© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
HEALTHCARE FOR SOCIETY
Achieving universal and comprehensive publicly funded prenatal screening and diagnostic algorithms in Hong Kong: an interview with Dr Wing-cheong Leung
Asta Lee1, Nicholas Chung2
1 Year 5, The University of Hong Kong, Hong Kong SAR, China
2 Year 2, The University of Hong Kong, Hong Kong SAR, China
In the field of obstetrics and gynaecology (O&G),
Dr Wing-cheong Leung is a distinguished leader
whose contributions have greatly advanced maternal
health. As Hong Kong’s first accredited subspecialist
in maternal fetal medicine (MFM), Dr Leung served
as the Chief of Service of the Department of O&G at
Kwong Wah Hospital from 2010 to 2021 and as the
President of the Hong Kong College of Obstetricians
and Gynaecologists (HKCOG) from 2016 to 2018.
His unwavering dedication to the public sector and
substantial contributions to O&G—encompassing
areas ranging from prenatal diagnosis and
postpartum haemorrhage to perinatal mental health
(including domestic violence)—earned him the title
of Honorary Fellow of the HKCOG in 2022 and the
Outstanding Staff Award of the Hospital Authority
(HA) in 2024.
Dr Leung’s journey in MFM began in 1999,
when he undertook overseas training in the
subspecialty at the Perinatal Centre of the University
of Toronto, Canada. An unexpected encounter with
the thesis topic of rapid aneuploidy testing ignited
his passion for prenatal diagnosis. He subsequently
earned his MD with a thesis Rapid aneuploidy testing or traditional karyotyping, or both, in
prenatal diagnosis and developed a novel algorithm
for prenatal diagnosis. This innovative work laid
the foundation for his current project, the FMPRG
platform (Fetal Medicine, Pathology, Radiology, and
Genetics/Genomics), which is transforming the
landscape of prenatal diagnosis in Hong Kong.
The concept of prenatal diagnosis for trisomy
21, widely known as Down syndrome, was first
introduced in the 1960s. At that time, only pregnant
women aged 35 years and older were eligible for
amniocentesis in the public sector because the
likelihood of having a child with Down syndrome
increases with maternal age. Although this
represented a substantial advancement in prenatal
diagnosis, the approach had important limitations.
Women aged 35 years and older faced the unsettling
risk of miscarriage associated with amniocentesis,
whereas those younger than 35 years were excluded
from screening. This exclusion was a considerable
oversight, considering that most expectant mothers
at the time were younger than 35 years. Among women
ineligible for public-sector screening, the financial
burden of self-financing tests in the private sector further exacerbated the stress associated with prenatal diagnosis.
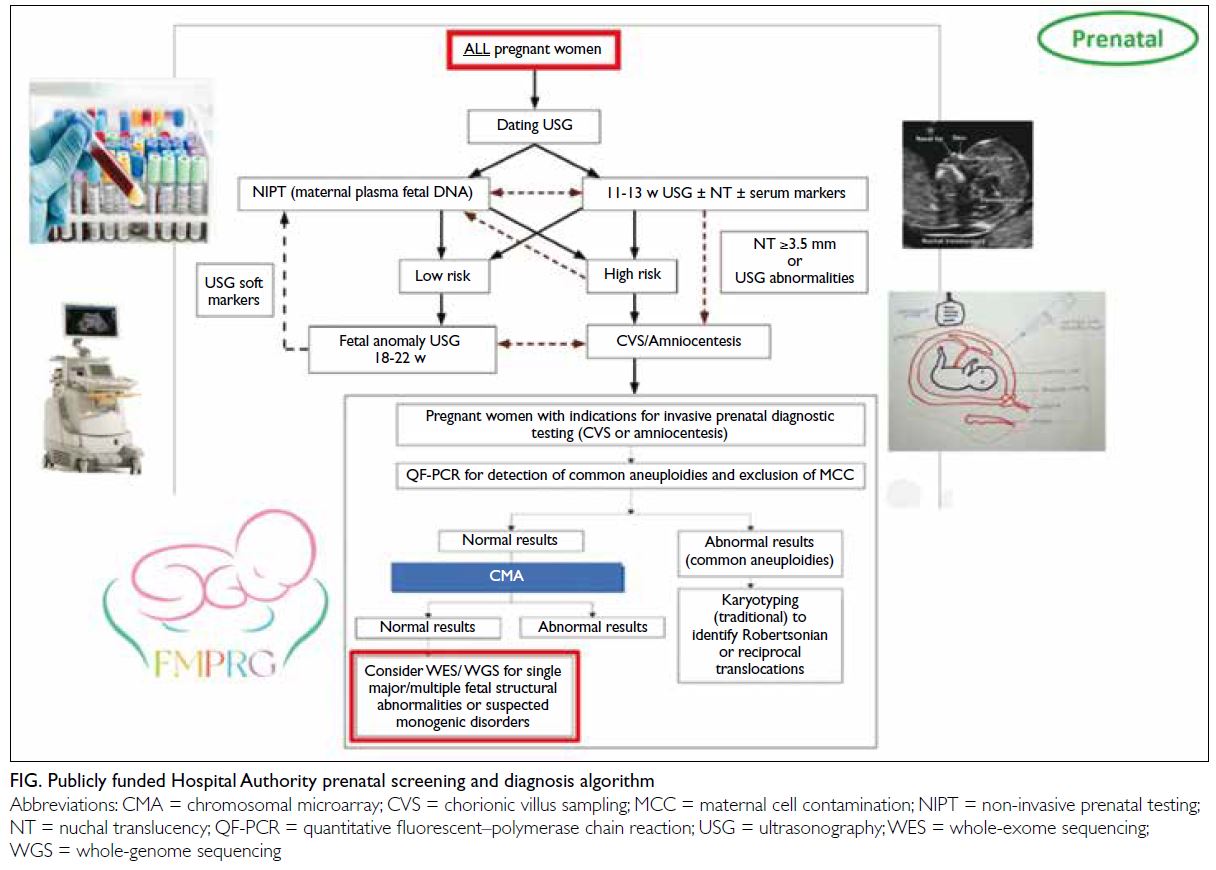
Recognising these inequities, Dr Leung and
his MFM seniors and colleagues developed a new
algorithm (Fig) to screen for Down syndrome in all
pregnant women, reserving amniocentesis for those
who met specific criteria. This strategy significantly
reduced the number of women exposed to the risk
of miscarriage associated with amniocentesis. The
initial screening process involves non-invasive
methods, including maternal serum markers and fetal
nuchal translucency measurements; amniocentesis
is required only if these methods show Down
syndrome positivity. To further refine the selection
process, a second tier comprising non-invasive
prenatal testing (ie, maternal plasma cell-free DNA
analysis with higher sensitivity and specificity) is
used for evaluation prior to amniocentesis. There
is also the potential to offer non-invasive prenatal
testing as a first-tier screening method if its cost
decreases and public funding becomes available.
After years of refinement, the approach to
prenatal diagnosis has evolved to encompass a
wider range of congenital conditions and genetic
disorders. The fetal anomaly ultrasound scan,
typically conducted between 18 and 22 weeks of
gestation, represents the next critical component of the algorithm for all pregnant women. This scan
evaluates fetal development and identifies potential
structural abnormalities, such as heart defects,
spina bifida, and other major organ anomalies.
The inclusion of the fetal anomaly ultrasound scan
is particularly important in regions such as Hong
Kong, where termination of pregnancy is legally
permissible only within 24 weeks of gestation. By
detecting major structural abnormalities within the
legal timeframe, pregnant women are enabled to
make informed decisions regarding their options and
to prepare for any required neonatal interventions.
Within this framework, pregnant women
who undergo invasive prenatal diagnostic testing,
such as chorionic villus sampling or amniocentesis,
are subsequently offered quantitative fluorescent
polymerase chain reaction. This test
detects common aneuploidies and excludes the
possibility of maternal cell contamination. If the
results are normal, chromosomal microarray analysis
(CMA) is performed to assess microdeletions and
microduplications associated with various genetic
conditions, including those that may result in
developmental delays and intellectual disabilities.
In each step of this comprehensive algorithm,
the diagnostic yield of prenatal diagnoses increases,
effectively mitigating potential risks for the expectant
mother while maximising the likelihood of detecting
any fetal conditions. However, it is important to note
that the cost of prenatal genetic tests remains high.
Although the costs of polymerase chain reaction and CMA tests are fully covered by the HA in Hong Kong, overall costs substantially
increase if whole-exome sequencing (WES) or
whole-genome sequencing (WGS) is indicated after
the CMA test. Dr Leung and Dr WF Ng (Senior
Pathologist, HA) are addressing this issue through
their current initiative—the FMPRG platform.
The FMPRG platform uses a multidisciplinary
approach to select complex fetal cases for publicly
funded WGS or WES. The FMPRG represents the
multidisciplinary team comprising specialists in
fetal medicine, pathology, radiology, and genetics/genomics. The voting team currently includes 15
core members, including MFM subspecialists from
all eight HA hospitals offering prenatal diagnosis
clinical services, clinical geneticists, the heads of
the two university prenatal diagnosis laboratories, pathologists, and radiologists. Complex fetal cases
are uploaded to the platform for online interactive
discussion and voting, enabling the team to select
appropriate cases for publicly funded WES or WGS
in a fair and timely manner. Not only does WES
or WGS increase the probability of identifying the
genetic cause of complex fetal abnormalities, but the
anonymised archiving of these cases on the platform
also creates a valuable database for future education
and research. The implications of this initiative
extend beyond the laboratory. As funding expands
from 20 to 60 cases annually, the initiative aims to
alleviate the financial burden on eligible mothers
while empowering families with critical genetic
insights to guide their pregnancies.
Looking to the future, Dr Leung envisions
the integration of artificial intelligence (AI) into
the consultation process as a transformative
advancement in prenatal care. Considering the
prolonged waiting times for consultations in Hong
Kong, AI chatbots could alleviate unnecessary stress
and anxiety for patients by addressing common
misconceptions and providing personalised
information about prenatal diagnosis, including
details about the algorithm and the FMPRG
platform. However, Dr Leung emphasises that AI is
intended to complement, rather than replace, face-to-face consultations. By thoughtfully integrating
AI within prenatal care, this approach combines the
efficiency of AI chatbots with the human touch of in-person
interactions, resulting in a more streamlined
and responsive care experience.
Dr Leung’s pioneering work in MFM is setting
a gold standard for equitable access to prenatal
diagnoses for all expectant mothers. He is a firm
advocate of the principle that financial circumstances
should never jeopardise a mother’s access to prenatal
diagnoses. Through the development of the HA
algorithms and the FMPRG platform, combined with
AI-driven consultations, he is committed to ensuring
equitable access to advanced prenatal screening and
diagnostic options for all expectant mothers. Dr
Leung’s vision is to establish a ‘universal safety net’
for all pregnant women, regardless of their economic
status, equipping them with the resources necessary
to make informed decisions about their health and
the health of their babies.

Dr Leung and three other members of the FMPRG voting team: (from left to right) Dr Anita Kan (Tsan Yuk Hospital Prenatal Diagnosis Laboratory), Dr WC Leung, Dr Elaine Kan (Hong Kong Children’s Hospital Radiology), and Dr HM Luk (Hong Kong Children’s Hospital Clinical Genetics) at the Hospital Authority Convention 2024