Hong Kong Med J 2024;30:Epub 17 Dec 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Public fertility preservation programme for cancer patients in Hong Kong
Dorothy TY Chan, MB, BS; Jennifer KY Ko, MB, BS, MRCOG; Kevin KW Lam, BSc, PhD; YW Tong, MB, BS, MRCOG; Evelyn Wong, MB, BS, MRCOG; Heidi HY Cheng, MB, BS, MRCOG; Sofie SF Yung, MB, BS, MRCOG; Raymond HW Li, MD, FRCOG; Ernest HY Ng, MD, FRCOG
Department of Obstetrics and Gynaecology, School of Clinical Medicine, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr Raymond HW Li (raymondli@hku.hk)
Abstract
Introduction: Fertility preservation (FP) offers
cancer patients the opportunity to have biological
children after completing treatment. This study was
performed to review the experience and changes in
service demand since the implementation of a public
FP programme for cancer patients in Hong Kong.
Methods: This retrospective study included men and
women who attended an assisted reproduction unit
for public FP services before cancer treatment from
August 2020 to February 2023. Their medical records
were reviewed and the results were compared with
findings from our previous study to evaluate trends
in service demand.
Results: During the study period, there were 48
consultations for female FP, compared with 72
women who presented for FP from 2010 to 2020 prior
to establishment of the public FP programme. The
median time from referral to consultation was 3 days
(interquartile range [IQR]=2-5). Eighteen women
(37.5%) underwent 19 cycles of ovarian stimulation
for oocyte or embryo cryopreservation. Thirty
women (62.5%) received gonadotropin-releasing
hormone agonists during cancer treatment. There
were 58 consultations for male FP during the study
period, compared with 265 men who presented for
sperm cryopreservation from 2005 to 2020. The median time from referral to consultation was 4
days (IQR=2-7). Fifty-five men (94.8%) attempted
sperm cryopreservation, and 49 (84.5%) successfully
preserved sperm.
Conclusion: Since the establishment of a public
FP programme for cancer patients, there has been
an increase in the demand for FP services at our
centre. Regular review of FP services is warranted
to assess changes in demand and identify areas for
improvement.
New knowledge added by this study
- Since the establishment of a public fertility preservation (FP) programme, there has been an increase in the number of patients seeking FP services at our centre.
- Reproductive-age men seeking FP were more likely than reproductive-age women to undergo gamete cryopreservation.
- Only 62.5% of women received gonadotropin-releasing hormone agonists during cancer treatment; the reasons for not receiving the agonists were not recorded.
- The cost of FP may be a barrier to patients considering this option.
- Public funding for medications and gamete storage can support reproductive-age patients in pursuing FP before cancer treatment.
- Further research is needed to improve FP, especially for reproductive-age women.
Introduction
Many individuals are diagnosed with cancer during
childhood, adolescence, and young adulthood.
Worldwide, there were approximately 1 335 100 new
cancer cases among adolescents and young adults
in 20191; the incidence rate was 44.99 per 100 000
people.1 In 2020, the incidence rate for cancer among Hong Kong children and adolescents (aged 0-19
years) was 160 cases per 1 000 000 people.2 There were
177 newly diagnosed cancer cases in this age-group
(92 in male patients and 85 in female patients).2 The
survival rates for childhood and adolescent cancers
are encouraging. In a retrospective cohort study
from a research hospital in the United States,3 the 5-year overall survival rate exceeded 83%. Similarly,
in Hong Kong, the 5-year survival rate among
women diagnosed with breast cancer, the most
common cancer in reproductive-age women, was
84% between 2010 and 2017.2
Chemotherapy or pelvic radiotherapy may
affect fertility, either temporarily or permanently.
Considering advances in cancer treatment and
improved post-treatment survival rates, fertility
should be discussed at the time of cancer diagnosis,
especially for younger patients who have not yet
completed their families. International guidelines
regarding fertility preservation (FP) recommend
that clinicians inform cancer patients about the
potential effects of cancer and its treatment on
reproductive function, as well as FP options.4 5
In a semi-structured phone interview study of
female cancer survivors who were diagnosed with
invasive cervical cancer, breast cancer, Hodgkin
lymphoma, or non-Hodgkin lymphoma at age ≤40
years, participants were interviewed an average of
10 years after diagnosis.6 Those who had wanted
children at the time of diagnosis but were unable
to conceive subsequently reported distress related
to their interrupted fertility.6 Additionally, patients
who do not receive accurate and timely information
regarding FP are at risk for psychological distress.7 In
our recently published cross-sectional questionnaire
study of reproductive-age women in Hong Kong
who had been diagnosed with breast cancer,8 only 44% of those women were aware of FP; however,
46% of the women felt that fertility concerns affected
their cancer treatment decisions.8
The most common FP options include sperm
cryopreservation for men and embryo or oocyte
cryopreservation for women. Other options for
women include pharmacological ovarian protection
using gonadotropin-releasing hormone (GnRH)
agonists, ovarian tissue cryopreservation, and
ovarian transposition. In Hong Kong, FP was
previously self-funded and only available through
private services. Sperm cryopreservation costs
approximately HK$4400 to HK$6600 for 2 years,
whereas oocyte and embryo cryopreservation costs
are approximately HK$15 000 to HK$20 000.9 Our
centre launched the first public FP programme for
cancer patients in Hong Kong, beginning in August
2020. Here, we review the two-and-a-half-year
experience of providing public FP services to cancer
patients in Hong Kong.
Methods
This retrospective study included men and women
who attended the Centre of Assisted Reproduction
and Embryology at The University of Hong Kong—Queen Mary Hospital for FP services before cancer
treatment, from the establishment of our public FP
programme in August 2020 until the end of February
2023.
Criteria for public fertility preservation
services
During the study period, we provided public FP for
cancer patients <35 years old, expressed a desire for
future fertility, had a survival rate exceeding 50%
after cancer treatment, had no living children, and
had not undergone prior chemotherapy or pelvic
radiotherapy. In women, an antral follicle count of
>7 on pelvic ultrasound was required. These criteria
were adapted from The Edinburgh Selection Criteria
for ovarian tissue cryopreservation.10
There was no minimum age requirement
for FP. Male adolescents could undergo sperm
freezing if they were able to provide sperm samples
for cryopreservation. For patients aged <18 years,
we included their parents in discussions prior to
proceeding with FP treatment.
During the study period, the public FP
programme offered up to 40 cycles of sperm freezing
and 20 cycles of oocyte/embryo freezing per year.
Referral process
Patients diagnosed with cancer who were expected
to undergo gonadotoxic treatments were referred
to our FP service by surgeons, oncologists,
paediatricians, haematologists, private practitioners,
and cancer support groups. Clinicians completed a referral letter, which can be downloaded from our
centre’s website.11 Patients or their doctors can also
contact us via email. Additionally, a chat group was
established between Hong Kong Children’s Hospital
and our centre to facilitate rapid referrals.
After we received a referral, the patient was
scheduled for an appointment in the public FP clinic
within 1 week. Our centre maintained a flexible
clinic schedule, which allowed urgent cases to be
accommodated within the existing clinic framework,
5 days per week.
Fertility preservation counselling
The details of our FP programme were previously
published.12 Information sheets and videos about
the FP services offered by our centre were readily
accessible to the general population and patients
through our website13 and YouTube channel.14
Patients were encouraged to review these materials
before attending the public FP clinic. For men, sperm
banking was arranged on the same day as counselling.
For women, the options of oocyte and embryo
preservation were discussed if feasible. Embryo
preservation was only offered to women who were
legally married. In Hong Kong, assisted reproductive
technology is regulated by the Human Reproductive
Technology Ordinance.15 This ordinance limits
the storage duration for frozen gametes in cancer
patients to 10 years or until the patient reaches the
age of 55 years, whichever is longer.15 The storage
duration for frozen embryos is limited to 10 years.15
Cryopreserved gametes and embryos can only be
used after a patient recovers from their illness and is
legally married.15 Posthumous use of cryopreserved
gametes and embryos is prohibited.15
The use of GnRH agonists for pharmacological
ovarian protection was discussed either after
cryopreservation or if cryopreservation was not
feasible. Such agonists were usually administered
monthly or every 3 months during chemotherapy.
The characteristics of men who underwent
sperm cryopreservation and women who underwent
ovarian stimulation for oocyte or embryo
cryopreservation were prospectively entered into
our database. Medical records (both in paper and
electronic formats), including data from the assisted
reproductive technology database at our centre
and the Hospital Authority’s electronic clinical
management system, were retrieved and reviewed.
These records encompassed demographic data,
cancer type, cancer treatment, FP method chosen,
ovarian stimulation cycle characteristics, semen
analysis, reproductive outcomes, and follow-up
information, if available.
All women who attended our centre for FP
were asked to return to our late-effects clinic for
gonadal function monitoring after the completion
of cancer treatment. All men were asked to undergo semen analysis when they wished to conceive after the completion of cancer treatment.
Statistical analysis
Data were analysed using SPSS (Windows version
26; IBM Corp, Armonk [NY], United States) and are
presented as median (interquartile range [IQR]) or
as number (percentage). P value was calculated by
Chi squared test.
Methods
Women
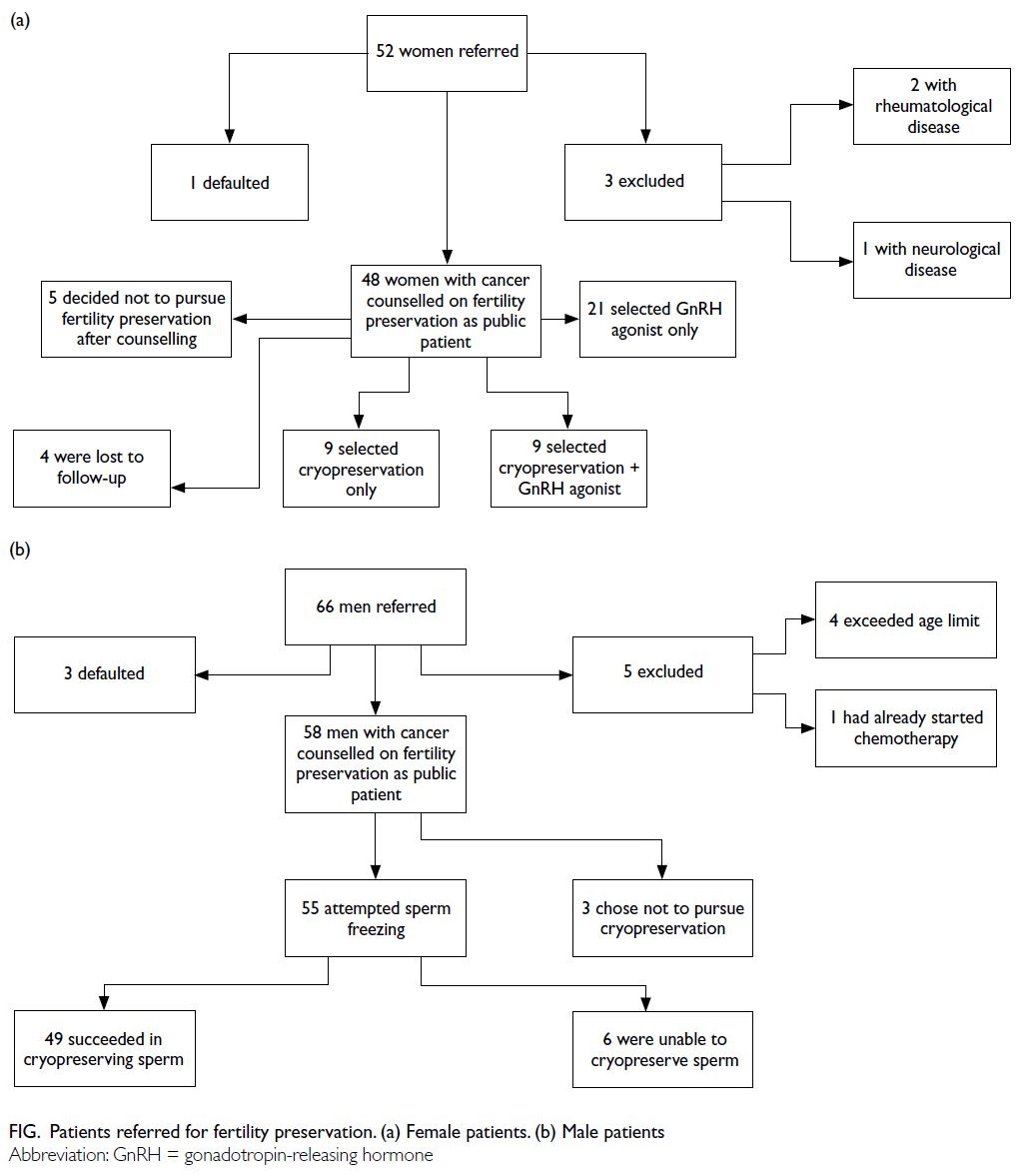
Fifty-two women were referred to our public FP
clinic between August 2020 and February 2023.
Three women were excluded from the analysis
because they had non-malignant conditions,
including rheumatological disease (systemic lupus
erythematosus) and neurological disease (multiple
sclerosis). Additionally, one woman missed her clinic
appointment. Therefore, the final analysis included
48 women (Fig a). The median age of these women
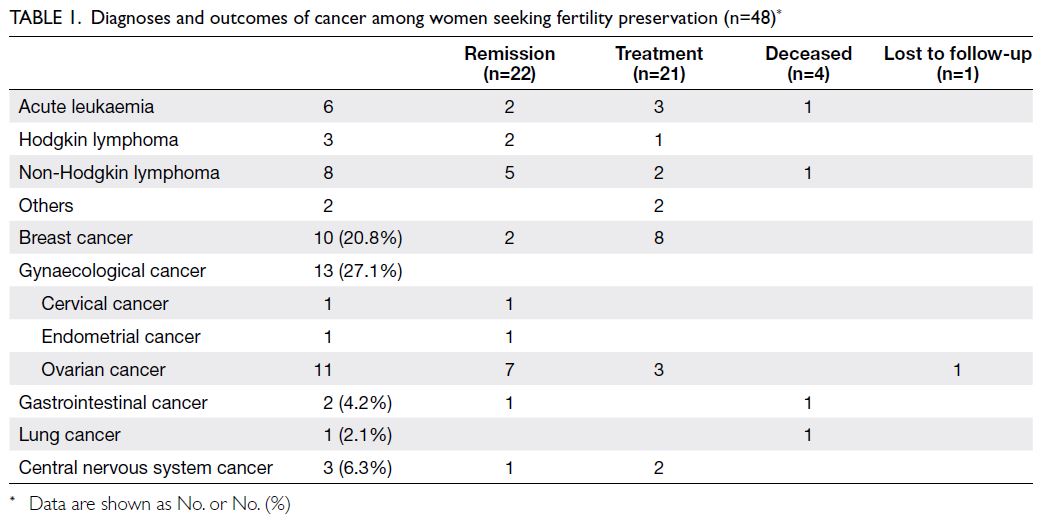
was 30 years (IQR=25-33). The cancer outcomes
of these women are shown in Table 1. Regarding
marital status, 36 women (75.0%) were single, 11
(22.9%) were married, and one (2.1%) was divorced.
All were nulliparous, except for one married woman
(2.1%) with a livebirth was ineligible for publicly
funded FP due to the programme’s criteria. She then
selected GnRH agonist treatment after counselling.
The median time from referral to consultation was 3
days (IQR=2-5).
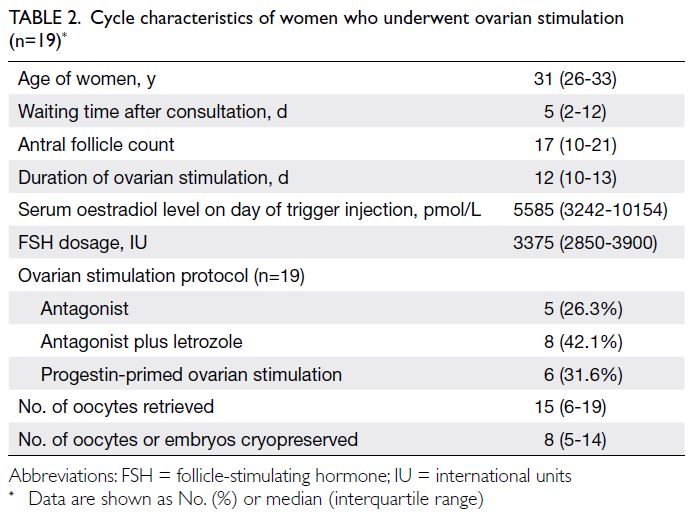
Eighteen women underwent 19 cycles
of ovarian stimulation for oocyte or embryo
cryopreservation (Table 2). One woman underwent
an additional self-financed stimulation cycle because
she only achieved two frozen oocytes in the first
cycle. She achieved two additional frozen oocytes in
the second attempt. Thirteen women cryopreserved
oocytes, whereas five women cryopreserved embryos
(three at the cleavage stage and two at the blastocyst
stage). The median time between consultation and
ovarian stimulation was 5 days (IQR=2-12) [Table 2].
All women with breast cancer received letrozole co-treatment
during ovarian stimulation.
One woman developed moderate ovarian
hyperstimulation syndrome requiring hospital
admission. Oocyte retrieval was uneventful, and
45 oocytes were retrieved. However, 3 days after
oocyte retrieval, she was admitted with abdominal
distension, shortness of breath, and vomiting.
She was diagnosed with moderate ovarian
hyperstimulation syndrome, which resolved with
conservative management.
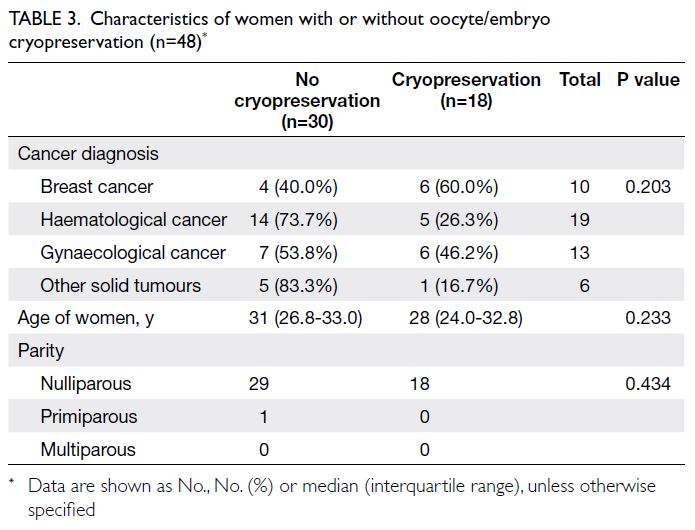
There was no significant age difference
between women who proceeded with oocyte/embryo cryopreservation and those who did not. The median age of women who proceeded with oocyte/embryo cryopreservation was 28 years (IQR=24.0-32.8), whereas the median age of women who did not proceed with oocyte/embryo cryopreservation was
31 years (IQR=26.8-33.0) [Table 3].
Among patients with breast and gynaecological
cancers, six of 10 (60.0%) and six of 13 (46.2%)
underwent oocyte/embryo cryopreservation,
respectively, compared with five of 19 (26.3%)
women with haematological cancers and one of six
(16.7%) women with other solid tumours (Table 3).
Among the 48 women who attended the clinic, nine (18.8%) proceeded with oocyte or embryo cryopreservation alone, nine (18.8%) underwent
cryopreservation followed by the use of GnRH
agonists, 21 (43.8%) received GnRH agonists alone,
five (10.4%) decided against FP after counselling, and
four (8.3%) were lost to follow-up. Those who chose
GnRH agonists received this treatment from their
primary oncology team.
At the end of February 2023, among the 48
women, 22 exhibited disease remission, 21 were
continuing treatment, four were deceased, and one had been lost to follow-up (Table 1). None of the
women have returned to use their frozen oocytes or
embryos, nor have any reported natural conception
since their cancer diagnosis.
Men
Sixty-six men were referred to our public FP clinic during the study period (Fig b). Five men were
excluded: four had exceeded the age limit and one had
already begun chemotherapy. Fertility preservation
counselling at a private clinic was offered to those
who were not eligible for the public FP service.
One man, who exceeded the age limit, underwent
self-funded sperm cryopreservation. Three men
missed their clinic appointments. Therefore, the
final analysis included 58 men (Fig b). The median
age of the men was 26 years (IQR=18.3-32.8). The
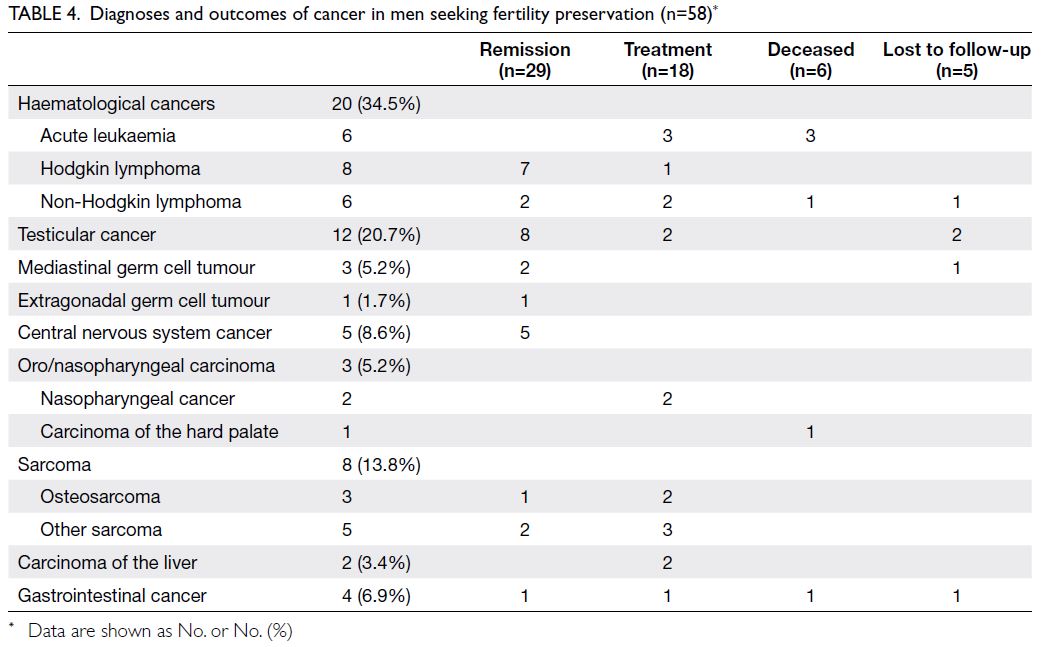
cancer outcomes of these men are shown in Table 4.
Regarding marital status, 51 men (87.9%) were single
and seven men (12.1%) were married. One man
(1.7%) had a child but was unmarried. The remaining
57 men (98.3%) had no offspring. The median time
from referral to consultation was 4 days (IQR=2-7).
Among the 58 men who attended the clinic,
55 attempted sperm freezing and three chose not to
undergo cryopreservation after counselling. Six men
were unable to cryopreserve sperm (Fig b). One,
aged 14 years, was unable to provide a semen sample;
four men submitted semen samples containing no
sperm. One man had previously attempted sperm
cryopreservation at a private hospital, but no
sperm were found in his ejaculate. He subsequently
underwent testicular sperm extraction at our
hospital; no sperm were retrieved. The ages of the
men with no sperm in their semen ranged from 15
to 34 years.
The median number of vials of cryopreserved
sperm was 5 (IQR=5-5) and the median sperm
concentration was 18.8 million/mL (IQR=4.3-52.8).
At the end of February 2023, among the
58 men, 29 exhibited disease remission, 18 were
continuing treatment, six were deceased, and five
had been lost to follow-up (Table 4). None of the
men have returned to use their frozen sperm.
As of this writing, six men and four woman
who attended the FP clinic have died.
Discussion
This is the first review of a public FP programme for
cancer patients in Hong Kong. Our study showed that among the 48 women who attended during
the study period, 37.5% (n=18) underwent oocyte/embryo cryopreservation and 62.5% (n=30) chose GnRH agonists for FP. In contrast, among the 58
men who attended for FP before cancer treatment,
>90% attempted sperm cryopreservation.
We previously published a review of our self-funded
FP service from 2010 to 2020.12 During
that period, 72 women attended consultations for FP, and 20 of them underwent 22 cycles of ovarian
stimulation for oocyte or embryo cryopreservation.12
Additionally, from 1995 to 2020, 265 men underwent
sperm cryopreservation.12 Over the years, there were
increases in the numbers of men and women seeking
FP; the increase was more prominent among women.
For comparison, we selected the period from
2018 to 2020 (ie, the 2.5 years immediately preceding
the launch of the public FP programme). During that
period, 19 women were referred for self-funded FP
prior to cancer treatment, and 10 (52.6%) underwent
oocyte or embryo cryopreservation. Fifty-eight
men were referred for FP and underwent sperm
cryopreservation. In the years prior to the launch
of the publicly funded FP programme, we had
already begun networking with various specialties,
which likely contributed to the gradual increase in
awareness and demand for FP services.
Public fertility preservation programme
A successful FP programme requires good
networking, flexibility, and a patient-friendly clinic
environment. During the establishment of the public
FP programme, we have networked with other
specialties to enhance collaboration. Our centre
aimed to simplify logistics so that consultations
could be arranged as quickly as possible, allowing FP
counselling and procedures to be completed within
the short window of opportunity before cancer
treatment. In our public FP clinic, the median waiting
times from referral to consultation were 3 days for women and 4 days for men. Among women who chose
oocyte or embryo cryopreservation, the median time
from consultation to the start of ovarian stimulation
was 5 days (IQR=2-12). In our previous study, the
time from consultation to oocyte retrieval was 17
days (IQR=13-30).12 Notably, our previous study
did not investigate the waiting time from referral to
consultation; therefore, direct comparisons cannot
be performed. Compared with our previous study
regarding FP for cancer patients at our centre,12 the
proportion of women who ultimately underwent
oocyte or embryo cryopreservation increased from
28% to 38% in the public FP programme. However,
further monitoring is needed to determine whether
this difference represents a true upward trend due to
increased awareness and easier access to the service.
Additionally, patient characteristics and cancer
types may vary across time periods.
For reproductive-age women with cancer, the
receipt of specialised counselling regarding fertility
issues, followed by FP, has been linked to less regret
and improved quality of life among survivors.16
Providing our patients with accessible FP counselling
and affordable treatments is an essential aspect of
comprehensive oncology care. A clinical practice
guideline from the American Society of Clinical
Oncology indicates that FP should be initiated as
early as possible in the treatment process to allow
for the widest range of options.17 Referral to FP
services enables patients to receive counselling from
reproductive medicine specialists, empowering
them to make informed decisions about fertility
treatment.
At our FP clinic, patients were able to consult
reproductive medicine specialists who discussed the
potential effects of gonadotoxic cancer treatments
on future fertility and described FP options. Local
regulations concerning gamete storage and assisted
reproduction were also explained. Patients were
informed that they must be legally married to use
frozen gametes in the future, and that gametes
cannot be used posthumously.
Gonadotropin-releasing hormone agonists
In our cohort, only 62.5% of women received
GnRH agonists during cancer treatment; the
reasons for not receiving GnRH agonists were
not recorded. Gonadotropin-releasing hormone
agonists are usually administered monthly or
every 3 months during cancer treatment, although
their effectiveness depends on the type of cancer
treatment. Some studies of breast cancer patients
have shown that GnRH agonists can reduce the risk
of premature ovarian insufficiency, but the fertility
benefit remains uncertain.18 19 20 Most studies have
focused on outcomes such as the maintenance
or resumption of menstruation, prevention of
treatment-related premature ovarian failure, and ovulation. In a Cochrane review20 which discussed
randomised controlled trials that examined the
effect of GnRH analogues for chemotherapy-induced
ovarian failure in premenopausal women, 12
randomised controlled trials were included. Eleven
studies reported rates of menstruation recovery or
maintenance, four studies measured treatment-related
premature ovarian failure, and seven studies
reported the rates of pregnancy.20 However, there
are limited data regarding live birth rates.20 A
meta-analysis of randomised studies concerning
ovarian suppression using GnRH agonists during
chemotherapy in breast cancer patients found that
temporary ovarian suppression with a GnRH agonist
in young breast cancer patients was associated with
a reduced risk of chemotherapy-induced premature
ovarian insufficiency; it also appeared to increase
the pregnancy rate without negatively influencing
prognosis.21 Thus far, the benefit of GnRH agonists
in other malignancies is unclear. A long-term
analysis of young female lymphoma patients showed
that GnRH agonists were not effective in preventing
chemotherapy-induced premature ovarian
insufficiency and did not improve future pregnancy
rates.22 According to the European Society of Human
Reproduction and Embryology guideline on female
FP,4 GnRH agonists should be offered as an option
for protecting ovarian function in premenopausal
breast cancer patients undergoing chemotherapy;
importantly, limited evidence exists regarding
their protective effects on ovarian reserve and
potential future pregnancies.4 In malignancies other
than breast cancer, GnRH agonists should not be
routinely offered as an option for protecting ovarian
function protection and FP without discussing
the uncertainty of their benefit.4 Gonadotropin-releasing
hormone agonists during chemotherapy
should not be considered as a substitute for
established FP techniques, such as cryopreservation.
They can be offered in addition to cryopreservation
or when such techniques are not feasible.4 Despite
the use of GnRH agonists, patients may experience
premature ovarian insufficiency. Gonadotropin-releasing
hormone agonists are currently provided
as a self-financed option; women are often referred
back to their oncology team, who prescribes and
administers these agonists after FP counselling.
The proportion of patients who underwent oocyte/embryo cryopreservation was higher among those
with gynaecological or breast cancers than among
those with haematological malignancies. This
difference is likely due to the nature of their diseases
and the urgency of initiating cancer treatment.
Oocyte or embryo cryopreservation
For women who chose to proceed with ovarian
stimulation for oocyte or embryo cryopreservation,
oocytes were retrieved during a stimulated cycle. Recombinant follicle-stimulating hormone could
be initiated on any day of the menstrual cycle for
ovarian stimulation (ie, ‘random-start’), using either
a GnRH antagonist or progestin-primed protocol.
This random-start approach allowed ovarian
stimulation without substantial delays and did not
affect the number or quality of retrieved oocytes.4
For women with hormone-sensitive cancers (eg,
breast cancer), letrozole was routinely used during
ovarian stimulation. The concomitant use of
letrozole reduced circulating oestrogen levels and
did not impair the efficacy of ovarian stimulation.23
A systematic review and meta-analysis regarding
the safety of hormonal stimulation in young women
with breast cancer before starting cancer treatment,
as well as survivors who underwent assisted
reproduction after cancer treatment, showed
no increased risk of breast cancer recurrence in
women who underwent ovarian stimulation with
concomitant letrozole treatment.24 Despite using
the ‘random-start’ approach, one cycle of ovarian
stimulation required approximately 2 weeks.
Limitations
This study had some limitations. It was a retrospective,
single-centre study conducted over a short period
of time; thus, it may not reflect situations in other
regions. Due to resource constraints, we only
included cancer patients who had not begun cancer
treatment. Patients who did not meet the criteria for
the public FP programme but still wished to pursue
FP were referred to private clinics or other private
centres upon receipt of their referral and therefore
were excluded from this review. Patients who had
already begun cancer treatment were also excluded
from the public FP service. However, they could still
be referred to our centre after stabilisation to assess
fertility and explore self-funded FP options before
undergoing more toxic chemotherapy, non–fertility-sparing
radiotherapy, or surgeries. At the time of
writing, our centre has not yet offered ovarian or
testicular tissue cryopreservation. A 2018 survey of
several Asian countries (eg, Australia, China, and
India) revealed that ovarian tissue cryopreservation
was available for prepubertal girls and postpubertal
women who were unable to delay the initiation of
chemotherapy.25 Testicular tissue cryopreservation
also was provided to prepubertal boys in Australia,
China, India, Indonesia, Japan, and Taiwan.25 A recently
published pilot study from Hong Kong demonstrated
the feasibility of ovarian tissue cryopreservation and
transplantation using xenografts in nude mice26;
ovarian tissue cryopreservation has recently become
available in Hong Kong.
The patients included in this study were
counselled for FP, and many are still undergoing
cancer treatment and monitoring; none have returned to use the stored material. They were
advised to return after cancer treatment for follow-up
regarding their gonadal function. Patient
satisfaction should also be evaluated. However, at
the time of cryopreservation—typically close to
the time of cancer diagnosis—patients may feel
overwhelmed by the diagnosis and planned cancer
treatments. Thus, patient satisfaction may be more
accurately evaluated when the cancer is controlled
or in remission.
Future outlook
Despite the presence of the public FP programme,
patients were required to pay for the medications
used in ovarian stimulation, as well as the fees
involved in oocyte handling, freezing, and storage
of frozen gametes or embryos; these costs were
considerably reduced compared with expenses in
private clinics. Cost remains a major barrier to
accessing FP services. Although a public healthcare
system has been established in Hong Kong, cancer
patients are often financially overwhelmed due to
the loss of income after a cancer diagnosis, along
with additional expenditures for various self-funded
investigations or treatments. We recently performed
a survey of the knowledge, attitudes, and intentions
regarding FP among breast cancer patients; most
participants thought that FP should be subsidised by
the government or provided at no cost.8
Conclusion
Since the establishment of a public FP programme
for cancer patients, there has been an increase in
the number of patients seeking FP services. More
than 90% of men attempted sperm cryopreservation,
whereas 37.5% of women underwent oocyte/embryo
cryopreservation and 62.5% of women received
GnRH agonists during cancer treatment. With
further promotion, changes in funding policies,
and a more accessible FP programme, the demand
for FP services is expected to increase. Fertility
preservation services should be regularly reviewed
to assess changes in demand and identify areas for
improvement.
Author contributions
Concept or design: JKY Ko, EHY Ng.
Acquisition of data: DTY Chan.
Analysis or interpretation of data: DTY Chan, JKY Ko, EHY Ng.
Drafting of the manuscript: DTY Chan.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: DTY Chan.
Analysis or interpretation of data: DTY Chan, JKY Ko, EHY Ng.
Drafting of the manuscript: DTY Chan.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study protocol was approved by the Institutional Review
Board of The University of Hong Kong/Hospital Authority
Hong Kong West Cluster, Hong Kong (Ref No.: UW 23-334).
The requirement for informed consent was waived by the
Board due to the retrospective nature of the research.
References
1. You L, Lv Z, Li C, et al. Worldwide cancer statistics of
adolescents and young adults in 2019: a systematic analysis
of the Global Burden of Disease Study 2019. ESMO Open
2021;6:100255. Crossref
2. Hong Kong Cancer Registry, Hospital Authority. Overview
of Hong Kong Cancer Statistics of 2020. Available
from: https://www3.ha.org.hk/cancereg/pdf/overview/Overview%20of%20HK%20Cancer%20Stat%202020.pdf. Accessed 7 May 2023.
3. Bhakta N, Liu Q, Ness KK, et al. The cumulative burden
of surviving childhood cancer: an initial report from
the St Jude Lifetime Cohort Study (SJLIFE). Lancet
2017;390:2569-82. Crossref
4. ESHRE Guideline Group on Female Fertility Preservation;
Anderson RA, Amant F, et al. ESHRE guideline:
female fertility preservation. Hum Reprod Open
2020;2020:hoaa052. Crossref
5. Practice Committee of the American Society for
Reproductive Medicine. Fertility preservation in patients
undergoing gonadotoxic therapy or gonadectomy: a
committee opinion. Fertil Steril 2019;112:1022-33. Crossref
6. Canada AL, Schover LR. The psychosocial impact of
interrupted childbearing in long-term female cancer
survivors. Psychooncology 2012;21:134-43. Crossref
7. Rosen A, Rodriguez-Wallberg KA, Rosenzweig L.
Psychosocial distress in young cancer survivors. Semin
Oncol Nurs 2009;25:268-77. Crossref
8. Ko JK, Cheung CS, Cheng HH, et al. Knowledge, attitudes
and intention on fertility preservation among breast cancer
patients. Sci Rep 2023;13:9645. Crossref
9. Yeung SY, Ng EY, Lao TT, Li TC, Chung JP. Fertility
preservation in Hong Kong Chinese society: awareness,
knowledge and acceptance. BMC Womens Health
2020;20:86. Crossref
10. Wallace WH, Smith AG, Kelsey TW, Edgar AE,
Anderson RA. Fertility preservation for girls and young
women with cancer: population-based validation of
criteria for ovarian tissue cryopreservation. Lancet Oncol
2014;15:1129-36. Crossref
11. Centre of Assisted Reproduction and Embryology, The
University of Hong Kong–Queen Mary Hospital. How to
make an appointment. Available from: https://hkuivf.hku.hk/en/services/fertility-preservation/how-to-make-an-appointment/. Accessed 27 Nov 2024.
12. Ko JK, Lam KK, Cheng HH, et al. Fertility preservation
programme in a tertiary-assisted reproduction unit in
Hong Kong. Fertil Reprod 2021;3:94-100. Crossref
13. Centre of Assisted Reproduction and Embryology, The
University of Hong Kong–Queen Mary Hospital. Fertility
preservation. Available from: https://hkuivf.hku.hk/en/services/fertility-preservation/. Accessed 27 Nov 2024.
14. Centre of Assisted Reproduction and Embryology, The
University of Hong Kong–Queen Mary Hospital. YouTube
Channel. Available from: https://www.youtube.com/@HKUIVF. Accessed 27 Nov 2024.
15. Hong Kong e-Legislation, Hong Kong SAR Government.
Cap 561 Human Reproductive Technology Ordinance.
Available from: https://www.elegislation.gov.hk/hk/cap561!en-zh-Hant-HK. Accessed 27 Nov 2024.
16. Letourneau JM, Ebbel EE, Katz PP, et al. Pretreatment
fertility counseling and fertility preservation improve
quality of life in reproductive age women with cancer.
Cancer 2012;118:1710-7. Crossref
17. Oktay K, Harvey BE, Partridge AH, et al. Fertility
preservation in patients with cancer: ASCO clinical
practice guideline update. J Clin Oncol 2018;36:1994-2001. Crossref
18. Blumenfeld Z. Fertility preservation using GnRH
agonists: rationale, possible mechanisms, and explanation
of controversy. Clin Med Insights Reprod Health
2019;13:1179558119870163. Crossref
19. Zong X, Yu Y, Yang H, et al. Effects of gonadotropin-releasing
hormone analogs on ovarian function
against chemotherapy-induced gonadotoxic effects in
premenopausal women with breast cancer in China: a
randomized clinical trial. JAMA Oncol 2022;8:252-8. Crossref
20. Chen H, Xiao L, Li J, Cui L, Huang W. Adjuvant
gonadotropin-releasing hormone analogues for the
prevention of chemotherapy-induced premature ovarian
failure in premenopausal women. Cochrane Database Syst
Rev 2019;3:CD008018. Crossref
21. Lambertini M, Ceppi M, Poggio F, et al. Ovarian
suppression using luteinizing hormone-releasing hormone
agonists during chemotherapy to preserve ovarian function
and fertility of breast cancer patients: a meta-analysis of
randomized studies. Ann Oncol 2015;26:2408-19. Crossref
22. Demeestere I, Brice P, Peccatori FA, et al. No evidence for
the benefit of gonadotropin-releasing hormone agonist
in preserving ovarian function and fertility in lymphoma
survivors treated with chemotherapy: final long-term
report of a prospective randomized trial. J Clin Oncol
2016;34:2568-74. Crossref
23. Moravek MB, Confino R, Lawson AK, et al. Predictors
and outcomes in breast cancer patients who did or did
not pursue fertility preservation. Breast Cancer Res Treat
2021;186:429-37. Crossref
24. Arecco L, Blondeaux E, Bruzzone M, et al. Safety of fertility
preservation techniques before and after anticancer
treatments in young women with breast cancer: a systematic
review and meta-analysis. Hum Reprod 2022;37:954-68. Crossref
25. Takae S, Lee JR, Mahajan N, et al. Fertility preservation for
child and adolescent cancer patients in Asian countries.
Front Endocrinol (Lausanne) 2019;10:655. Crossref
26. Chung JP, Chan DY, Song Y, et al. Implementation of
ovarian tissue cryopreservation in Hong Kong. Hong Kong
Med J 2023;29:121-31. Crossref