Hong Kong Med J 2025 Apr;31(2):154–8 | Epub 8 Apr 2025
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
PERSPECTIVE
Anaemia and iron deficiency in advanced prostate cancer: revisiting a common morbidity
Chris HM Wong, MRCS, MB, ChB; Ivan CH Ko, MRCS, MB, ChB; David KW Leung, FRCSEd; HC Chan, FRCSEd, FHKAM (Surgery); KM Li, FRCSEd, FHKAM (Surgery); YS Chan, FRCSEd, FHKAM (Surgery); Francis CH Wong, FRCSEd, FHKAM (Surgery); Peter KF Chiu, FRCSEd, FHKAM (Surgery);
Jeremy YC Teoh, FRCSEd, FHKAM (Surgery); CF Ng, FRCSEd, FHKAM (Surgery)
SH Ho Urology Centre, Department of Surgery, The Chinese University of Hong Kong, Hong Kong SAR, China
Corresponding author: Prof CF Ng (ngcf@surgery.cuhk.edu.hk)
Introduction
Anaemia is a common morbidity associated with
malignancies at various stages. The reported
prevalence of anaemia ranges from 20% to 60%
in solid organ tumours.1 The pathophysiology of
cancer-related anaemia (CRA) is multifactorial.
Contributing mechanisms include chronic
inflammatory reactions; activation of
proinflammatory cytokines such as interleukin-6,
interleukin-1, and tumour necrosis factor alpha;
nutritional deficiencies; bone marrow infiltration;
and haemolysis. The relative contributions of these
factors vary across malignancies that affect different
organ systems.2 Cancer-related anaemia can result
in substantial morbidity and impaired quality of
life. Its symptoms are broad, encompassing fatigue,
depression, cognitive impairment, syncope, and
falls.3 Symptoms may emerge when haemoglobin
levels fall below 11.5 g/dL, a threshold classified as
mild anaemia by most physicians.4 In oncological
contexts, there is evidence of negative relationships
between anaemia and overall outcomes. A meta-analysis
of solid and haematological malignancies
revealed a 65% increase in overall mortality among
patients with anaemia relative to those without.5
Anaemia in prostate cancer: causes and incidence
Anaemia has been widely associated with advanced
prostate cancer (CaP), particularly in metastatic
stages treated with androgen deprivation therapy
(ADT). Haematuria resulting from tumour invasion
into the urethra is one potential cause.6 Marrow
suppression due to diffuse bone metastases, as well
as CaP therapies (eg, radiotherapy, chemotherapy,
and long-term ADT) may also contribute to reduced
erythropoietin production and, consequently, lower
haemoglobin levels.
The incidence of anaemia in CaP remains
largely uncertain, despite its common association
with clinical management. Based on inferences from
published studies, the prevalence of anaemia in CaP
patients ranges from 13% to 78%.7 8 9 These figures substantially vary due to differences in inclusion
criteria and distinct definitions of prostate CRA.
Efforts to identify the true incidence of anaemia in
CaP are challenging because it arises from multiple
causes and produces symptoms analogous to those
of advanced malignancy. Limitations concerning
population- or registry-based databases further
complicate assessment, given that exact blood
parameters are often omitted. We share our
findings regarding the incidence of anaemia in a
local cohort of CaP patients and identify potential
associated factors. We aim to enhance recognition
of this common co-morbidity and facilitate timely
management.
Prostate cancer-associated
anaemia: why does it matter?
The impact of anaemia in CaP appears to extend
beyond quality-of-life-related symptoms. A metaanalysis
of 15 retrospective and prospective studies
concluded that anaemia was associated with worse
overall survival and progression-free survival.7 There
is speculation that anaemia-induced hypoxia within
cancer colonies reduces reactive oxygen species
levels. The downstream effect is dimerisation of
hypoxia-inducible factor 1 alpha and beta, leading to
the transcription of treatment resistance-associated
oncogenes8 and, ultimately, the development
of castration-resistant prostate cancer (CRPC).
These findings highlight the importance of early
identification and prompt treatment of prostate
CRA in the outpatient setting to preserve long-term
oncological outcomes in advanced CaP treatment.
Implications of androgen
deprivation therapy
In the management of high-risk localised, locally
advanced, and metastatic prostate cancer, ADT
plays a crucial role; treatment durations range from
2 years to lifelong. Its influence on the development
of anaemia in this patient population cannot be
understated. In a randomised controlled study
involving 141 patients scheduled for radiotherapy, Asbell et al9 investigated changes in haemoglobin
levels after radiotherapy and ADT with maximal
androgen blockade among patients with localised
and advanced CaP. A decrease in haemoglobin level
of up to 2 g/L was reported after the initiation of
maximal androgen blockade; differences emerged
as early as 2 months post-initiation. A Taiwanese
population-based registry study by Wu et al,10 examining the relationship between iron deficiency
and prostate cancer treatment in 10 893 cases with
and without ADT (segregated according to registry
coding), revealed a hazard ratio of 1.60, indicating
increased likelihood of iron deficiency among
patients receiving ADT. Similar effects have been
detected in Western populations. Timilshina et al11
identified ADT use as an independent predictor of
haemoglobin decline over 1 year, whereas Choo et al12
reported a decline in haemoglobin levels over 2 years
in a cohort of 72 patients receiving adjuvant ADT
plus radiotherapy.
The relationship between testosterone and
haemoglobin synthesis has been suggested to arise
from a synergistic effect on erythropoietin. Through
its downstream effects, testosterone enhances the
action of polychromatophilic erythroblasts and
supports the activities of polymerases I and II in
conjunction with erythropoietin.7 An ADT-induced
lack of testosterone may impair haematopoiesis and
contribute to anaemia.
Associations of prostate cancer
with anaemia and iron deficiency:
findings from a local cohort
Consecutive patients were recruited from the
prostate cancer clinic within a general urology unit.
Ethics approval was approved by the Joint Chinese
University of Hong Kong–New Territories East
Cluster Clinical Research Ethics Committee (Ref
No.: 2014.251) and registered in ClinicalTrials.
gov (Identifier: NCT03344835). Patients with a
history of gastrointestinal or haematological cancer
were excluded because these conditions represent
significant confounders in the development of
anaemia. Individuals exhibiting active symptoms
of gastrointestinal bleeding or a positive faecal
occult blood test result pending further evaluation
were also excluded. Additionally, patients with a
prior diagnosis of iron deficiency and ongoing iron
supplementation were excluded.
The definition of anaemia in this study
adhered to World Health Organization criteria;
a haemoglobin level of 13 g/dL served as the
threshold.13 Iron deficiency was defined as a ferritin
level <100 μg/L or transferrin saturation <20%. Iron deficiency anaemia was classified as the
coexistence of both anaemia and iron deficiency.14
Anaemia of chronic disease was characterised by a
low serum iron level without evidence of depleted iron stores.15 A state of CRPC was defined by a
testosterone level <1.7 nmol/dL, in accordance
with European Association of Urology guidelines.16
For sample size calculation, we regarded
the proportion of anaemia within the cohort as a
proportional variable. Assuming d=0.08, α=0.2, and
p=0.5, the calculated sample size required for the
survey was 65. Statistical analysis was performed
using SPSS software (Windows version 24.0; IBM
Corp, Armonk [NY], United States). Missing data
were handled using mean substitution. Because
no significant missing data were anticipated,
sensitivity analysis was not planned. Categorical
variables were represented as percentages, whereas
continuous variables were expressed as mean values.
Multivariate regression analysis was planned to
identify confounding factors influencing outcomes. A
two-tailed P value <0.05 was considered statistically
significant.
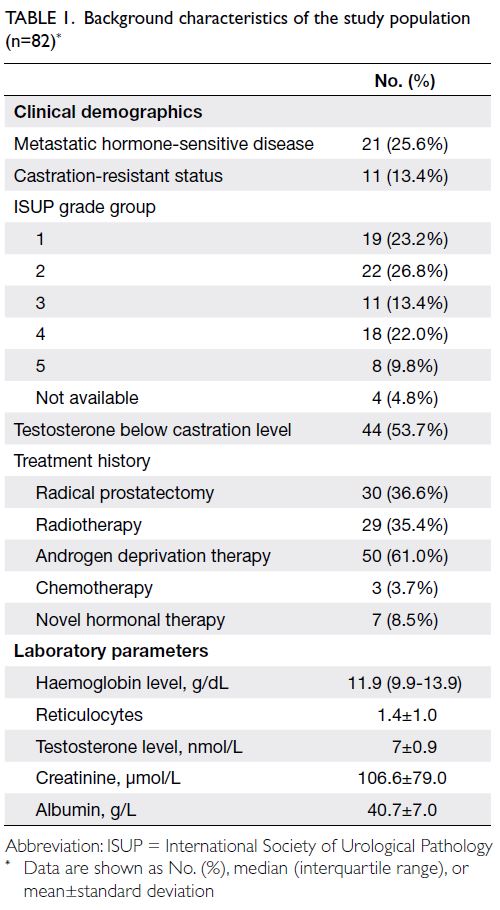
Overall, 82 eligible patients recruited from
September to December 2022 were included in
the analysis (Table 1). The mean age of the cohort was 74.2 years. Most patients had a performance
status of 1 (67.1%). Concomitant diabetes mellitus,
ischaemic heart disease, and chronic kidney disease
were present in 26.8%, 7.3%, and 31.7% of patients,
respectively. At the time of analysis, 25.6% of
patients had metastatic hormone-sensitive CaP;
13.4% of patients had CRPC. The mean prostate-specific
antigen value at diagnosis was 98.0 ng/mL,
and International Society of Urological Pathology
grade group 2 was most common (26.8%). More than
half of the patients had castrated testosterone levels
(mean testosterone level=7.0 nmol/L).
A substantial proportion of patients had
anaemia (43/82, 52.4%). In total, 20.7% of patients
had iron deficiency without anaemia. Among
individuals with anaemia, 14.0% had iron deficiency
anaemia, 7.0% had B12 deficiency, and 16.3% had
folate deficiency. Anaemia of chronic disease was
present in 39.5% of patients. Patients exhibiting
iron deficiency anaemia were scheduled to undergo
a faecal occult blood test if they had not recently
completed upper and lower gastrointestinal
investigations. None of the patients displayed
faecal occult blood positivity. Further analysis
was conducted to identify risk factors predictive
of anaemia in this cohort. Univariate analysis
identified a castrated state (odds ratio [OR]=1.99; P=0.002), metastatic disease (OR=1.78; P=0.004),
and hypoalbuminaemia (OR=2.05; P=0.015) as
statistically significant predictors of anaemia (online supplementary Table 1). Considering the observed
association between a castrated state and anaemia,
we performed an additional analysis to examine the
relationship between haemoglobin and testosterone
levels. No association was identified in analysis of the entire cohort. However, when cases were stratified
by disease stage (localised, metastatic hormonesensitive,
and castration-resistant), a significant
association was observed within the localised
disease subgroup (effect coefficient=0.323; P=0.024)
[online supplementary Table 2]; lower testosterone
levels tended to be present in anaemic patients.
Multivariate analysis found that patients in
a castrated state (ie, those receiving ADT) had
a higher likelihood of iron deficiency (OR=1.30; P=0.006). More than half of the CRPC patients
exhibited iron deficiency (OR=2.55; P=0.017).
Chronic kidney disease and metastatic status were
not significantly associated with iron deficiency,
although slight trends were observed. Other factors,
including patient age, prostate-specific antigen level
at diagnosis or follow-up, performance status, and
International Society of Urological Pathology grade
group, were not associated with iron deficiency
(Table 2).
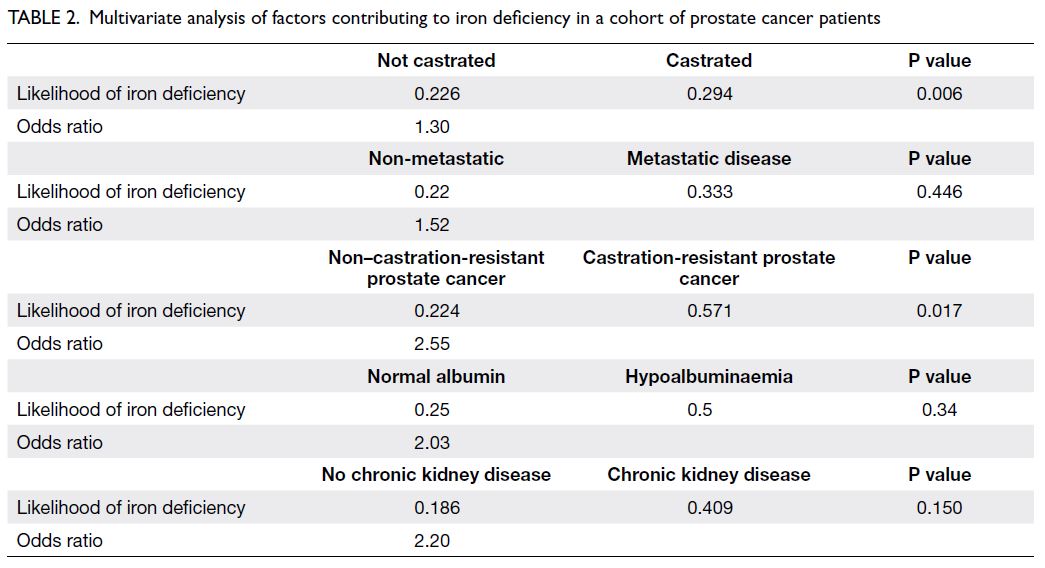
Table 2. Multivariate analysis of factors contributing to iron deficiency in a cohort of prostate cancer patients
Future directions
This study provided a snapshot of the prevalences of
anaemia and iron deficiency in CaP. The incidence
of anaemia was 52.4%, whereas iron deficiency was
present in 20.7% of the cohort. Castration-resistant
prostate cancer was associated with iron deficiency.
An association between metastatic or castrated
status and anaemia was also observed, reinforcing
the notion that anaemia and iron deficiency are
common in advanced CaP. A high level of vigilance is
required among physicians responsible for the care
of patients with advanced CaP, particularly during
management of CRA. As highlighted in several multicentre reviews of Asian CaP cohorts,17 18 19 the
consequences of anaemia may include profound
adverse effects, such as metabolic complications or
diminished glycaemic control. Increased awareness
of CaP-related anaemia—a readily treatable
condition—could improve quality of life and long-term
patient outcomes.
Since the introduction of multiple antitumour
treatments during earlier phases of CaP management,
the treatment landscape has drastically changed in
recent decades. Published studies concerning the
association between prostate cancer and anaemia are
largely outdated.10 20 Future research could explore
the impact of novel treatments—including androgen
receptor signalling inhibitors, poly (ADP-ribose)
polymerase 1 inhibitors, and prostate- or metastasis-directed
radiotherapy—on CaP-related anaemia.
Further studies might also assess the effectiveness
of iron replacement therapy. Intravenous iron or
erythropoietin21 plays a role in the management of
malignancy-related anaemia by improving patient-reported
quality of life. Finally, future studies could
investigate potential effects of anaemia treatment on
quality-of-life measures and oncological outcomes
in patients with advanced CaP.
Conclusion
Anaemia and iron deficiency are commonly
observed in Asian Chinese patients with prostate
cancer. Castrated and CRPC states were identified
as predictors of iron deficiency in this patient
population. Physicians are encouraged to monitor
the development of anaemia after initiation of
prostate cancer treatment. Large-scale studies may
be warranted to evaluate the benefits of anti-anaemia
treatment.
Author contributions
Concept or design: CF Ng, CHM Wong.
Acquisition of data: All authors.
Analysis or interpretation of data: CHM Wong.
Drafting of the manuscript: CHM Wong.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: CHM Wong.
Drafting of the manuscript: CHM Wong.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As editors of the journal, JYC Teoh and CF Ng were not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplementary material
The supplementary material was provided by the authors, and
some information may not have been peer reviewed. Accepted
supplementary material will be published as submitted by the
authors, without any editing or formatting. Any opinions or
recommendations discussed are solely those of the author(s)
and are not endorsed by the Hong Kong Academy of
Medicine and the Hong Kong Medical Association. The Hong
Kong Academy of Medicine and the Hong Kong Medical
Association disclaim all liability and responsibility arising
from any reliance placed on the content.
References
1. Xu H, Xu L, Page JH, et al. Incidence of anemia in patients
diagnosed with solid tumors receiving chemotherapy,
2010-2013. Clin Epidemiol 2016;8:61-71. Crossref
2. Busti F, Marchi G, Ugolini S, Castagna A, Girelli D.
Anemia and iron deficiency in cancer patients: role of iron
replacement therapy. Pharmaceuticals (Basel) 2018;11:94. Crossref
3. van Eeden R, Rapoport BL. Current trends in the
management of anaemia in solid tumours and
haematological malignancies. Curr Opin Support Palliat
Care 2016;10:189-94. Crossref
4. Crawford J, Cella D, Cleeland CS, et al. Relationship
between changes in hemoglobin level and quality of life
during chemotherapy in anemic cancer patients receiving
epoetin alfa therapy. Cancer 2002;95:888-95. Crossref
5. Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent
prognostic factor for survival in patients with cancer: a
systemic, quantitative review. Cancer 2001;91:2214-21. Crossref
6. Nalesnik JG, Mysliwiec AG, Canby-Hagino E. Anemia in
men with advanced prostate cancer: incidence, etiology,
and treatment. Rev Urol 2004;6:1-4.
7. Dai D, Han S, Li L, et al. Anemia is associated with poor
outcomes of metastatic castration-resistant prostate
cancer, a systematic review and meta-analysis. Am J Transl
Res 2018;10:3877-86.
8. Varlotto J, Stevenson MA. Anemia, tumor hypoxemia, and
the cancer patient. Int J Radiat Oncol Biol Phys 2005;63:25-36. Crossref
9. Asbell SO, Leon SA, Tester WJ, Brereton HD, Ago CT,
Rotman M. Development of anemia and recovery in
prostate cancer patients treated with combined androgen
blockade and radiotherapy. Prostate 1996;29:243-8. Crossref
10. Wu FJ, Li IH, Chien WC, et al. Androgen deprivation
therapy and the risk of iron-deficiency anaemia among
patients with prostate cancer: a population-based cohort
study. BMJ Open 2020;10:e034202. Crossref
11. Timilshina N, Hussain S, Breunis H, Alibhai SM. Predictors
of hemoglobin decline in non-metastatic prostate cancer
patients on androgen deprivation therapy: a matched
cohort study. Support Care Cancer 2011;19:1815-21. Crossref
12. Choo R, Chander S, Danjoux C, et al. How are hemoglobin
levels affected by androgen deprivation in non-metastatic
prostate cancer patients? Can J Urol 2005;12:2547-52.
13. DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia
in the world [in English, French]. World Health Stat Q
1985;38:302-16.
14. Cappellini MD, Musallam KM, Taher AT. Iron deficiency
anaemia revisited. J Intern Med 2020;287:153-70. Crossref
15. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG,
Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of
unexplained anemia. Blood 2004;104:2263-8. Crossref
16. Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines
on prostate cancer. Part II: Treatment of advanced,
relapsing, and castration-resistant prostate cancer. Eur
Urol 2014;65:467-79. Crossref
17. Wong C, Chu P, Teoh J, et al. Risks of metabolic diseases
and androgen deprivation therapy for prostate cancer in
a Chinese population: a prospective multi-centre cohort
study. Int Urol Nephrol 2022;54:993-1000. Crossref
18. Wong CH, Xu N, Lim J, et al. Adverse metabolic
consequences of androgen deprivation therapy (ADT) on Asian patients with prostate cancer: primary results from
the real-life experience of ADT in Asia (READT) study.
Prostate 2023;83:801-8. Crossref
19. Ng CF, Chiu PK, Yee CH, Lau BS, Leung SC, Teoh JY.
Effect of androgen deprivation therapy on cardiovascular
function in Chinese patients with advanced prostate
cancer: a prospective cohort study. Sci Rep 2020;10:18060. Crossref
20. Fonseca R, Rajkumar SV, White WL, Tefferi A, Hoagland HC.
Anemia after orchiectomy. Am J Hematol 1998;59:230-3. Crossref
21. Zhao F, Wang Y, Liu L, Bian M. Erythropoietin for cancer-associated
malignant anemia: a meta-analysis. Mol Clin
Oncol 2017;6:925-30. Crossref