Hong Kong Med J 2024;30:Epub 14 Oct 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Alectinib-induced haemolytic anaemia in anaplastic lymphoma kinase–positive non–small-cell lung cancer: a case report
Toby CH Leung , MB, ChB; Tommy CY So, MB, BS, FHKAM (Radiology)
Department of Clinical Oncology, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
Corresponding author: Dr Toby CH Leung (lch423@ha.org.hk)
Case presentation
A 70-year-old Chinese woman was diagnosed
with stage IV anaplastic lymphoma kinase (ALK)–positive non–small-cell lung carcinoma with
intrapulmonary, pleural and lymph node metastasis in December 2022. Her private oncologist first
prescribed lorlatinib in mid-December 2022. The
drug had been well tolerated by the patient and there
was no evidence of myelotoxicity.
The patient attended a public hospital for
further care in February 2023. Lorlatinib was
switched to alectinib on 17 March 2023. Her baseline
haemoglobin level was 11.1 g/dL on 16 March 2023
prior to commencement of alectinib. Subsequent
follow-ups on 13 April and 3 May revealed that her
haemoglobin level had fallen to 10 g/dL and 8.7 g/dL,
respectively (Fig 1a). Mean cell volume was 65.4 fL.
She had no clinical signs or symptoms suggestive of
acute blood loss.
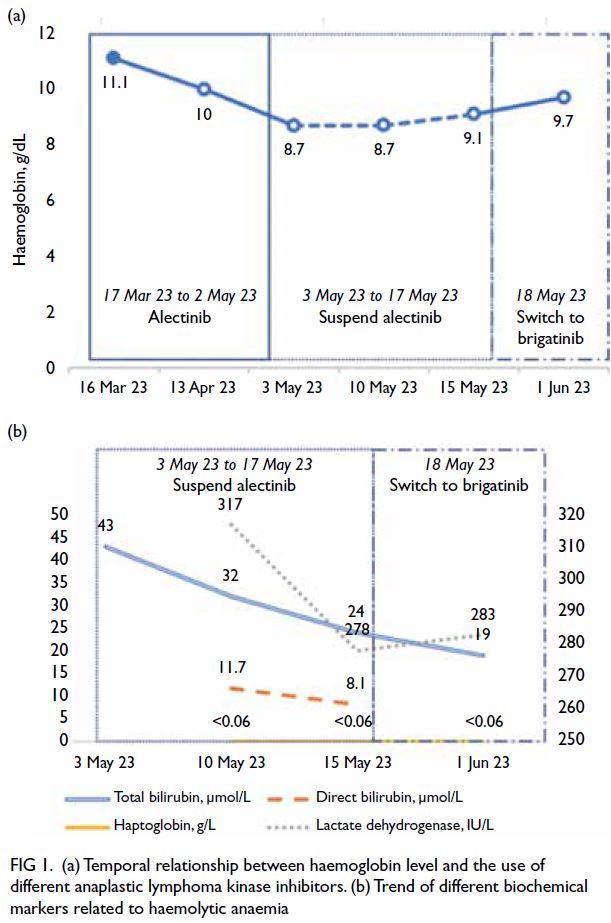
Figure 1. (a) Temporal relationship between haemoglobin level and the use of different anaplastic lymphoma kinase inhibitors. (b) Trend of different biochemical markers related to haemolytic anaemia
Peripheral blood smear showed marked red
cell sphero-acanthocytosis (Fig 2). The previously
normal bilirubin level rose to 43 μmol/L and
lactate dehydrogenase (LDH) level to 317 IU/L.
Haptoglobin level was very low (<0.06 g/L) [Fig 1b].
Direct Coombs test (DAT) was negative. Alectinib
had been withheld since 3 May 2023 in view of the
potential differential diagnosis of drug-induced
haemolytic anaemia. After suspending alectinib,
the haemoglobin level remained static for 1 week
(8.7 g/dL on 10 May 2023). It then increased slowly
over the following week (9.1 g/dL on 15 May 2023)
[Fig 1a]. Lactate dehydrogenase level also decreased
to 278 IU/L, with total bilirubin level decreased to
24 μmol/L and direct bilirubin level normalised
(Fig 1b).
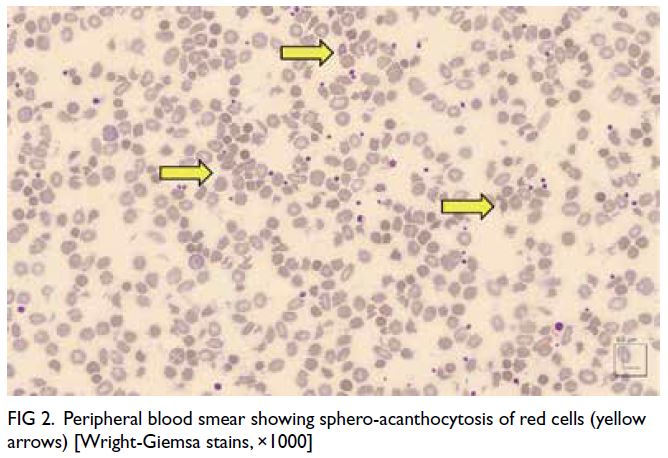
Figure 2. Peripheral blood smear showing sphero-acanthocytosis of red cells (yellow arrows) [Wright-Giemsa stains, ×1000]
Since the anaemia of the patient had improved,
another ALK inhibitor, brigatinib, was prescribed
on 18 May 2023, at 90 mg daily. Her haemoglobin
level increased to 9.7 g/dL after 2 weeks. Total
bilirubin level was normalised on 1 June 2023 (Fig 1a). Brigatinib was then stepped up to 180 mg daily (full dose) as it was well tolerated.
The patient demonstrated a favourable
response to ALK inhibitors as evidenced by a
continuously decreasing cancer embryonic antigen
level. A contrast computed tomography scan of the
thorax, abdomen and pelvis on 30 May 2023 showed
partial response after 5 months of lorlatinib and
alectinib. The primary lung tumour at the right lower
lobe showed a partial response, decreasing from 6.2 cm to 1.5 cm in size. There was no evidence of bone metastasis.
Discussion
Anaemia caused by alectinib is uncommon although
clinical trials have reported clinically significant
anaemia (grade ≥3) in around 7% of cases.1
Nonetheless there are limited reports of alectinib-induced
haemolytic anaemia.2 Misawa et al3 described a case of grade 4 anaemia due to drug-induced
haemolytic anaemia in 2023. No such case
has been reported in Hong Kong to date.
The index patient demonstrated alectinib-induced
haemolytic anaemia with morphological
change to erythrocytes and negative DAT result. The
blood smear of sphero-acanthocytosis combined
with altered bilirubin, LDH and haptoglobin was
strongly suggestive of haemolytic anaemia. The
improvement in haemoglobin, bilirubin and LDH
levels following suspension of alectinib suggested
that the haemolytic anaemia was drug induced.
Drug-induced haemolytic anaemia is usually
due to drug-induced immune haemolytic anaemia.4
Nonetheless in this case, an autoimmune cause was
excluded due to the patient’s negative DAT result
even though 5% to 10% of DAT-negative cases may
have an immune component.2 Differential diagnoses
of DAT-negative haemolytic anaemia include
membranopathies (eg, hereditary spherocytosis),
thrombotic microangiopathies (eg, thrombotic
thrombocytopenic purpura), enzymopathies (eg,
glucose-6-phosphate dehydrogenase), infection (eg,
malaria or Clostridium), and haemoglobinopathies
(eg, sickle cell disease).5 Our patient had no signs of
infection or history of haematological disease. The
temporal relationship between administration of
alectinib and the occurrence of haemolytic anaemia
favoured a diagnosis of drug-induced haemolysis. The laboratory findings were also consistent with other case reports.6
The precise mechanism is uncertain. It has
been postulated that alectinib induces erythrocyte
membrane changes.3 The presence of spherocytes
in the peripheral blood film may arise from these
membrane changes (Fig 2). Additional investigation
is warranted to further understand the underlying
mechanism.
Our patient developed haemolytic anaemia
within 2 months of commencing alectinib. Misawa
et al3 reported that grade 4 haemolytic anaemia
could occur after 3 years. Regular monitoring of
haemoglobin should be undertaken in patients
prescribed alectinib. Haemolytic anaemia workup,
including peripheral blood smear, bilirubin,
haptoglobin and LDH levels, should be considered if
indicated.
This case demonstrated no cross reactivity
among other ALK-positive first-line targeted
therapies (lorlatinib and brigatinib). Our patient
first commenced lorlatinib following a private
consultation and later switched to alectinib
with funding support. The patient continues her
treatment with brigatinib. There was no documented
drop in haemoglobin level after lorlatinib, and
following discontinuation of alectinib and initiation
of brigatinib, her haemoglobin level showed an
improving trend. Limited case reports of alectinib-induced
haemolytic anaemia have been managed
by discontinuation of therapy or rechallenge
with alectinib at a reduced dosage.2 3 6 To the best
of our knowledge, cross reactivity among first-line
ALK tyrosine inhibitors (lorlatinib, alectinib
and brigatinib) has not been reported. Our case
demonstrates that it is safe to switch treatment to
an alternative ALK inhibitor when alectinib-induced
haemolytic anaemia occurs. The drug-induced
haemolytic anaemia is specific to alectinib.
Conclusion
This case highlights the importance of interval
haemoglobin monitoring. A persistent drop
in haemoglobin level following initiation of
alectinib warrants prompt investigations for
possible differential diagnosis of alectinib-induced
haemolytic anaemia. This case also suggests that it is
safe to switch to an alternative ALK inhibitor in the
presence of alectinib-induced haemolytic anaemia.
Author contributions
Both authors contributed to the concept or design of the
study, acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. Both authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
Both authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank Dr Kenneth Mung from the Department of Pathology of Pamela Youde Nethersole Eastern Hospital for providing microscopic photos of the blood smear of the patient.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration
of Helsinki. The patient provided consent for all treatments
and procures, and consent for publication of this case report.
References
1. Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for
patients with treatment-naive advanced ALK-positive
non–small-cell lung cancer in the ALEX study. Ann Oncol
2020;31:1056-64. Crossref
2. Okumoto J, Sakamoto S, Masuda T, et al. Alectinib-induced
immune hemolytic anemia in a patient with lung
adenocarcinoma. Intern Med 2021;60:611-5. Crossref
3. Misawa, K, Nakamichi S, Iida H, et al. Alectinib-induced
severe hemolytic anemia in a patient with ALK-positive
non–small cell lung cancer: a case report. Onco Targets
Ther 2023;16:65-9. Crossref
4. Garbe E, Andersohn F, Bronder E, et al. Drug induced
immune haemolytic anaemia in the Berlin Case-Control
Surveillance Study. Br J Haematol 2011;154:644-53. Crossref
5. Palmer D, Seviar D. How to approach haemolysis:
haemolytic anaemia for the general physician. Clin Med
(Lond) 2022;22:210-3. Crossref
6. Gullapalli V, Xu W, Lewis CR, Anazodo A, Gerber GK. A
multi-centre case series of alectinib-related erythrocyte
membrane changes and associated haemolysis. J Hematop
2021;14:131-6. Crossref

