Hong Kong Med J 2024 Oct;30(5):380–5 | Epub 29 Aug 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Validation of diagnostic coding for chronic obstructive pulmonary disease in an electronic health record system in Hong Kong
WC Kwok, MB, BS, FHKAM (Medicine)1; Terence CC Tam, MB, BS, FHKAM (Medicine)1; CW Sing, PhD2; Esther WY Chan, PhD2; CL Cheung, PhD2
1 Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong SAR, China
2 Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr WC Kwok (kwokwch@hku.hk)
Abstract
Introduction: Electronic health record databases
can facilitate epidemiology research regarding
diseases such as chronic obstructive pulmonary
disease (COPD), a common medical condition
worldwide. We aimed to assess the validity of
International Classification of Diseases, 9th Revision
(ICD-9) code algorithms for identifying COPD in
Hong Kong’s territory-wide electronic health record
system, the Clinical Data Analysis and Reporting
System (CDARS).
Methods: Adult patients diagnosed with COPD at
all public hospitals in Hong Kong and specifically
at Queen Mary Hospital from 2011 to 2020 were
identified using the ICD-9 code 496 (Chronic
airway obstruction, not elsewhere classified) within
the CDARS. Two respiratory specialists reviewed
clinical records and spirometry results to confirm
the presence of COPD in a randomly selected group
of cases.
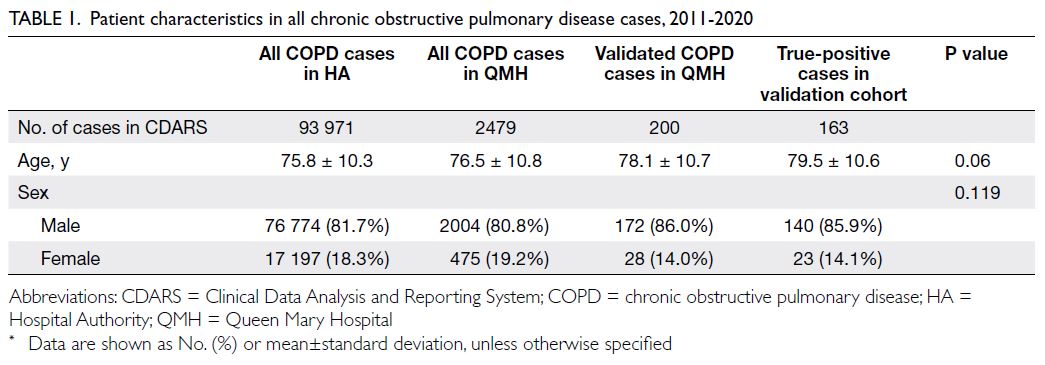
Results: During the study period, 93 971 and 2479
patients had the diagnostic code for COPD at all
public hospitals in Hong Kong and specifically at
Queen Mary Hospital, respectively. Two hundred
cases were randomly selected from Queen Mary Hospital for validation using medical records
and spirometry results. The overall positive
predictive value was 81.5% (95% confidence
interval=76.1%-86.9%). We also developed an
algorithm to identify COPD cases in our cohort.
Conclusion: This study represents the first
validation of ICD-9 coding for COPD in the CDARS.
Our findings demonstrated that the ICD-9 code 496
is a reliable indicator for identifying COPD cases,
supporting the use of the CDARS database for
further clinical research concerning COPD.
New knowledge added by this study
- This is the first validation study of International Classification of Diseases, 9th Revision (ICD-9) coding for chronic obstructive pulmonary disease (COPD) in the Hong Kong Clinical Data Analysis and Reporting System (CDARS).
- The ICD-9 code 496 demonstrated a high positive predictive value for identifying COPD cases in the CDARS.
- This study established an algorithm for identifying COPD cases in the CDARS.
- The findings provide a basis for territory-wide analysis of COPD in Hong Kong.
Introduction
Chronic obstructive pulmonary disease (COPD) is
a chronic inflammatory lung disease characterised
by airflow limitation, which causes symptoms
such as difficulty breathing, productive cough,
and wheezing. Smoking is the primary risk factor
for COPD development.1 Patients with COPD
experience gradual deterioration of lung function,
with potential intermittent exacerbations.
Although COPD is preventable and
manageable, it was ranked as the fourth leading cause
of death worldwide in the 2019 Global Initiative for Chronic Obstructive Lung Disease guidelines.2
The Global Burden of Disease Study estimated that
there were 3.2 million COPD-related deaths in 2015,
an increase of 11.6% compared with 1990.3 The
prevalence of COPD also increased by 44.2% during
the same period, reaching 174.5 million cases in
2015.3 In Hong Kong, the Population Health Survey
2014/15 revealed that 0.5% (0.6% in male individuals;
0.4% in female individuals) of non-institutionalised
persons aged ≥15 years had physician-diagnosed
COPD.4
The prevalence of COPD in Hong Kong among adults aged ≥60 years is 25.9% or 12.4%,
depending on the spirometric criteria used (post-bronchodilator
ratio of forced expiratory volume
in 1 second to forced vital capacity [ie, FEV1/FVC
ratio] <70% or lower limit of normal).5 In 2005, the
crude mortality rate for COPD was 29.1 per 100 000
population, whereas the crude hospitalisation rate
was 193 per 100 000 population.6 From January 2017
to December 2020, there were 78 693 admissions for
COPD across all public hospitals in Hong Kong.7 8
Population-based or large database studies
are valuable for understanding the epidemiology,
clinical characteristics, and burden of COPD.9 10 11 12 13 14 15
In countries/regions with electronic health record
(EHR) systems, the EHR databases offer extensive
information for clinical management, research,
and big data analysis of various diseases, including
COPD. Studies in the US and the United Kingdom
have validated diagnostic codes for COPD and acute
exacerbation of COPD. A study of the diagnostic code
for COPD in the US showed a positive predictive value
(PPV) of 91.7%, sensitivity of 71.7%, and specificity
of 94.4%.16 In the United Kingdom, the diagnostic
code for acute exacerbation of COPD had a PPV of
85.5% and sensitivity of 62.9%.17 Electronic health
records typically contain diagnostic information,
associated morbidity and mortality data, and
possible longitudinal follow-up data, allowing the
evaluation of COPD trends and associated health
outcomes. Before research can be conducted using EHR data, the diagnostic coding must be validated.
The Clinical Data Analysis and Reporting System
(CDARS), an EHR database managed by the Hospital
Authority (HA; a public healthcare service provider
that manages 43 hospitals/institutions and 123
outpatient clinics18), has covered >90% of the Hong
Kong population since 1993. The CDARS captures
medical information including diagnoses, drug
prescriptions, demographics, admissions, medical
procedures, and laboratory results. Although the
accuracy of diagnostic coding has been demonstrated
for some conditions in Hong Kong,19 20 21 it has not
been validated for COPD. In this study, we aimed to
assess the validity of International Classification of
Diseases, 9th Revision (ICD-9) code algorithms for
identifying COPD in the CDARS.
Methods
This study was conducted at Queen Mary Hospital
(QMH), a territory-wide tertiary and quaternary
referral centre under HA for advanced medical
services and respiratory diseases. All medical
information regarding its patients is captured within
the CDARS.
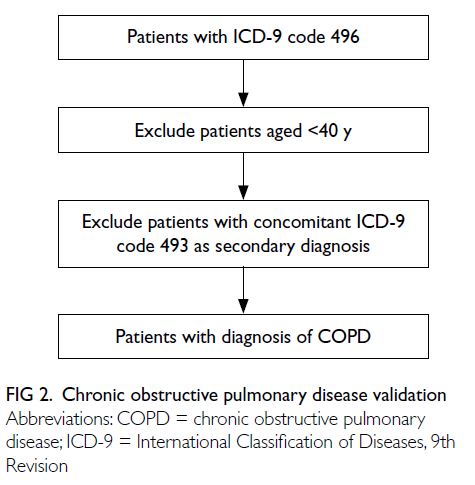
Firstly, all adult patients aged ≥40 years with a
principal diagnosis of COPD in HA from 1 January
2011 to 31 December 2020 were identified through
the CDARS. Then, in the ICD-9 coding validation
session, it included adult patients aged ≥40 years
with a principal diagnosis of COPD recorded at
QMH from 1 January 2011 to 31 December 2020.
Potential COPD cases in the CDARS were initially
identified using the ICD-9 code 496 (Chronic airway
obstruction, not elsewhere classified). Cases with a
secondary diagnosis of ICD-9 code 493 (Asthma;
indicating potential asthma-COPD overlap [ACO]
or asthma) were excluded. The clinical information
and spirometry results for all potential COPD cases
during the study period were retrieved for validation
from the CDARS. The algorithm used for case
identification is depicted in Figure 1.
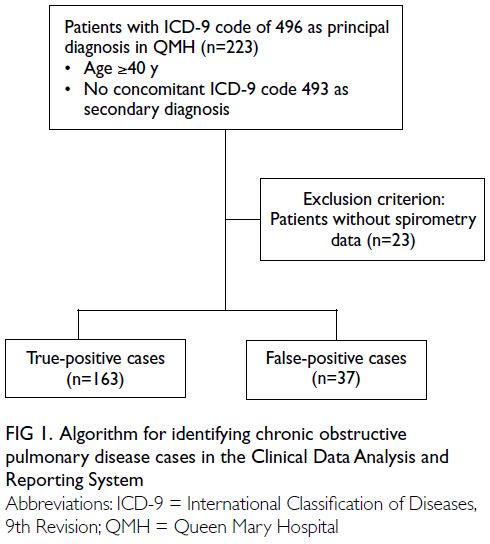
Figure 1. Algorithm for identifying chronic obstructive pulmonary disease cases in the Clinical Data Analysis and Reporting System
Among potential cases identified in the QMH
cohort, 200 were randomly selected for validation.
Case validation was performed by two respiratory
specialists, based on the clinical information,
spirometry results, physician notes, and clinical
examination reports. A potential COPD case was
regarded as true positive if the specialist concluded
that the patient had definite COPD according to
the Global Initiative for Chronic Obstructive Lung
Disease guidelines.22 A valid case was defined as
the presence of symptoms compatible with COPD,
along with spirometry results demonstrating airflow
limitation (ie, FEV1/FVC ratio <0.7) that could not
be fully reversed by the administration of an inhaled
bronchodilator. Potential cases not meeting these
criteria were regarded as false positive. Patients
without spirometry data were excluded from the case validation process. The flow of patient selection is illustrated in Figure 1.
The PPV was computed to assess the validity
of COPD diagnostic codes in the CDARS, using the
definition of the number of true positives (ie, cases
identified by ICD-9 codes which met the above
criteria) divided by the total number of true positives
plus false positives (ie, cases identified by ICD-9
codes which did not meet the above criteria).
Cohen’s kappa was used to estimate inter-rater
reliability and the 95% confidence interval
was estimated using a binomial distribution. All
statistical analyses were performed using SPSS
software (Windows 26.0; IBM Corp, Armonk [NY], US).
Results
In total, 2479 potential cases were identified in
QMH between 2011 and 2020. During the same
period, there were 93 971 cases with a principal
diagnostic code of COPD across all public hospitals
in Hong Kong. There were no significant differences
in age or sex between QMH cases and overall cases
throughout the HA (Table 1). Of the QMH cases, 200
were randomly selected for detailed validation. The
validation process showed that 163 cases were true
positives, resulting in an overall PPV of 81.5% (95%
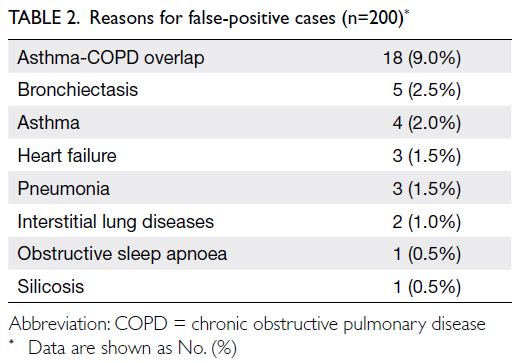
confidence interval=76.1%-86.9%). Major reasons
for false positives included ACO, asthma, and
bronchiectasis (Table 2). Cohen’s kappa was 0.77,
suggesting substantial agreement. The proposed
algorithm for identifying COPD cases in the CDARS
is illustrated in Figure 2.
Discussion
In this validation study, the estimated overall PPV
was 81.5% when ICD-9 coding was used to identify
COPD cases within the CDARS, the territory-wide
EHR system in Hong Kong.
A PubMed search using the terms ‘COPD’ AND
‘validation’ OR ‘international classification of disease
codes’ did not identify any literature regarding
validation of diagnostic codes for COPD in EHRs
within Hong Kong. Validation of local diagnostic
codes for COPD will facilitate large-scale studies in
Hong Kong, which are needed considering the high
local prevalence of this disease. Our study showed a
PPV >70%, which is the typical validation criterion
for case-finding algorithms in population-based
cohort studies.23 24 The high PPV in our study may
be attributable to the nature of the CDARS database,
with high PPV also reported in other local validation
studies involving other diseases.21 25 The CDARS
database contains EHRs from all public hospitals,
where diagnostic facilities and diagnostic protocols
are well-established; in contrast, data from claims databases and general practitioners are expected to
have lower accuracy. As such, in prior local validation
studies with CDARS, they had high reported PPV of
79%25 and 100%21 for interstitial lung diseases and hip
fracture, respectively. Also, COPD is a disease that
is easier to be recognised by demonstrating airflow
obstruction on spirometry, which contributed to the
high PPV. Additionally, regular audits by the HA of
diagnostic codes in patient discharge summaries to
make sure the correct diagnosis were entered further
enhance the accuracy of CDARS data.
Among the false-positive cases, ACO was the
most frequent cause (Table 2). This relationship
could be due to incorrect entry of COPD diagnostic
codes or to patients with childhood asthma who
developed COPD later in life. The lack of a separate
ICD-9 diagnostic code for ACO and the absence
of diagnostic criteria for this condition contribute
to these challenges.26 27 28 29 30 31 32 33 34 Considering the current
difficulties in accurate diagnosis of ACO, the actual PPV for COPD could be higher. Thus, our proposed
algorithm excludes cases with a secondary diagnosis
of asthma in the CDARS to avoid including
patients with ACO. Proper education to address
this miscoding issue is essential. Asthma was the
second most common incorrectly coded diagnosis.
This result could be related to initial misdiagnosis
at presentation, such as attributing shortness of
breath in a smoker to COPD, rather than asthma.
Heart failure, which also presents with dyspnoea
and wheezing, could be misclassified as COPD in
rare instances. Bronchiectasis, pneumonia, silicosis,
and interstitial lung disease can also present with
chronic productive cough and dyspnoea, similar to
COPD.
Strengths and limitations
The strengths of this study include its use of territorywide
database with >11 million records, which
allowed the identification of a sufficient number of
cases. The methodology utilised to confirm true-positive
COPD cases was both feasible and practical:
the medical records and spirometry results for all
cases with the COPD diagnostic code were reviewed
by respiratory specialists.
However, this study had some limitations.
First, the patient population mostly comprised adult
Chinese patients, consistent with the demographics
of patients with COPD in Hong Kong. This ethnicity
component may limit generalisability to other
populations. Second, only QMH cases were selected
for validation. However, because all hospitals and
clinics within the HA use a single diagnostic coding
system, the diagnostic coding consistency is expected
to be high. The high accuracy of ICD-9 coding within
the Hong Kong CDARS has been demonstrated in
other studies.20 21
Conclusion
This study represents the first validation of ICD-9
coding for COPD in Hong Kong. Our findings
demonstrated that use of ICD-9 code 496, in
conjunction with our algorithm to identify COPD,
results in a PPV with sufficient reliability to support
utilisation of the CDARS database for future COPD
research.
Author contributions
Concept or design: WC Kwok, CL Cheung.
Acquisition of data: WC Kwok.
Analysis or interpretation of data: WC Kwok.
Drafting of the manuscript: WC Kwok, CL Cheung.
Critical revision of the manuscript for important intellectual content: TCC Tam, CW Sing, EWY Chan, CL Cheung.
Acquisition of data: WC Kwok.
Analysis or interpretation of data: WC Kwok.
Drafting of the manuscript: WC Kwok, CL Cheung.
Critical revision of the manuscript for important intellectual content: TCC Tam, CW Sing, EWY Chan, CL Cheung.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The research was approved by the Institutional Review Board
of The University of Hong Kong / Hospital Authority Hong
Kong West Cluster, Hong Kong (Ref No.: UW22-716). The
requirement for informed consent is waived by the Board due
to the retrospective nature of the research.
References
1. Gershon AS, Warner L, Cascagnette P, Victor JC, To
T. Lifetime risk of developing chronic obstructive
pulmonary disease: a longitudinal population study. Lancet
2011;378:991-6. Crossref
2. Singh D, Agusti A, Anzueto A, et al. Global strategy for
the diagnosis, management, and prevention of chronic
obstructive lung disease: the GOLD Science Committee
Report 2019. Eur Respir J 2019;53:1900164. Crossref
3. Benziger CP, Roth GA, Moran AE. The Global Burden of
Disease study and the preventable burden of NCD. Glob
Heart 2016;11:393-7. Crossref
4. Centre for Health Protection, Department of Health, Hong
Kong SAR Government. Non-Communicable Diseases
Watch. Chronic obstructive pulmonary disease: an
overview. November 2018. Available from: https://www.chp.gov.hk/files/pdf/ncd_watch_november_2018.pdf. Accessed 9 Aug 2024.
5. Chan HS, Ko FW, Chan JW, et al. Hospital burden of
chronic obstructive pulmonary disease in Hong Kong—the
trend from 2006 to 2014. Int J Chron Obstruct Pulmon Dis
2023;18:507-19. Crossref
6. Chan-Yeung M, Lai CK, Chan KS, et al. The burden of
lung disease in Hong Kong: a report from the Hong Kong
Thoracic Society. Respirology 2008;13 Suppl 4:S133-65. Crossref
7. Ko FW, Lau LH, Ng SS, et al. Respiratory admissions before
and during the COVID-19 pandemic with mediation
analysis of air pollutants, mask-wearing and influenza
rates. Respirology 2023;28:47-55. Crossref
8. Chan KP, Ma TF, Kwok WC, et al. Significant reduction
in hospital admissions for acute exacerbation of chronic
obstructive pulmonary disease in Hong Kong during
coronavirus disease 2019 pandemic. Respir Med
2020;171:106085. Crossref
9. Buist AS, McBurnie MA, Vollmer WM, et al. International
variation in the prevalence of COPD (the BOLD study): a
population-based prevalence study. Lancet 2007;370:741-50. Crossref
10. Grahn K, Gustavsson P, Andersson T, et al. Occupational
exposure to particles and increased risk of developing
chronic obstructive pulmonary disease (COPD): a
population-based cohort study in Stockholm, Sweden.
Environ Res 2021;200:111739. Crossref
11. Lindberg A, Lindberg L, Sawalha S, et al. Large
underreporting of COPD as cause of death-results
from a population-based cohort study. Respir Med
2021;186:106518. Crossref
12. Landt E, Çolak Y, Lange P, Laursen LC, Nordestgaard BG, Dahl M. Chronic cough in individuals with COPD: a population-based cohort study. Chest 2020;157:1446-54. Crossref
13. Lee SC, Son KJ, Han CH, Park SC, Jung JY. Impact of
COPD on COVID-19 prognosis: a nationwide population-based
study in South Korea. Sci Rep 2021;11:3735. Crossref
14. Du Y, Li Q, Sidorenkov G, et al. Computed tomography
screening for early lung cancer, COPD and cardiovascular
disease in Shanghai: rationale and design of a population-based
comparative study. Acad Radiol 2021;28:36-45. Crossref
15. Bahremand T, Etminan M, Roshan-Moniri N, De Vera MA,
Tavakoli H, Sadatsafavi M. Are COPD prescription patterns
aligned with guidelines? Evidence from a Canadian
population-based study. Int J Chron Obstruct Pulmon Dis
2021;16:751-9. Crossref
16. Chu SH, Wan ES, Cho MH, et al. An independently
validated, portable algorithm for the rapid identification
of COPD patients using electronic health records. Sci Rep
2021;11:19959. Crossref
17. Rothnie KJ, Müllerová H, Hurst JR, et al. Validation of
the recording of acute exacerbations of COPD in UK
primary care electronic healthcare records. PLoS One
2016;11:e0151357. Crossref
18. Hospital Authority, Hong Kong SAR Government.
Introduction. Caring for our community's health. 2024.
Available from: https://www.ha.org.hk/visitor/ha_visitor_index.asp?Content_ID=10008&Lang=ENG&Dimension=100&Parent_ID=10004. Accessed 9 Aug 2024.
19. Chan SM, Chung GK, Chan YH, et al. Resilience and
coping strategies of older adults in Hong Kong during
COVID-19 pandemic: a mixed methods study. BMC
Geriatr 2022;22:299. Crossref
20. Cheung CL, Tan KC, Kung AW. Cohort profile: the Hong
Kong Osteoporosis study and the follow-up study. Int J
Epidemiol 2018;47:397-8f. Crossref
21. Sing CW, Woo YC, Lee AC, et al. Validity of major
osteoporotic fracture diagnosis codes in the Clinical
Data Analysis and Reporting System in Hong Kong.
Pharmacoepidemiol Drug Saf 2017;26:973-6. Crossref
22. Global Initiative for Chronic Obstructive Lung Disease.
2022 Global Strategy for Prevention, Diagnosis and
Management of COPD.
23. Cho SK, Doyle TJ, Lee H, et al. Validation of claims-based
algorithms to identify interstitial lung disease in
patients with rheumatoid arthritis. Semin Arthritis Rheum
2020;50:592-7. Crossref
24. Papani R, Sharma G, Agarwal A, et al. Validation of claims-based
algorithms for pulmonary arterial hypertension.
Pulm Circ 2018;8:2045894018759246. Crossref
25. Ye Y, Hubbard R, Li GH, et al. Validation of diagnostic
coding for interstitial lung diseases in an electronic health
record system in Hong Kong. Pharmacoepidemiol Drug
Saf 2022;31:519-23. Crossref
26. Leung JM, Sin DD. Asthma-COPD overlap syndrome:
pathogenesis, clinical features, and therapeutic targets.
BMJ 2017;358:j3772. Crossref
27. Cosio BG, Soriano JB, López-Campos JL, et al. Defining the
asthma-COPD overlap syndrome in a COPD cohort. Chest
2016;149:45-52. Crossref
28. Gibson PG, Simpson JL. The overlap syndrome of asthma
and COPD: what are its features and how important is it?
Thorax 2009;64:728-35. Crossref
29. Sin DD, Miravitlles M, Mannino DM, et al. What is asthma-COPD overlap syndrome? Towards a consensus definition from a round table discussion. Eur Respir J 2016;48:664-73. Crossref
30. Cataldo D, Corhay JL, Derom E, et al. A Belgian survey on
the diagnosis of asthma-COPD overlap syndrome. Int J
Chron Obstruct Pulmon Dis 2017;12:601-13. Crossref
31. Soler-Cataluña JJ, Cosío B, Izquierdo JL, et al. Consensus
document on the overlap phenotype COPD-asthma
in COPD [in English, Spanish]. Arch Bronconeumol
2012;48:331-7. Crossref
32. Koblizek V, Chlumsky J, Zindr V, et al. Chronic obstructive
pulmonary disease: official diagnosis and treatment
guidelines of the Czech Pneumological and Phthisiological
Society; a novel phenotypic approach to COPD with patient-oriented care. Biomed Pap Med Fac Univ Palacky
Olomouc Czech Repub 2013;157:189-201. Crossref
33. Miravitlles M, Alvarez-Gutierrez FJ, Calle M, et al.
Algorithm for identification of asthma-COPD overlap:
consensus between the Spanish COPD and asthma
guidelines. Eur Respir J 2017;49:1700068. Crossref
34. Global Initiative for Asthma; Global Initiative for Chronic
Obstructive Lung Disease. Diagnosis of Diseases of
Chronic Airflow Limitation: Asthma, COPD and Asthma-COPD Overlap Syndrome. Updated 2015. Available from:
https://goldcopd.org/wp-content/uploads/2016/04/GOLD_ACOS_2015.pdf. Accessed 2 Aug 2024.