Hong Kong Med J 2025;31:Epub 9 Apr 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Impact of iron deficiency on attention among
school-aged adolescents in Hong Kong
YT Cheung, PhD1; Dorothy FY Chan, MB, ChB2; CK Lee, MB, BS, MD3; WC Tsoi, MB, ChB3; CW Lau, MB, ChB3; Jennifer NS Leung, MB, BS3; Jason CC So, MB, BS4; Stella TY Tsang, PhD5; Chris LP Wong, PhD6; Yvonne YL Chu, MB7; CK Li, MB, BS, MD7
1 School of Pharmacy, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
2 Department of Paediatrics, Prince of Wales Hospital, Hong Kong SAR, China
3 Hong Kong Red Cross Blood Transfusion Service, Hospital Authority, Hong Kong SAR, China
4 Department of Pathology, Hong Kong Children’s Hospital, Hong Kong SAR, China
5 Department of Pathology, Hong Kong Molecular Pathology Diagnostic Centre, Hong Kong SAR, China
6 Amber Medical Group Limited, Hong Kong SAR, China
7 Department of Paediatrics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Prof CK Li (ckli@cuhk.edu.hk)
Abstract
Introduction: Adolescence is a critical period for
higher-order cognitive function development. The
adverse effects of low iron reserves on attention are
particularly relevant to school-aged students. Based
on our previous study identifying a 11.1% prevalence
of iron deficiency (ID) among Chinese school-aged
adolescents aged 16 to 19 years in Hong Kong, the
present study examined the association between iron
status and attention outcomes in these adolescents.
Methods: This cross-sectional study recruited
523 adolescents (65.0% female; mean age=17.5
years) from 16 local schools. Serum ferritin levels
and complete blood counts were measured.
Iron deficiency was defined as serum ferritin
concentration <15 μg/L. The Conners Continuous
Performance Test Third Edition was administered
to assess impairments in three attention domains,
namely, sustained attention, inattention, and
impulsivity. Multivariable analyses, conducted both
for the overall cohort and stratified by sex, were used
to evaluate the associations between serum ferritin
levels and attention outcomes, adjusting for fatigue
and dietary patterns.
Results: In the overall cohort, a lower serum
ferritin concentration was significantly associated
with sustained attention impairment (risk ratio
[RR]=0.825, 95% confidence interval [95% CI]=0.732-0.946; P=0.040). Among female participants,
those with sustained attention impairment had
significantly lower serum ferritin concentrations
than those with intact attention function
(median=40.0 μg/L; interquartile range [IQR]=18.8-52.1 vs median=48.5 μg/L; IQR=21.8-73.8; P=0.038).
Multivariable analysis showed a similar trend, though the association was not statistically significant
(RR=0.954, 95% CI=0.904-1.005; P=0.073). Among
male adolescents, iron reserves were not significantly
associated with attention outcomes.
Conclusion: These findings highlight the importance
of timely ID screening and correction in school-aged
adolescents, particularly among female adolescents.
New knowledge added by this study
- The prevalence of iron deficiency among Chinese school-aged adolescents aged 16 to 19 years in Hong Kong is 11.1%.
- Lower serum ferritin reserves were associated with sustained attention impairment in the overall cohort.
- The consequences of low iron reserves on health and functional outcomes should be emphasised among school-aged adolescents.
- Adolescents with low ferritin concentrations should receive counselling on the consumption of iron-rich foods and iron supplementation.
- Future research should evaluate the effects of iron supplementation on functional outcomes.
Introduction
Adolescence marks a critical stage of physical
growth, lean body mass development, and pubertal
maturation. These biological and physiological
changes increase the demand for micronutrients.
In particular, iron deficiency (ID) remains a global
public health concern.1 Iron deficiency is the most
common nutritional deficiency and the leading
cause of iron deficiency anaemia (IDA). Because
dietary intake is the primary source of iron for most
individuals, inadequate dietary iron intake is the
main cause of IDA, particularly in adolescents, who
are more likely to have poor dietary patterns.2 The
Global Burden of Disease 2020 report estimated
that approximately 60% of the total global burden of
anaemia in 2019 arose from inadequate dietary iron
intake.3 Consequently, ID was identified as the most
important cause of anaemia-related disability.3 4
In addition to its essential role in haemoglobin
synthesis, iron is a key element in brain metabolism
and is vital for multiple cellular processes, including
neurotransmitter synthesis, neuron myelination,
and mitochondrial function.5 Studies in young
children have demonstrated that ID during early
life adversely affects psychomotor development,
concentration, memory, and learning ability.6 7
Notably, the attention domain has received considerable research interest because iron plays
a crucial role in the regulation of dopaminergic
activity, which is implicated in the pathogenesis
and symptoms of attention-deficit hyperactivity
disorder (ADHD). Some studies have detected lower
ferritin concentrations in children diagnosed with
ADHD than in non-ADHD controls.8 9 However,
many cognitive studies regarding ID have involved
children aged ≤15 years.8 10 Few population-based
studies have examined the effect of iron status on
cognitive outcomes in adolescents and young adults,
and no such studies have been conducted in Chinese
populations.
Our previous study11 reported a prevalence of
11.1% for ID among Chinese school-aged adolescents
aged 16 to 19 years in Hong Kong, with ID and IDA
affecting 17.1% and 10.9% of girls, respectively, while
no male participants were affected More than one-third
of these adolescents reported regularly skipping
at least one meal per day.11 Notably, lower serum
ferritin concentrations were observed in adolescents
who skipped meals, reported infrequent intake of
iron-rich foods, or had heavy menstrual bleeding.11
Consistent with findings from other studies, poor
iron reserves were associated with greater self-reported
fatigue, reduced physical functioning, and
worse school performance.11 Adolescence represents
the second most critical period for the development
of higher-order cognitive functions, including
attention, self-control, and executive function. The
adverse effects of low iron reserves on attention span
and attentiveness are particularly relevant to upper
secondary students in Hong Kong, who are expected
to excel academically and prepare for the Hong Kong
Diploma of Secondary Education Examination, the
city’s university entrance examination. This study
aimed to examine the association between iron
status and attention outcomes among school-aged
adolescents in Hong Kong.
Methods
This cross-sectional study recruited healthy
adolescent students through the Hong Kong Red
Cross Blood Transfusion Service blood donation
campaigns at 16 secondary schools between
October 2020 and December 2021. The detailed
methodology was described in our previous report,11
which aimed to identify the risk factors of ID and
IDA in this cohort to facilitate future association
studies on health and functional outcomes. In the
present study, the dataset was used to delineate
the impact of iron reserves on performance-based
attention functioning, which is distinct from the
self-reported daily functioning outcomes presented
in the previous report.11
Study population
Students eligible for this study were aged ≥16 years and had agreed to participate in blood donation
screening. Students were excluded if they exhibited
signs or symptoms of an active infection, reported
a history of anaemia, or were receiving treatment
for anaemia. Students who did not pass the blood
donation screening were still permitted to participate
in the study.
Prevalences of iron deficiency and iron
deficiency anaemia
A serum ferritin concentration <15 μg/L was
considered indicative of ID in both male and
female participants, based on the World Health
Organization definition.12 Iron deficiency anaemia
was defined as the presence of both ID and anaemia.
In accordance with the recommendations of the
World Health Organization, anaemia was defined
as a haemoglobin concentration <12 g/dL in female
participants and <13 g/dL in male participants.13
All assays were conducted on the same day in the
Department of Pathology Laboratory at Hong
Kong Children’s Hospital. The specifications of the
instruments and tests have been reported in our
prior study.11
Attention outcomes
Before blood donation, participants completed the
Conners Continuous Performance Test Third Edition
(CPT-III), a validated assessment commonly used in
clinical and research settings to evaluate attention.14
The CPT-III requires 14 minutes to complete and
generates specific CPT attention measures (online supplementary Tables 1 to 3). Raw scores for each
CPT measure were converted into T-scores based
on normative samples (mean=50, standard deviation
[SD]=10). Each CPT measure was classified as
indicating no/mild (T-score within <1 SD), moderate
(T-score within 1-2 SDs), or severe (T-score within
>2 SDs) impairment.
Based on the CPT-III manual and the clinical
discretion of a developmental specialist (the second
author), attention measures were categorised
into three clinically relevant attention domains of
interest,14 namely, sustained attention impairment
(inability to maintain attention), inattention
(inability to focus or concentrate), and impulsivity
(difficulty with response inhibition).
Covariates
Fatigue, a recognised risk factor for diminished
neurocognitive function, is associated with
ID.11 15 Participants completed the PedsQL
Multidimensional Fatigue Scale, which has been
validated in young adults up to 25 years of age.16
Each item was scored on a 100-point reverse scale,
where lower scores indicated more severe fatigue.
The Traditional Chinese version of the PedsQL Multidimensional Fatigue Scale has demonstrated
good internal consistency, reliability, and content
validity in the Chinese population.17 18
We previously reported that dietary patterns
are associated with iron reserves in Hong Kong
adolescents.11 All participants self-reported their
dietary patterns, including meal-skipping habits
(breakfast, lunch, or dinner) and the frequency
of consuming common iron-rich foods, namely,
seafood, meat, iron-fortified cereal, leafy vegetables,
beans, nuts, dried fruits, and eggs.11
Statistical analyses
The demographic and haematological characteristics
of the cohort, along with their attention outcomes,
were summarised using descriptive analysis.
The primary outcome was attention
impairment. Serum ferritin concentration was used
as the predictor of interest, rather than a comparison
of attention outcomes between participants with
and without ID or IDA, considering that clinical
thresholds for diagnosing ID and IDA may not
be applicable when evaluating the effect of iron
on functional outcomes. Even if an adolescent is
not clinically diagnosed with ID or IDA, a low-to-normal
ferritin concentration may affect functional
outcomes; previous studies have shown that the
impact of ID on neurodevelopment may occur
before ID manifests as clinical anaemia.19 20 The
Mann-Whitney U test was utilised to compare
serum ferritin concentrations between participants
with normal attention function (ie, those who did
not exhibit impairment in any of the three attention
domains) and those with moderate or severe
impairment in sustained attention, inattention, or
impulsivity.
Multivariable analysis using a log-binomial
regression model was conducted, with serum ferritin
concentration, fatigue, dietary pattern, and dietary
iron intake as predictors. Models were adjusted for
age and sex. Risk ratios (RRs) and 95% confidence
intervals (95% CIs) were calculated.
Given that previous studies have shown a
positive association between iron reserves and
functional outcomes regardless of sex,8 15 20 21 we
first conducted all analyses in the overall cohort.
Subsequently, analyses were performed separately
for male and female participants.
The significance threshold was set at P<0.05.
All statistical analyses were performed using SAS 9.4
(SAS Institute, Cary [NC], US) and were two-tailed.
Results
As reported in our previous study,11 a total of 523
students were recruited (participation rate: 70%).
Twenty-nine students were deferred from blood
donation due to low haemoglobin concentrations but still completed the study procedures. Two-thirds
of participants were female (n=340, 65.0%). The
demographics of the study cohort, stratified by sex,
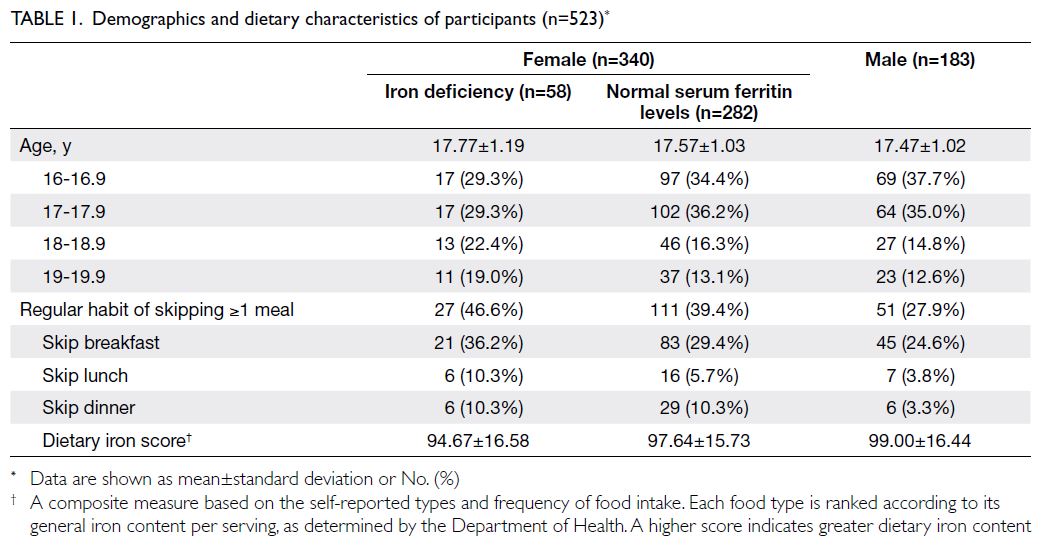
are presented in Table 1.
The median ferritin concentration in male
participants was 136.17 μg/L (interquartile range
[IQR]=89.89-219.83; Fig a); no male participants
were diagnosed with ID. Among female participants
diagnosed with ID (n=58/340, 17.1%), the median
haemoglobin concentration was 11.6 g/dL (IQR=11.1-12.2; Fig b). Among female participants with normal
serum ferritin concentrations (n=282/340, 82.9%),
the median serum ferritin concentration was 56.07
μg/L (IQR=33.82-84.11; Fig c).
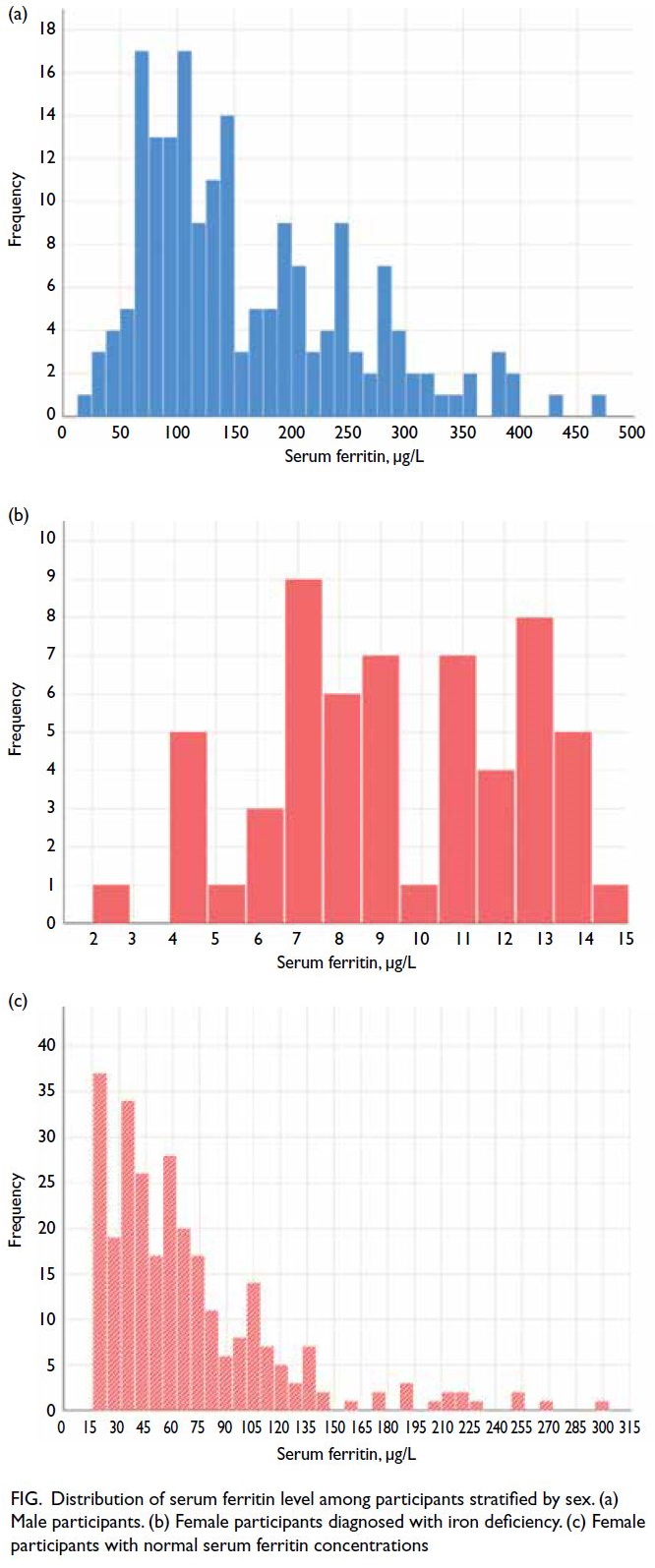
Figure. Distribution of serum ferritin level among participants stratified by sex. (a) Male participants. (b) Female participants diagnosed with iron deficiency. (c) Female participants with normal serum ferritin concentrations
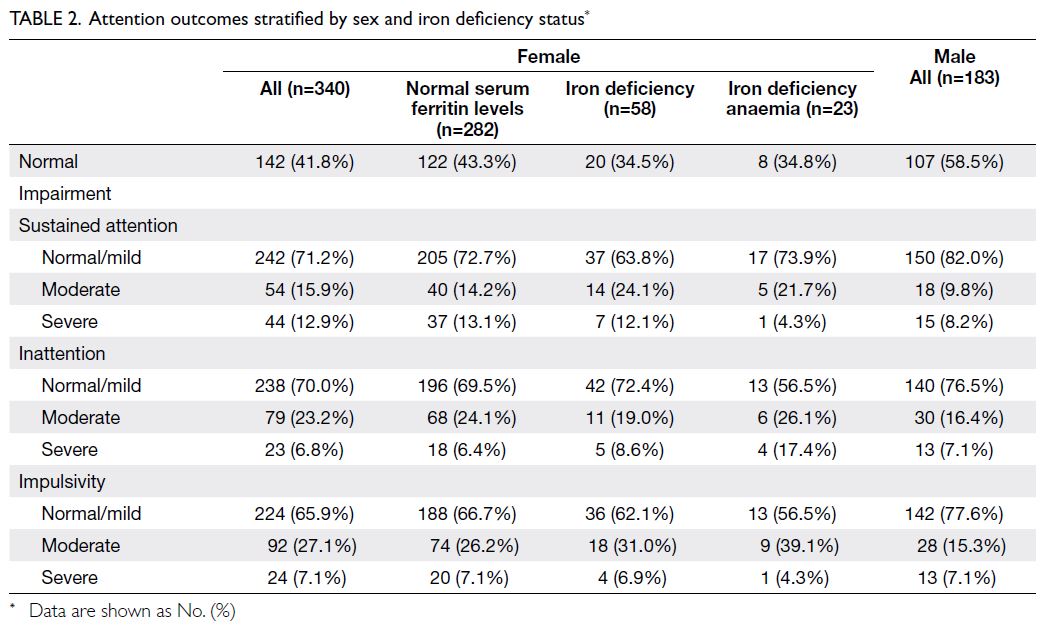
Attention outcomes
Overall, 249 participants (47.6%) exhibited
normal function in all three attention domains.
Approximately one-quarter of the participants
demonstrated moderate-to-severe impairment in
sustained attention (n=131/523, 25.0%), inattention
(n=145/523, 27.7%), and impulsivity (n=157/523,
30.0%).
Among female participants with ID, the rates
of moderate-to-severe impairment in sustained
attention, inattention, and impulsivity were 36.2%
(n=21/58), 27.6% (n=16/58), and 37.9% (n=22/58),
respectively. The rates of moderate-to-severe
impairment in inattention and impulsivity among
female participants with IDA were numerically
higher at 43.5% (n=10/23 for both domains). Among
male participants, the rates of moderate-to-severe
impairment in sustained attention, inattention,
and impulsivity were 18.0% (n=33/183), 23.5%
(n=43/183), and 22.4% (n=41/183), respectively
(Table 2).
Association between iron reserves and
attention outcomes in the overall cohort
In the overall cohort, participants with sustained
attention impairment had significantly lower
serum ferritin concentrations relative to those
with intact attention function (median=51.2 μg/L,
IQR=27.1-106.8 vs median=73.9 μg/L, IQR=37.8-138.0; P=0.020). Although the associations were not
statistically significant, trends of lower serum ferritin
concentrations were also observed in participants
with impulsivity impairment (median=68.1 μg/L,
IQR=29.0-114.8 vs median=73.9 μg/L, IQR=37.8-138.0; P=0.067) and inattention impairment
(median=69.9 μg/L, IQR=32.0-110.8 vs median=73.9
μg/L, IQR=37.8-138.0; P=0.142) relative to those
with intact attention function.
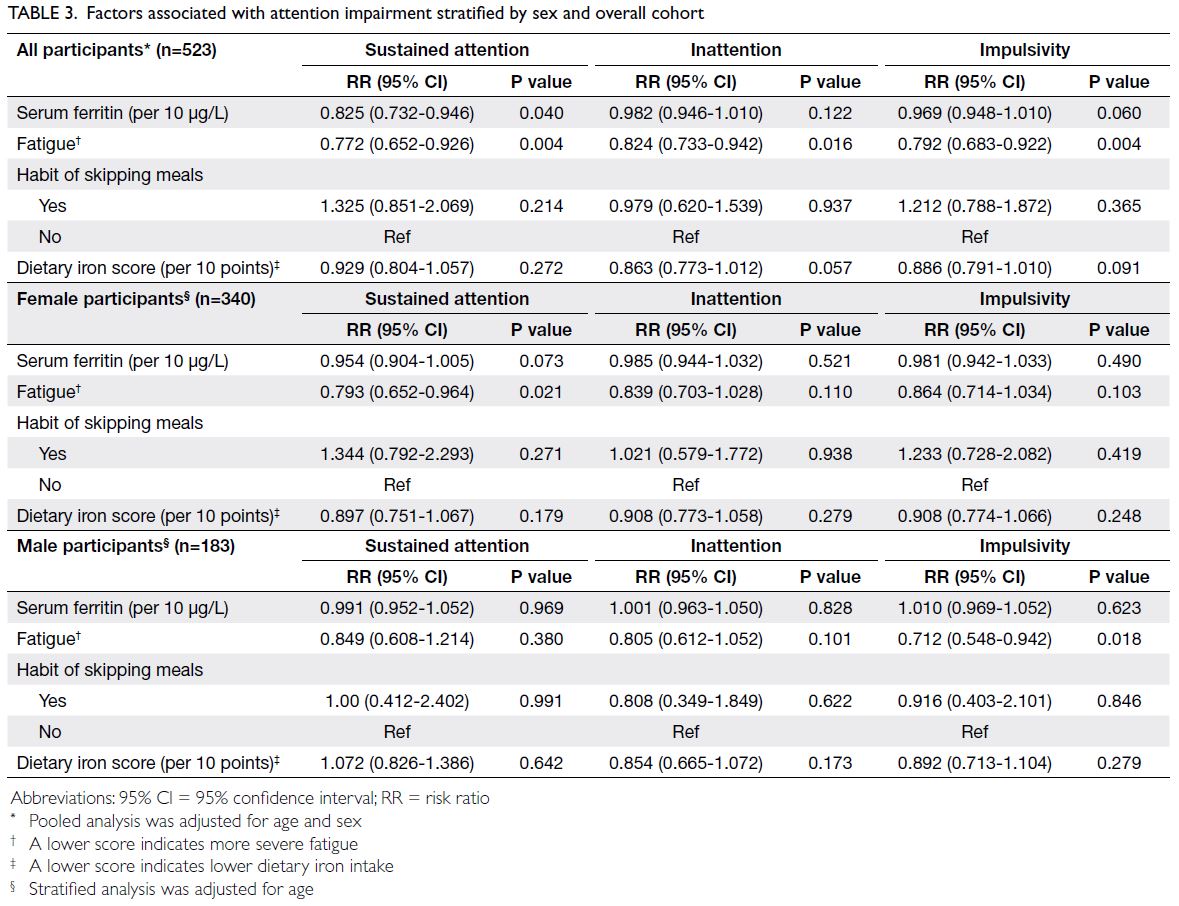
Pooled analysis of the overall cohort, adjusted
for age and sex, showed a significant association
between lower serum ferritin concentration and
sustained attention impairment (RR=0.825, 95%
CI=0.732-0.946; P=0.040), suggesting that each
10 μg/L increase in serum ferritin concentration
was associated with a 17.6% decrease in the risk of
sustained attention impairment. A higher level of
fatigue was associated with impairment in sustained
attention (RR=0.772, 95% CI=0.652-0.926; P=0.004),
inattention (RR=0.824, 95% CI=0.733-0.942;
P=0.016), and impulsivity (RR=0.792, 95% CI=0.683-0.922; P=0.004). Serum ferritin concentration was
not significantly associated with risks of impairment
in inattention or impulsivity (Table 3).
Association between iron reserves and
attention outcomes stratified by sex
Female participants with sustained attention
impairment had marginally lower serum ferritin concentrations relative to those with intact attention
function (median=40.0 μg/L, IQR=18.8-52.1 vs
median=48.5 μg/L, IQR=21.8-73.8; P=0.038).
Although the associations were not statistically
significant, trends for lower serum ferritin
concentrations were also observed in participants
with impulsivity impairment (median=43.0 μg/L,
95% CI=19.5-63.2 vs median=48.5 μg/L, IQR=21.8-73.8; P=0.071) relative to those with intact attention
function. No significant difference was observed for
inattention impairment. Additionally, no significant
association was detected between iron reserves and
attention impairment in male participants.
Multivariable analysis revealed that the
association between iron reserves and sustained
attention impairment in female participants
was attenuated and not statistically significant
(RR=0.954, 95% CI=0.904-1.005; P=0.073). A higher
level of fatigue was associated with an increased
risk of sustained attention impairment (RR=0.793,
95% CI=0.652-0.964; P=0.021). Among male
participants, iron reserves did not affect attention
outcomes, but fatigue was associated with impulsivity
impairment (RR=0.712, 95% CI=0.548-0.942;
P=0.018). Dietary patterns were not significantly
associated with attention outcomes in either male or
female participants (Table 3).
Discussion
In the overall cohort, a lower serum ferritin
concentration was associated with a higher risk
of sustained attention impairment, consistent
with previous reports that iron reserves play
an essential role in functional performance in
adolescents.6 7 8 21 When the analysis was stratified
by sex, a similar but modest association between
low iron reserves and sustained attention
impairment was observed in female school-aged
adolescents. This finding is supported by studies
regarding the neurobiology of attention-related
developmental disorders associated with ID.6 7 9 10
A meta-analysis of 10 studies, comprising 2191
healthy children and 1196 children with ADHD,
showed that serum ferritin concentrations were
0.4-fold lower in children with ADHD than in those
without developmental disorders.8 Iron deficiency
may be associated with disruptions in monoamine
synthesis and monoamine signal transduction, which
manifest as attention deficits.10 22 Adequate iron
intake and iron stores may, therefore, be important
factors influencing the onset of attention problems
in the developing brain. This finding should be
prospectively validated in larger cohorts with a
comprehensive assessment of cognitive domains
beyond attention. However, from a developmental
perspective, sustained attention is closely related
to performance on targeted assessments, such as
mathematical fluency and reading comprehension, as well as broader academic measures in national
standardised examinations.23 24 This relationship
is particularly relevant because the Hong Kong
educational system is well known for its examination-dominated
culture. Most examinations range from
2 to 3 hours, requiring students to maintain a high
level of sustained attention. Therefore, these findings
may have long-term implications for students’
academic success. Future research should investigate
the effects of ID and IDA on subsequent academic
achievement in Hong Kong adolescents.
Evidence regarding the effectiveness of
iron supplementation in terms of improving
neurocognitive function in children and adolescents
has been inconclusive. Furthermore, iron
supplements are associated with gastrointestinal
symptoms and constipation, which contribute to
non-adherence, particularly in adolescents.25 A
systematic review of 14 randomised controlled
trials indicated that iron supplementation improved
attention and intelligence quotient in anaemic older
children and adults.26 However, these effects were
inconsistent across studies; they were influenced
by socio-economic factors, participant age, and the
clinical thresholds used to define ID and IDA.20 25 26
The benefits for cognitive development in older
adolescents remain uncertain and warrant further
investigation.26
In this study, we found that students who
reported higher levels of fatigue were more
likely to have worse attention outcomes. We also
previously reported that lower serum ferritin
concentrations are associated with self-reported
fatigue in adolescents.11 Evidence supporting the
role of iron supplementation in fatigue reduction
is more consistent than its effects on cognitive
function in young adults, particularly among non-anaemic
menstruating women with low ferritin
concentrations.21 27 Notably, iron supplementation
has been associated with reductions in subjective
measures of fatigue among non-anaemic iron-deficient
adults.21 The present findings suggest that
ID correction in adolescents could reduce fatigue
levels, which may indirectly improve attention
outcomes. Using a serum ferritin concentration
threshold of 15 μg/L to diagnose clinical ID,
some researchers have demonstrated that iron
supplementation can improve fatigue and physical
performance among individuals with serum ferritin
concentrations at the lower end of the normal range
(30-50 μg/L).21 Collectively, the known health risks of
ID, including impaired physical growth, fatigue, and
reduced fitness in adolescents, underscore the need
to educate students about maintaining a balanced
diet with adequate iron intake. Adolescents with low
ferritin concentrations should receive counselling
focused on the consumption of iron-rich foods and
iron supplementation to alleviate fatigue, even in the absence of documented anaemia.
Dietary patterns and self-reported intake of
iron-rich foods were not directly associated with
attention outcomes in the multivariate analysis, likely
because neurocognitive function is a multifactorial
and complex phenotype influenced by both
nutritional and non-nutritional factors. Additionally,
we did not use a comprehensive measure of dietary
iron intake. However, we previously showed that
skipping at least one meal per day or exhibiting low
dietary iron intake was associated with lower iron
reserves.11 Iron deficiency prevention in adolescents
requires effective management of knowledge gaps
related to food nutrition, dieting, and body image.
Collectively, these findings highlight the importance
of developing nutrition education programmes to
encourage proactive adoption of dietary and other
nutrition-related behaviours that promote health
and well-being.
Limitations
Despite the relatively large cohort of school-aged
adolescents and the well-characterised
haematological assessments, this study had several
important limitations. First, the participation rate
in the blood donation programme was affected by
the coronavirus disease 2019 pandemic and school
closures. This change in participation rate may
have introduced sampling bias because students
with worse health statuses may have been more
likely to decline blood donation. Second, we only
assessed attention measures in this study. It was
not feasible to administer a full neurocognitive
test battery, which typically requires >1 hour, in
a school-based environment with limited time,
space, and supervisory personnel. Future studies
should include a more comprehensive evaluation of
neurocognitive function. Finally, we did not evaluate
factors potentially associated with the causes of
anaemia and cognitive function, such as markers
of socio-economic status, family functioning, living
environment, and physical activity.28 29 Nevertheless,
our findings regarding the association between iron
status and attention outcomes provide valuable
local population data and guidance for future iron
supplementation initiatives.
Conclusion
Lower serum ferritin concentrations and self-reported
fatigue were associated with an increased
risk of sustained attention impairment among school-aged
adolescents in Hong Kong. The potential health
consequences of ID without anaemia, particularly
its effects on physical well-being and school
performance, should be effectively communicated
to the Hong Kong population, especially to female
adolescents. Dietary interventions should target
Author contributions
Concept or design: All authors.
Acquisition of data: CK Lee, WC Tsoi, CW Lau, JNS Leung, STY Tsang, CLP Wong, YYL Chu, CK Li.
Analysis of data: YT Cheung, DFY Chan.
Interpretation of data: All authors.
Drafting of the manuscript: YT Cheung.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: CK Lee, WC Tsoi, CW Lau, JNS Leung, STY Tsang, CLP Wong, YYL Chu, CK Li.
Analysis of data: YT Cheung, DFY Chan.
Interpretation of data: All authors.
Drafting of the manuscript: YT Cheung.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank the principals and staff of the participating
schools, as well as Mr Calvin Lam from Department of
Paediatrics of The Chinese University of Hong Kong for
assistance with data collection.
Declaration
Part of the results was presented at the Joint Annual Scientific
Meeting 2022 (hybrid meeting) of The Hong Kong Paediatric
Society, Hong Kong College of Paediatricians, Hong Kong
Paediatric Nurses Association, and Hong Kong College of
Paediatric Nursing in Hong Kong on 26 September 2022.
Funding/support
This research was funded by the Health and Medical Research
Fund, the former Food and Health Bureau, Hong Kong SAR
Government (Ref No.: 17180441). The funder had no role
in study design, data collection, analysis, interpretation, or
manuscript preparation.
Ethics approval
This research was approved by the Joint Chinese University
of Hong Kong—New Territories East Cluster Clinical
Research Ethics Committee, Hong Kong (Ref No.: 2019.107).
Participants aged ≥18 years provided written informed
consent, whereas those aged <18 years provided written assent
along with informed consent from a parent or legal guardian.
Supplementary material
The supplementary material was provided by the authors and
some information may not have been peer reviewed. Accepted
supplementary material will be published as submitted by the
authors, without any editing or formatting. Any opinions
or recommendations discussed are solely those of the
author(s) and are not endorsed by the Hong Kong Academy
of Medicine and the Hong Kong Medical Association.
The Hong Kong Academy of Medicine and the Hong Kong Medical Association disclaim all liability and responsibility
arising from any reliance placed on the content.
References
1. Powers JM, O’Brien S, Berlan ED, Hoppin AG, editors.
Iron requirements and iron deficiency in adolescents.
UpToDate. Available from: https://www.uptodate.com/contents/iron-requirements-and-iron-deficiency-in-adolescents. Accessed 1 Apr 2025.
2. Camaschella C, Girelli D. The changing landscape of iron
deficiency. Mol Aspects Med 2020;75:100861. Crossref
3. Safiri S, Kolahi AA, Noori M, et al. Burden of anemia and
its underlying causes in 204 countries and territories,
1990-2019: results from the Global Burden of Disease
Study 2019. J Hematol Oncol 2021;14:185. Crossref
4. Institute for Health Metrics and Evaluation, University of
Washington. GBD results. 2020. Available from: https://vizhub.healthdata.org/gbd-results/. Accessed 6 May 2023.
5. Hare D, Ayton S, Bush A, Lei P. A delicate balance: iron
metabolism and diseases of the brain. Front Aging
Neurosci 2013;5:34. Crossref
6. Jáuregui-Lobera I. Iron deficiency and cognitive functions.
Neuropsychiatr Dis Treat 2014;10:2087-95. Crossref
7. Pivina L, Semenova Y, Doşa MD, Dauletyarova M,
Bjørklund G. Iron deficiency, cognitive functions, and
neurobehavioral disorders in children. J Mol Neurosci
2019;68:1-10. Crossref
8. Wang Y, Huang L, Zhang L, Qu Y, Mu D. Iron status in
attention-deficit/hyperactivity disorder: a systematic
review and meta-analysis. PLoS One 2017;12:e0169145. Crossref
9. Bener A, Kamal M, Bener H, Bhugra D. Higher prevalence
of iron deficiency as strong predictor of attention deficit
hyperactivity disorder in children. Ann Med Health Sci Res
2014;4(Suppl 3):S291-7. Crossref
10. Tseng PT, Cheng YS, Yen CF, et al. Peripheral iron levels
in children with attention-deficit hyperactivity disorder: a
systematic review and meta-analysis. Sci Rep 2018;8:788. Crossref
11. Cheung YT, Chan DF, Lee CK, et al. Iron deficiency
among school-aged adolescents in Hong Kong: prevalence,
predictors, and effects on health-related quality of life. Int J
Environ Res Public Health 2023;20:2578. Crossref
12. World Health Organization. WHO guideline on use of
ferritin concentrations to assess iron status in individuals
and populations. 2020. Available from: https://apps.who.int/iris/handle/10665/331505. Accessed 6 Oct 2023.
13. World Health Organization. Haemoglobin concentrations
for the diagnosis of anaemia and assessment of severity.
2011 May 31. Available from: https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1. Accessed 6 Oct 2023.
14. Conners CK, Sitarenios G. Conners’ Continuous
Performance Test (CPT). In: Kreutzer JS, DeLuca J, Caplan
B, editors. Encyclopedia of Clinical Neuropsychology. New
York: Springer; 2011: 681-3. Crossref
15. Sulheim D, Fagermoen E, Sivertsen ØS, Winger A, Wyller VB,
Øie MG. Cognitive dysfunction in adolescents with
chronic fatigue: a cross-sectional study. Arch Dis Child
2015;100:838-44. Crossref
16. Varni JW, Limbers CA. The PedsQL Multidimensional
Fatigue Scale in young adults: feasibility, reliability and
validity in a university student population. Qual Life Res 2008;17:105-14. Crossref
17. Yeung NC, Lau JT, Yu X, et al. Psychometric properties
of the Chinese version of the Pediatric Quality of Life
Inventory 4.0 Generic Core Scales among pediatric cancer
patients. Cancer Nurs 2013;36:463-73. Crossref
18. Hao Y, Tian Q, Lu Y, Chai Y, Rao S. Psychometric
properties of the Chinese version of the Pediatric Quality
of Life Inventory 4.0 Generic Core Scales. Qual Life Res
2010;19:1229-33. Crossref
19. Camaschella C. Iron deficiency. Blood 2019;133:30-9. Crossref
20. Hermoso M, Vucic V, Vollhardt C, et al. The effect of iron
on cognitive development and function in infants, children
and adolescents: a systematic review. Ann Nutr Metab
2011;59:154-65. Crossref
21. Houston BL, Hurrie D, Graham J, et al. Efficacy of iron
supplementation on fatigue and physical capacity in non-anaemic
iron-deficient adults: a systematic review of
randomised controlled trials. BMJ Open 2018;8:e019240. Crossref
22. Kim J, Wessling-Resnick M. Iron and mechanisms of
emotional behavior. J Nutr Biochem 2014;25:1101-7. Crossref
23. Gallen CL, Schaerlaeken S, Younger JW; Project iLEAD
Consortium; Anguera JA, Gazzaley A. Contribution of
sustained attention abilities to real-world academic skills
in children. Sci Rep 2023;13:2673. Crossref
24. Schmengler H, Peeters M, Stevens GW, et al. Educational level, attention problems, and externalizing behaviour
in adolescence and early adulthood: the role of social
causation and health-related selection—the TRAILS study.
Eur Child Adolesc Psychiatry 2023;32:809-24. Crossref
25. Finkelstein JL, Herman HS, Guetterman HM, Peña-Rosas JP,
Mehta S. Daily iron supplementation for prevention
or treatment of iron deficiency anaemia in infants,
children, and adolescents. Cochrane Database Syst Rev
2018;2018:CD013227. Crossref
26. Falkingham M, Abdelhamid A, Curtis P, Fairweather-Tait S,
Dye L, Hooper L. The effects of oral iron supplementation
on cognition in older children and adults: a systematic
review and meta-analysis. Nutr J 2010;9:4. Crossref
27. Vaucher P, Druais PL, Waldvogel S, Favrat B. Effect of iron
supplementation on fatigue in nonanemic menstruating
women with low ferritin: a randomized controlled trial.
CMAJ 2012;184:1247-54. Crossref
28. Hess SY, Owais A, Jefferds ME, Young MF, Cahill A,
Rogers LM. Accelerating action to reduce anemia: review
of causes and risk factors and related data needs. Ann N Y
Acad Sci 2023;1523:11-23. Crossref
29. Meredith WJ, Cardenas-Iniguez C, Berman MG,
Rosenberg MD. Effects of the physical and social
environment on youth cognitive performance. Dev
Psychobiol 2022;64:e22258. Crossref