduced the risk of the
primary cardiovascular composite outcome of death
from cardiovascular causes, non-fatal myocardial
infarction, non-fatal stroke, or hospitalisation for
heart failure in T2DM patients with an eGFR of
≥25 mL/min/1.73 m2 (12.4% vs 14.2% in the placebo
group; P=0.03).49
A meta-analysis of the efficacy and safety of
finerenone in patients with CKD concluded that,
compared with placebo, finerenone significantly
reduced the uACR (mean difference: -0.30; P<0.05)
while decreasing the risk of cardiovascular disorders
and increasing the risk of hyperkalaemia (RR=0.92;
95% CI=0.85-0.99; P<0.05 and RR=2.04; 95%
CI=1.77-2.34; P<0.00001, respectively).50
Statement 12: Patients with type 2 diabetes mellitus
and chronic kidney disease who have not achieved
individualised glycaemic targets despite metformin
and sodium-glucose cotransporter-2 inhibitor
treatment or who cannot use those medications can
be treated with glucagon-like peptide-1 receptor
agonists.
A 2022 trial compared the effectiveness of four
commonly used glucose-lowering medications in
patients with T2DM, namely, insulin glargine U-100,
glimepiride (sulfonylurea), liraglutide (glucagon-like
peptide-1 receptor agonist [GLP-1 RA]), and
sitagliptin (dipeptidyl peptidase-4 inhibitor).51 All
four medications reduced glycated haemoglobin
levels in combination with metformin, although
glargine and liraglutide were modestly more effective
in terms of achieving and maintaining glycaemic
targets.51 Severe hypoglycaemia was rare in all
treatment groups: glimepiride (2.2% of participants),
glargine (1.3%), liraglutide (1.0%), and sitagliptin
(0.7%).51 There were no differences in the rates of
major adverse cardiac events, hospitalisation for
heart failure, cardiovascular mortality, and all-cause
mortality.51
A meta-analysis of randomised trials concluded
that GLP-1 RAs are effective for cardiovascular and
renal protection.52 Similarly, Sattar et al52 reported
that GLP-1 RAs significantly reduced major
adverse cardiac events by 14% (P<0.0001), all-cause
mortality by 12% (P=0.0001), hospital admission for
heart failure by 11% (P=0.013), and composite renal
outcomes by 21% (P<0.0001), without increasing
the risks of severe hypoglycaemia, retinopathy, or
pancreatic adverse effects. The cardiovascular and
renal protective effects of GLP-1 RAs in patients with
T2DM were confirmed by a second meta-analysis.46
The expert panel noted that all available
evidence regarding the renal protective effects of
GLP-1 RAs was derived from secondary analyses
of cardiovascular outcome trials. However, there is
an ongoing renal outcome–specific trial involving
semaglutide to further investigate its effects on renal
outcomes.
Statement 13: Non-diabetic chronic kidney disease
patients with hypertension and a urine albumin-creatinine
ratio of ≥200 mg/g (≥20 mg/mmol)
should be treated with the maximum tolerated dose
of an angiotensin-converting enzyme inhibitor or an
angiotensin II receptor blocker for blood pressure
control and renal protection.
Angiotensin-converting enzyme inhibitors
produce antihypertensive and renal protective
effects while reducing proteinuria in non-diabetic
nephropathy patients.53 The strong and consistent
effects of ACEis in terms of slowing non-diabetic
renal disease progression and decreasing blood
pressure were confirmed in a meta-analysis of 11
randomised controlled trials.53
Coronel et al54 showed that irbesartan usage
in non-diabetic patients with advanced CKD had
effects on disease progression and blood pressure
control similar to those of ACEis. Irbesartan also
showed a stronger antiproteinuric effect compared
with ACEis.54
Statement 14: Non-diabetic chronic kidney disease
patients with an estimated glomerular filtration
rate of ≥20 mL/min/1.73 m2 and a urine albumin-creatinine
ratio of ≥200 mg/g (≥20 mg/mmol) can
be treated with a sodium-glucose cotransporter-2
inhibitor for renal and cardiovascular protection.
There is increasing evidence of the renal and
cardiovascular protective effects of SGLT2is in non-diabetic
patients with CKD. The renal protective
effects of SGLT2is in patients with CKD were
demonstrated in the DAPA-CKD (Dapagliflozin and
Prevention of Adverse Outcomes in Chronic Kidney
Disease)41 and EMPA-KIDNEY (Study of Heart
and Kidney Protection with Empagliflozin) trials.55
In the DAPA-CKD trial, dapagliflozin conferred a
composite renal benefit (sustained eGFR decline of
≥50%, end-stage kidney disease, or death from renal
causes; hazard ratio [HR]=0.56; 95% CI=0.45-0.68)
in CKD patients with or without T2DM, an eGFR
of 25 to 75 mL/min/1.73 m2, and an uACR of 200 to
5000 mg/g (20-500 mg/mmol).41 Dapagliflozin also
reduced all-cause mortality (HR=0.69; 95% CI=0.53-0.88).41 In the EMPA-KIDNEY trial, empagliflozin
lowered the risk of kidney disease progression
(defined as end-stage kidney disease, sustained
eGFR decline to <10 mL/min/1.73 m2, sustained
eGFR decline of ≥40% from baseline, or death from
renal causes; HR=0.71; 95% CI=0.62-0.81).55
Cardiovascular outcome trials showed
that SGLT2is lowered the risks of heart failure
hospitalisation and cardiovascular death by 30% to
35%.56 The results of the DAPA-CKD36 and EMPA-KIDNEY
trials55 confirmed that SGLT2is can benefit
patients with CKD, regardless of T2DM status.
Dapagliflozin is the only SGLT2i with evidence of
reducing all-cause mortality in a clinical trial (ie,
DAPA-CKD) specifically focused on patients with
CKD, with or without T2DM. In the DAPA-CKD
trial,41 dapagliflozin lowered the composite risk of
death from cardiovascular causes or hospitalisation
for heart failure (HR=0.71; 95% CI=0.55-0.92).
Conclusion
Chronic kidney disease is a major health problem
worldwide and in Hong Kong. Our consensus group
developed this initial set of recommendations to
familiarise Hong Kong clinicians with strategies for
early CKD management. In this article, we discussed
the current status of CKD management in Hong
Kong; based on the limited local evidence and
international evidence, we also highlighted the need
for early diagnosis and treatment of CKD. Finally,
we recommended appropriate treatment strategies
for patients with CKD who present with co-morbid
diabetes or hypertension.
Author contributions
All authors contributed to the concept or design, acquisition of data, analysis or interpretation of data, drafting of the manuscript, and critical revision of the manuscript for
important intellectual content. All authors had full access to
the data, contributed to the study, approved the final version
for publication, and take responsibility for its accuracy and
integrity.
Conflicts of interest
RCW Ma has received research funding from AstraZeneca,
Bayer, Merck Sharp & Dohme, Novo Nordisk, Pfizer, Roche
Diagnostics, and Tricida Inc for carrying out clinical trials or
studies, and from AstraZeneca, Bayer, Boehringer Ingelheim,
and Merck for speaker honoraria or consultancy on advisory
boards. All proceeds have been donated to The Chinese
University of Hong Kong to support diabetes research.
CC Szeto receives research support from AstraZeneca,
Boehringer Ingelheim, and Otsuka Pharmaceutical. KCB Tan
has participated in advisory boards and speakers bureaus
for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, and
Sanofi. MCS Wong is an advisory committee member for
Pfizer; an external expert for GlaxoSmithKline Limited,
a member of the advisory board of AstraZeneca, and an
honorary advisor of GenieBiome Limited. He was paid
consultancy fees for providing research advice and delivering
talks. Also, as an editor of the journal, he was not involved
in the peer review process. Other authors have disclosed no
conflicts of interest.
Acknowledgement
English language editing and writing support, funded by an
unrestricted educational grant from AstraZeneca Hong Kong
Limited, was provided by Mr Poh Sien Ooi of MIMS Medica
Sdn Bhd and Dr Mita Pabari of MIMS (Hong Kong) Limited.
The funder had no role in study design, data collection,
analysis, interpretation, or manuscript preparation.
Disclaimer
This article references the Kidney Disease: Improving Global
Outcomes (KDIGO) 2012 Clinical Practice Guideline for
the Evaluation and Management of Chronic Kidney Disease.
Please note that the KDIGO 2024 Clinical Practice Guideline
for the Evaluation and Management of Chronic Kidney
Disease57 has been published during the development of
this work. As such, readers are advised to consult the most
recent KDIGO guideline for the latest recommendations and
information on chronic kidney disease.
References
1. Kovesdy CP. Epidemiology of chronic kidney disease: an
update 2022. Kidney Int Supp (2011) 2022;12:7-11. Crossref
2. Non-communicable Disease Branch, Centre for Health
Protection, Department of Health, Hong Kong SAR
Government. Report of Population Health Survey 2020-22
(Part I). 2022. Available from: https://www.chp.gov.hk/files/pdf/dh_phs_2020-22_part_1_report_eng_rectified.pdf. Accessed 14 May 2023.
3. Kidney Disease: Improving Global Outcomes. KDIGO 2012 Clinical Practice Guideline for the Evaluation and
Management of Chronic Kidney Disease. Kidney Int Suppl
2013;3:1-150.
4. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 clinical practice
guideline for diabetes management in chronic kidney
disease. Kidney Int 2022;102(5S):S1-127. Crossref
5. Shlipak MG, Tummalapalli SL, Boulware LE, et al. The case
for early identification and intervention of chronic kidney
disease: conclusions from a Kidney Disease: Improving
Global Outcomes (KDIGO) Controversies Conference.
Kidney Int 2021;99:34-47. Crossref
6. Kidney Disease: Improving Global Outcomes (KDIGO)
Glomerular Diseases Work Group. KDIGO 2021 clinical
practice guideline for the management of glomerular
diseases. Kidney Int 2021;100(4S):S1-276. Crossref
7. Banerjee D, Winocour P, Chowdhury TA, et al. Management
of hypertension in patients with diabetic kidney disease:
summary of the Joint Association of British Clinical
Diabetologists and UK Kidney Association (ABCD-UKKA)
Guideline 2021. Kidney Int Rep 2022;7:681-7. Crossref
8. Liew A, Bavanandan S, Prasad N, et al. Asian Pacific
Society of Nephrology clinical practice guideline on
diabetic kidney disease—executive summary. Nephrology
(Carlton) 2020;25 Suppl 2:3-11. Crossref
9. Howick J, Chalmers I, Glasziou P, et al. The 2011 Oxford
CEBM Evidence Levels of Evidence (Introductory
Document). 2011. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. Accessed 27 May 2024.
10. OCEBM Levels of Evidence Working Group. Oxford
Centre for Evidence-Based Medicine 2011 Levels of
Evidence. Available from: https://www.cebm.ox.ac.uk/files/levels-of-evidence/cebm-levels-of-evidence-2-1.pdf. Accessed 27 Aug 2024.
11. Leung CB, Cheung WL, Li PK. Renal registry in Hong
Kong—the first 20 years. Kidney Int Suppl (2011)
2015;5:33-8. Crossref
12. Mok KY, Chan PF, Lai LK, Chow KL, Chao DV. Prevalence
of diabetic nephropathy among Chinese patients with
type 2 diabetes mellitus and different categories of their
estimated glomerular filtration rate based on the Chronic
Kidney Disease Epidemiology Collaboration (CKD-EPI)
equation in primary care in Hong Kong: a cross-sectional
study. J Diabetes Metab Disord 2019;18:281-8. Crossref
13. Tang SC, Wong AK, Mak SK. Clinical practice guidelines
for the provision of renal service in Hong Kong: general
nephrology. Nephrology (Carlton) 2019;24 Suppl 1:9-26. Crossref
14. Seidu S, Barrat J, Khunti K. Clinical update: the important
role of dual kidney function testing (ACR and eGFR) in
primary care: identification of risk and management in
type 2 diabetes. Prim Care Diabetes 2020;14:370-5. Crossref
15. Heerspink HJ, Greene T, Tighiouart H, et al. Change in
albuminuria as a surrogate endpoint for progression of
kidney disease: a meta-analysis of treatment effects in
randomised clinical trials. Lancet Diabetes Endocrinol
2019;7:128-39. Crossref
16. Vassalotti JA, Centor R, Turner BJ, et al. Practical approach
to detection and management of chronic kidney disease for
the primary care clinician. Am J Med 2016;129:153-62.e7. Crossref
17. Levey AS, Stevens LA, Schmid CH, et al. A new equation
to estimate glomerular filtration rate. Ann Intern Med
2009;150:604-12. Crossref
18. Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K.
Estimating equations for glomerular filtration rate in the
era of creatinine standardization: a systematic review. Ann
Intern Med 2012;156:785-95. Crossref
19. Jafar TH, Chaturvedi N, Hatcher J, Levey AS. Use of
albumin-creatinine ratio and urine albumin concentration
as a screening test for albuminuria in an Indo-Asian
population. Nephrol Dial Transplant 2007;22:2194-200. Crossref
20. Kulasooriya PN, Bandara SN, Priyadarshani C, et al.
Prediction of microalbuminuria by analysing total urine
protein-to-creatinine ratio in diabetic nephropathy
patients in rural Sri Lanka. Ceylon Med J 2018;63:72-7. Crossref
21. Kamińska J, Dymicka-Piekarska V, Tomaszewska J,
Matowicka-Karna J, Koper-Lenkiewicz OM. Diagnostic
utility of protein-to-creatinine ratio (P/C ratio) in spot
urine sample within routine clinical practice. Crit Rev Clin
Lab Sci 2020;57:345-64. Crossref
22. Webster AC, Nagler EV, Morton RL, Masson P. Chronic
kidney disease. Lancet 2017;389:1238-52. Crossref
23. Tsang JY, Blakeman T, Hegarty J, Humphreys J, Harvey G.
Understanding the implementation of interventions
to improve the management of chronic kidney disease
in primary care: a rapid realist review. Implement Sci
2016;11:47. Crossref
24. Smekal MD, Tam-Tham H, Finlay J, et al. Patient and
provider experience and perspectives of a risk-based
approach to multidisciplinary chronic kidney disease care:
a mixed methods study. BMC Nephrol 2019;20:110. Crossref
25. Sperati CJ, Soman S, Agrawal V, et al. Primary care
physicians’ perceptions of barriers and facilitators to
management of chronic kidney disease: a mixed methods
study. PLoS One 2019;14:e0221325. Crossref
26. Elihimas Júnior UF, Elihimas HC, Lemos VM, et al. Smoking
as risk factor for chronic kidney disease: systematic review
[in English, Portuguese]. J Bras Nefrol 2014;36:519-28. Crossref
27. Xue L, Lou Y, Feng X, Wang C, Ran Z, Zhang X. Prevalence
of chronic kidney disease and associated factors among
the Chinese population in Taian, China. BMC Nephrol
2014;15:205. Crossref
28. Lin YC, Lai YJ, Lin YC, et al. Effect of weight loss on the
estimated glomerular filtration rates of obese patients at
risk of chronic kidney disease: the RIGOR-TMU study. J
Cachexia Sarcopenia Muscle 2019;10:756-66. Crossref
29. Milam RH. Exercise guidelines for chronic kidney disease
patients. J Ren Nutr 2016;26:e23-5. Crossref
30. Mallamaci F, Pisano A, Tripepi G. Physical activity in
chronic kidney disease and the EXerCise Introduction To
Enhance trial. Nephrol Dial Transplant 2020;35 (Suppl
2):ii18-22. Crossref
31. Bennett WL, Maruthur NM, Singh S, et al. Comparative
effectiveness and safety of medications for type 2 diabetes:
an update including new drugs and 2-drug combinations.
Ann Intern Med 2011;154:602-13. Crossref
32. Strippoli GF, Bonifati C, Craig M, Navaneethan SD,
Craig JC. Angiotensin converting enzyme inhibitors and
angiotensin II receptor antagonists for preventing the
progression of diabetic kidney disease. Cochrane Database
Syst Rev 2006;2006:CD006257. Crossref
33. Song YH, Cai GY, Xiao YF, Liu JQ, Chen XM. The clinical
characteristics and antihypertensive medications for
mortality of elderly hospitalized hemodialysis patients: a
multicenter retrospective study in China. Saudi J Kidney
Dis Transpl 2021;32:637-44. Crossref
34. Zhao M, Qu H, Wang R, et al. Efficacy and safety of dual
vs single renin-angiotensin-aldosterone system blockade
in chronic kidney disease: an updated meta-analysis
of randomized controlled trials. Medicine (Baltimore) 2021;100:e26544. Crossref
35. Palevsky PM, Zhang JH, Seliger SL, Emanuele N, Fried LF;
VA NEPHRON-D Study. Incidence, severity, and outcomes
of AKI associated with dual renin-angiotensin system
blockade. Clin J Am Soc Nephrol 2016;11:1944-53. Crossref
36. McMurray JJ, Solomon SD, Inzucchi SE, et al. Dapagliflozin
in patients with heart failure and reduced ejection fraction.
N Engl J Med 2019;381:1995-2008. Crossref
37. Packer M, Anker SD, Butler J, et al. Cardiovascular and
renal outcomes with empagliflozin in heart failure. N Engl
J Med 2020;383:1413-24. Crossref
38. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and
cardiovascular and renal events in type 2 diabetes. N Engl J
Med 2017;377:644-57. Crossref
39. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and
cardiovascular outcomes in type 2 diabetes. N Engl J Med
2019;380:347-57. Crossref
40. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and
renal outcomes in type 2 diabetes and nephropathy. N Engl
J Med 2019;380:2295-306. Crossref
41. Heerspink HJ, Stefánsson BV, Correa-Rotter R, et al.
Dapagliflozin in patients with chronic kidney disease. N
Engl J Med 2020;383:1436-46. Crossref
42. Bakris G, Oshima M, Mahaffey KW, et al. Effects of
canagliflozin in patients with baseline eGFR <30 ml/min per 1.73 m2: subgroup analysis of the randomized
CREDENCE trial. Clin J Am Soc Nephrol 2020;15:1705-14. Crossref
43. Chertow GM, Vart P, Jongs N, et al. Effects of dapagliflozin
in stage 4 chronic kidney disease. J Am Soc Nephrol
2021;32:2351-61. Crossref
44. Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients
with diabetes and chronic kidney disease. N Engl J Med
2021;384:129-39. Crossref
45. Li N, Zhou G, Zheng Y, et al. Effects of SGLT2 inhibitors on
cardiovascular outcomes in patients with stage 3/4 CKD: a
meta-analysis. PLoS One 2022;17:e0261986. Crossref
46. Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose
cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like
peptide-1 (GLP-1) receptor agonists for type 2
diabetes: systematic review and network meta-analysis of
randomised controlled trials. BMJ 2021;372:m4573. Crossref
47. Cherney DZ, Cooper ME, Tikkanen I, et al. Pooled analysis
of phase III trials indicate contrasting influences of renal
function on blood pressure, body weight, and HbA1c
reductions with empagliflozin. Kidney Int 2018;93:231-44. Crossref
48. Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone
on chronic kidney disease outcomes in type 2 diabetes. N
Engl J Med 2020;383:2219-29. Crossref
49. Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events
with finerenone in kidney disease and type 2 diabetes. N
Engl J Med 2021;385:2252-63. Crossref
50. Fu Z, Geng X, Chi K, et al. Efficacy and safety of finerenone
in patients with chronic kidney disease: a systematic
review with meta-analysis and trial sequential analysis.
Ann Palliat Med 2021;10:7428-39. Crossref
51. GRADE Study Research Group; Nathan DM, Lachin JM,
et al. Glycemia reduction in type 2 diabetes—glycemic
outcomes. N Engl J Med 2022;387:1063-74. Crossref
52. Sattar N, Lee MM, Kristensen SL, et al. Cardiovascular,
mortality, and kidney outcomes with GLP-1 receptor
agonists in patients with type 2 diabetes: a systematic
review and meta-analysis of randomised trials. Lancet
Diabetes Endocrinol 2021;9:653-62. Crossref
53. Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting
enzyme inhibitors and progression of
nondiabetic renal disease. A meta-analysis of patient-level
data. Ann Intern Med 2001;135:73-87. Crossref
54. Coronel F, Cigarrán S, García-Mena M, Herrero J, Calvo N,
Pérez-Flores I. Irbesartan in hypertensive non-diabetic
advanced chronic kidney disease. Comparative study with
ACEi [in Spanish]. Nefrologia 2008;28:56-60.
55. The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388:117-27. Crossref
56. Joshi SS, Singh T, Newby DE, Singh J. Sodium-glucose co-transporter
2 inhibitor therapy: mechanisms of action in
heart failure. Heart 2021;107:1032-8. Crossref
57. Kidney Disease: Improving Global Outcomes (KDIGO)
CKD Work Group. KDIGO 2024 Clinical Practice
Guideline for the Evaluation and Management of Chronic
Kidney Disease. Kidney Int 2024;105(4S):S117-314. Crossref