Hong Kong Med J 2024 Oct;30(5):418–21 | Epub 16 Oct 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
COMMENTARY
Protein induced by vitamin K absence-II for the
surveillance and monitoring of hepatocellular carcinoma in Hong Kong
Rashid NS Lui, MB, ChB, FHKAM (Medicine)1,2,3; Loey LY Mak, MB, BS, FHKAM (Medicine)4,5 KN Kung, MB, ChB, FHKAM (Medicine)6; CM Leung, MB, BS, FHKAM (Medicine)7; Reggie ST Li, MB, BS, FHKAM (Medicine)8; YK Ma, MB, ChB, FHKAM (Medicine)9; Carmen KM Ng, MB, BS, FHKAM (Medicine)8; Henry LY Chan, MB, ChB, FHKAM (Medicine)10; MF Yuen, MB, BS, FHKAM (Medicine)4,5; Grace LH Wong, MB, ChB, FHKAM (Medicine)1,3; James Fung, MB, ChB, FHKAM (Medicine)4,5; on behalf of the Hong Kong Association for the Study of Liver Diseases Hepatocellular Carcinoma Surveillance Expert Meeting
1 Division of Gastroenterology and Hepatology, Department of Medicine and Therapeutics, Prince of Wales Hospital, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
2 Department of Clinical Oncology, Prince of Wales Hospital, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
3 Institute of Digestive Disease, State Key Laboratory of Digestive Disease, The Chinese University of Hong Kong, Hong Kong SAR, China
4 Division of Gastroenterology and Hepatology, Department of Medicine, Queen Mary Hospital, School of Clinical Medicine, The University of Hong Kong, Hong Kong SAR, China
5 State Key Laboratory of Liver Research, The University of Hong Kong, Hong Kong SAR, China
6 Department of Medicine and Geriatrics, United Christian Hospital, Hong Kong SAR, China
7 Department of Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong SAR, China
8 Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong SAR, China
9 Department of Medicine, Tuen Mun Hospital, Hong Kong SAR, China
10 Division of Gastroenterology and Hepatology, Department of Internal Medicine, Union Hospital, Hong Kong SAR, China
Corresponding author: Dr James Fung (jfunggastro@gmail.com)
Introduction
In Hong Kong, primary liver cancer is the fifth most
common cancer and third leading cause of cancer-related
deaths.1 Moreover, the age- and sex-adjusted
prevalence of hepatitis B virus (HBV) infection is
6.2%.2 Locally, >75% of liver cancers are linked to
chronic HBV infection.3 Although cirrhosis remains
the leading risk factor for hepatocellular carcinoma
(HCC) worldwide, its epidemiology is transitioning
from viral to non-viral factors because of increases
in metabolic dysfunction–associated steatotic liver
disease (MASLD) and alcohol-associated liver
disease.4 It is estimated that three-quarters of global deaths related to HCC occur in the Asia-Pacific region.5
Unmet needs in Hong Kong
In 2016, the World Health Organization published
an advocacy brief aimed at combating viral
hepatitis.6 This brief set ambitious global targets, including a 90% reduction in the number of new cases and a 65% decrease in deaths by 2030.6 In
recognition of the public health threat posed by
viral hepatitis, the Hong Kong SAR Government
introduced the Hong Kong Viral Hepatitis Action
Plan 2020–2024, which provides a comprehensive
strategy for reducing the local public health burden of the disease.7 Considering that most HBV-related
deaths are caused by HCC, it is important for at-risk
individuals to undergo surveillance for the early
detection of cancer; this approach enables potentially
curative therapies.8 The most widely used modalities
include liver ultrasonography (USG), either alone
or in combination with alpha-fetoprotein (AFP)
monitoring.9 The overall sensitivity of these methods
in patients with HBV is reported to exceed 80% for
any stage of HCC. However, they exhibit suboptimal
sensitivity for early-stage HCC, cirrhosis, obesity,
MASLD, and alcohol-related disease.10 Alpha-fetoprotein
is the most widely used serologic
biomarker for HCC surveillance. However, almost
one-third of HCCs do not secrete AFP.11 Additionally,
AFP may be elevated in benign conditions such
as active hepatitis and liver cirrhosis, as well as
non-HCC malignancies (eg, cholangiocarcinoma).9
Despite these limitations, studies conducted in Hong
Kong using USG and AFP every 6 months, primarily
in patients with HBV, have shown that this approach
can detect smaller and earlier-stage HCCs; it is also
associated with improved survival.12 13 The situation is less clear for MASLD, where HCC surveillance is
not yet routinely recommended.14
The Hong Kong Association for the Study of
Liver Diseases convened an HCC Surveillance Expert
Meeting on 29 June 2023. Eleven hepatologists from academia and the public sector participated
to explore new strategies for HCC surveillance in
at-risk individuals. The relevant discussion points
and recommendations from this meeting are
presented below.
Value and application of new biomarkers in hepatocellular carcinoma surveillance
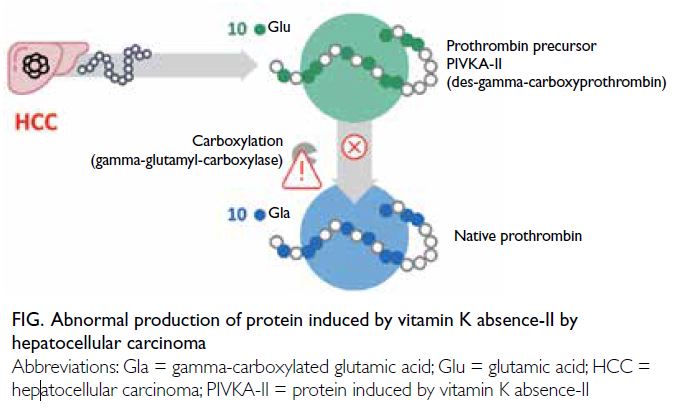
Protein induced by vitamin K absence-II (PIVKA-II)
is an abnormal form of the coagulation protein
prothrombin. Elevated levels of PIVKA-II can be
detected in individuals with vitamin K deficiency
and individuals taking vitamin K antagonists (eg,
warfarin). In the context of HCC, PIVKA-II is an
abnormal prothrombin molecule known as des-gamma-carboxyprothrombin, which is thought
to arise from an acquired defect in the post-translational
carboxylation of the prothrombin
precursor in malignant cells. Protein induced by
vitamin K absence-II has been identified as an
HCC-associated serum biomarker and serves as an
independent predictor of microvascular invasion in
HCC (Fig).15
When a PIVKA-II cut-off value of 28.4 ng/mL
was used, the sensitivity of HCC detection was 86.9%,
and the specificity was 83.7%.16 For early-stage HCC
detection, the sensitivity and specificity were 77.9%
and 83.7%, respectively.16 In contrast, when an AFP
cut-off value of 20 ng/mL was used, the sensitivity
and specificity for early-stage HCC were 36.4% and
98.1%, respectively.16 These findings indicate that
PIVKA-II has greater sensitivity for early-stage
HCC. The use of PIVKA-II in combination with AFP
increases the HCC detection sensitivity to 92%.16
There may also be a role for the combined use of
PIVKA-II and AFP in detecting HCC in non-viral
chronic liver diseases, such as MASLD or alcoholic
liver disease.17 Consequently, a panel of experts
from the Asia-Pacific region strongly agreed that
PIVKA-II in combination with AFP enables optimal
detection of HCC; they also agreed that PIVKA-II
is valuable for HCC detection in AFP-negative
patients,18 and it may be useful when monitoring
treatment response and recurrence. Notably,
PIVKA-II assays generally are not affected by renal
impairment or haemodialysis.19
A health economics study from Hong Kong20
showed that PIVKA-II-based surveillance strategies
are feasible options for the local healthcare system.
The combination of PIVKA-II and AFP exhibited
greater sensitivity for early-stage HCC compared
with the combination of USG and AFP. In patients
who had compensated liver cirrhosis, the use of
PIVKA-II and AFP for HCC detection resulted
in cost savings of HK$3328 and a gain of 0.016 in
quality-adjusted life years compared with the use of USG and AFP.20 It was projected that an additional
521 cases of early-stage HCC per 100 000 patients
could be detected by the combination of PIVKA-II
and AFP compared with the combination of USG
and AFP.20 Among patients with chronic hepatitis
B, the combination of PIVKA-II and AFP for HCC
detection led to cost savings of HK$4351 and a gain
of 0.008 in quality-adjusted life years compared with
the use of USG and AFP.20 It was projected that an
additional 247 cases of early-stage HCC per 100 000
patients could be detected by the combination of
PIVKA-II and AFP compared with the combination
of USG and AFP.20 The study concluded that all
PIVKA-II–based surveillance strategies, including
the GAAD (gender, age, AFP, and des-gamma-carboxyprothrombin)
score, provided superior
early-stage HCC detection compared with the
current approach of USG in combination with AFP.20
Local experience using protein induced by vitamin K absence-II in clinical practice
In Hong Kong, a pilot programme involving the
use of PIVKA-II in combination with AFP was
conducted in several acute hospitals from 2022 to
2023. Generally, patients in this programme were
HBV carriers, had advanced fibrosis or cirrhosis,
and/or had a high index of suspicion for HCC with
chronically elevated AFP levels or abnormal imaging
findings.
We carried out a clinical audit of 165 patients
who underwent PIVKA-II testing. The pooled
analysis revealed an overall sensitivity of 85.7%,
specificity of 96.2%, positive predictive value of 50%
and negative predictive value of 99.3%, consistent
with findings reported in the literature16 (Table).
Among the six patients with false-positive results, five had chronic hepatitis B (two of whom also had
cirrhosis), and one had alcoholic liver cirrhosis. One
patient with non-cirrhotic chronic HBV experienced
a non-icteric flare with alanine transaminase level
reaching 159 U/L. In the single patient with false-negative
results, the AFP level showed an increasing
trend, rising from 8.6 μg/L in July 2022 to 180 μg/L
in June 2023. The patient’s initial triphasic computed
tomography scan only showed a non-specific lesion;
2 months later, the lesion was identified as a 1.4-cm
HCC on a follow-up scan. This case provides real-world
evidence to support the use of PIVKA-II in
combination with AFP.
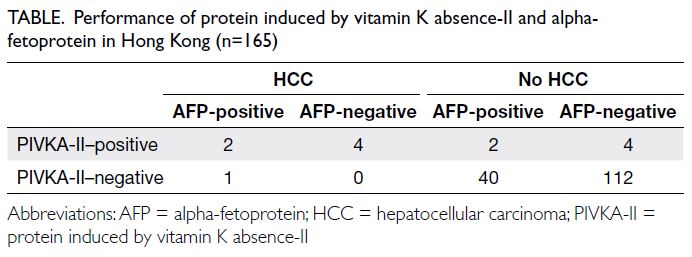
Table. Performance of protein induced by vitamin K absence-II and alpha-fetoprotein in Hong Kong (n=165)
Recommendations of the Expert Meeting
Based on the above data and local experience with PIVKA-II, the Expert Meeting established the following recommendations:
- Most experts recommended using PIVKA-II in addition to AFP because this combination has better diagnostic performance compared with either biomarker alone. However, PIVKA-II cannot entirely replace AFP due to limited evidence and differing biological mechanisms.
- Regardless of the biomarker(s) used, this approach cannot serve as a substitute for semi-annual liver USG in HCC surveillance. This recommendation is particularly pertinent considering the long wait times for USG in the public sector. Protein induced by vitamin K absence-II may be useful in prioritising patient referrals to the private sector for imaging.
- Protein induced by vitamin K absence-II is recommended for special patient populations, such as those with cirrhosis, normal AFP levels, and non-viral aetiologies of chronic liver disease (eg, MASLD and alcoholic liver disease), particularly when accompanied by cirrhosis.
- The utility of the GAAD score has been demonstrated, but it is considered difficult to interpret for continuous monitoring because age increases each year.21
- Other potential roles for PIVKA-II include its use in difficult or borderline cases, where it may serve as a helpful adjunct to clarify the diagnosis, and for monitoring HCC recurrence in patients who have undergone HCC resection.
Practical considerations and future directions
Currently, most experts in Hong Kong would
consider using PIVKA-II for surveillance in patients
who exhibit MASLD-related cirrhosis or newly
diagnosed HCC with normal AFP levels. The cost
of the PIVKA-II test is perceived as a substantial
barrier to wider implementation of this surveillance
and monitoring strategy. More evidence, such as a
cost-effectiveness study, is needed to justify its use
as a replacement for USG and facilitate broader
adoption. It was suggested that the test should
initially be made available to a small group of high-risk
patients before expansion to a larger population.
In the near future, additional data regarding PIVKA-II-based detection of MASLD-related HCC should
be collected and analysed.
Conclusion
The PIVKA-II test, when used in combination
with AFP, is likely to be both cost-effective and
clinically useful for HCC surveillance in Hong
Kong, particularly among patients with small and
AFP-negative HCC. Use of this test should be
prioritised in certain at-risk patient groups, such
as those with cirrhosis and non-viral aetiologies.
Considering the existing service gaps in providing
timely USG surveillance within the public sector,
better diagnostic, surveillance, and risk stratification
tools (eg, PIVKA-II) are needed to meet the targets
established by the World Health Organization and
the Hong Kong Viral Hepatitis Action Plan.
Author contributions
Concept or design: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: RNS Lui, LLY Mak.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: RNS Lui, LLY Mak.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
RNS Lui has served as an advisory board member for Gilead
Sciences and speaker for GenieBiome; he also holds equity in
Pfizer. As an editor of the journal, RNS Lui was not involved in
the peer review process. LLY Mak has served as a speaker for
Roche. HLY Chan is an advisor for Aligos, GlaxoSmithKline,
Roche, Vaccitech, and Virion Therapeutics; a speaker for
Echosens, Gilead Sciences, Roche, and Viatris; and a data
management board member for Aligos, Arbutus, Roche,
Vaccitech, and Zhimeng Therapeutics. MF Yuen has served as an advisor/consultant for and/or received grant/research
support from AbbVie, Aligos, AiCuris, Antios Therapeutics,
Arbutus Biopharma, Arrowhead Pharmaceuticals, Assembly
Biosciences, Bristol Myers Squibb, ClearB Therapeutics,
Dicerna Pharmaceuticals, Finch Therapeutics, Fujirebio,
GlaxoSmithKline, Gilead Sciences, Immunocore, Janssen,
Roche, Silverback Therapeutics, Sysmex Corporation, Tune
Therapeutics, Vir Biotechnology, and Visirna Therapeutics.
GLH Wong has served as an advisory board member for
AstraZeneca, Gilead Sciences, and Janssen, as well as a
speaker for Abbott, AbbVie, Ascletis, Bristol Myers Squibb,
Echosens, Gilead Sciences, Janssen, and Roche; she has also
received a research grant from Gilead Sciences. Other authors
have disclosed no conflicts of interest.
Funding/support
The authors thank Roche for their support in organising the meeting and providing free protein induced by vitamin K absence-II assays. The funder had no role in study design, data collection/analysis/interpretation or manuscript preparation.
References
1. Hong Kong Cancer Registry, Hospital Authority. 10 most
common cancers in Hong Kong in 2021. Available from:
https://www3.ha.org.hk/cancereg/. Accessed 11 Mar 2024.
2. Viral Hepatitis Control Office, Centre for Health Protection,
Department of Health, Hong Kong SAR Government.
Thematic Report on Viral Hepatitis (Population Health
Survey 2020-22). 2023. Available from: https://www.hepatitis.gov.hk/english/health_professionals/files/Full_Report_Thematic_report_PHS_2020-22.pdf. Accessed 27 Mar 2024.
3. Yuen MF, Hou JL, Chutaputti A; Asia Pacific Working
Party on Prevention of Hepatocellular Carcinoma.
Hepatocellular carcinoma in the Asia pacific region. J
Gastroenterol Hepatol 2009;24:346-53. Crossref
4. Danpanichkul P, Suparan K, Tothanarungroj P, et al.
Epidemiology of gastrointestinal cancers: a systematic
analysis from the Global Burden of Disease Study 2021.
Gut 2024 Sep 6:gutjnl-2024-333227. Epub ahead of print. Crossref
5. Mak LY, Liu K, Chirapongsathorn S, et al. Liver diseases
and hepatocellular carcinoma in the Asia-Pacific region:
burden, trends, challenges and future directions. Nat Rev
Gastroenterol Hepatol 2024 Aug 15. Epub ahead of print. Crossref
6. World Health Organization. Combating hepatitis B and
C to reach elimination by 2030: advocacy brief. 2016.
Available from: https://iris.who.int/handle/10665/206453. Accessed 15 Mar 2024.
7. Hong Kong SAR Government. Hong Kong Viral Hepatitis
Action Plan 2020–2024. 2020. Available from: https://www.hepatitis.gov.hk/doc/action_plan/Action%20Plan_Full%20Version_PDF_en.pdf. Accessed 27 Mar 2024.
8. Reig M, Forner A, Rimola J, et al. BCLC strategy for
prognosis prediction and treatment recommendation: the
2022 update. J Hepatol 2022;76:681-93. Crossref
9. Kanwal F, Singal AG. Surveillance for hepatocellular
carcinoma: current best practice and future direction.
Gastroenterology 2019;157:54-64. Crossref
10. Huang DQ, Singal AG, Kanwal F, et al. Hepatocellular
carcinoma surveillance—utilization, barriers and the
impact of changing aetiology. Nat Rev Gastroenterol
Hepatol 2023;20:797-809. Crossref
11. El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. Crossref
12. Wong GL, Wong VW, Tan GM, et al. Surveillance
programme for hepatocellular carcinoma improves the
survival of patients with chronic viral hepatitis. Liver Int
2008;28:79-87. Crossref
13. Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL.
Early detection of hepatocellular carcinoma increases the
chance of treatment: Hong Kong experience. Hepatology
2000;31:330-5. Crossref
14. Yip TC, Lee HW, Chan WK, Wong GL, Wong VW.
Asian perspective on NAFLD-associated HCC. J Hepatol
2022;76:726-34. Crossref
15. Poté N, Cauchy F, Albuquerque M, et al. Performance of
PIVKA-II for early hepatocellular carcinoma diagnosis
and prediction of microvascular invasion. J Hepatol
2015;62:848-54. Crossref
16. Chan HL, Vogel A, Berg T, et al. Performance evaluation
of the Elecsys PIVKA-II and Elecsys AFP assays for
hepatocellular carcinoma diagnosis. JGH Open 2022;6:292-300. Crossref
17. Ricco G, Cavallone D, Cosma C, et al. Impact of etiology
of chronic liver disease on hepatocellular carcinoma
biomarkers. Cancer Biomark 2018;21:603-12. Crossref
18. Kim DY, Toan BN, Tan CK, et al. Utility of combining
PIVKA-II and AFP in the surveillance and monitoring of
hepatocellular carcinoma in the Asia-Pacific region. Clin
Mol Hepatol 2023;29:277-92. Crossref
19. Kato A, Yasuda H, Togawa A, et al. Measurement of des-gamma-carboxy prothrombin levels in hemodialysis patients positive for anti-hepatitis virus C antibody. Clin Nephrol 2002;58:296-300. Crossref
20. Leung MK, Ko M, Chen J, et al. Surveillance of
hepatocellular cancer among hepatitis B and cirrhosis
patients using protein induced by vitamin K absence-II
(PIVKA-II): a cost-utility analysis for Hong Kong as an
example of endemic regions. Hepatol Int 2023;17:S1-267.
21. Piratvisuth T, Hou J, Tanwandee T, et al. Development and
clinical validation of a novel algorithmic score (GAAD)
for detecting HCC in prospective cohort studies. Hepatol
Commun 2023;7:e0317. Crossref