Hong Kong Med J 2024 Apr;30(2):94-101 | Epub 5 Apr 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Impact of a novel pre-hospital stroke notification programme on acute stroke care key performance indicators in Hong Kong: a multicentre prospective cohort study with historical controls
KY Cheng, FHKCEM, FHKAM (Emergency Medicine)1; Ellen LM Yu, BSc, MSc (Epi/Biostat)2; Tafu Yamamoto, MB, ChB1; Julie CL Kwong, BHS, MBA3; YK Ho, MB, BS, FHKAM (Emergency Medicine)4; HK Ngan, MB, BS, FHKAM (Emergency Medicine)1; WH Lin, MB, BS1; Jessica MT Lau, FHKCEM, FHKAM (Emergency Medicine)5; CH Cheung, MB, ChB, MRCP (UK)6; Gordon PC Lee, FHKCEM, FHKAM (Emergency Medicine)4; LH Siu, FHKAM (Medicine), FHKCP3; Bun Sheng, MSc, MB, ChB6; Winnie WY Wong, FHKAM (Medicine), FRCP3; WY Man, BNurs, MSc6; Cathy CC Cheung, BNurs, MSc5; CT Tse, MB, BS, FHKAM (Medicine)6
1 Department of Accident and Emergency, Yan Chai Hospital, Hong Kong SAR, China
2 Clinical Research Centre, Kowloon West Cluster, Hospital Authority, Hong Kong SAR, China
3 Division of Neurology, Department of Medicine and Geriatrics, Caritas Medical Centre, Hong Kong SAR, China
4 Department of Accident and Emergency, Caritas Medical Centre, Hong Kong SAR, China
5 Department of Accident and Emergency, North Lantau Hospital, Hong Kong SAR, China
6 Division of Neurology, Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong SAR, China
Corresponding author: Dr KY Cheng (pkycheng31@fellow.hkam.hk)
Abstract
Introduction: Early identification and initiation of
reperfusion therapy is essential for suspected acute
ischaemic stroke. A pre-hospital stroke notification
(PSN) protocol using FASE (facial drooping, arm
weakness, speech difficulties, and eye palsy) was
implemented to improve key performance indicators
(KPIs) in acute stroke care delivery. We assessed
KPIs and clinical outcomes before and after PSN
implementation in Hong Kong.
Methods: This prospective cohort study with
historical controls was conducted in the Accident
and Emergency Departments of four public hospitals
in Hong Kong. Patients were screened using the PSN
protocol between August 2021 and February 2022.
Suspected stroke patients between August 2020 and
February 2021 were included as historical controls.
Door-to-needle (DTN) and door-to–computed
tomography (DTC) times before and after PSN
implementation were compared. Clinical outcomes
including National Institutes of Health Stroke Scale
score at 24 hours and modified Rankin Scale score at
3 months after intravenous recombinant tissue-type
plasminogen activator (IV-rtPA) were also assessed.
Results: Among the 715 patients (266 PSN and
449 non-PSN) included, 50.8% of PSN patients and
37.7% of non-PSN patients had a DTC time within
25 minutes (P<0.001). For the 58 PSN and 134
non-PSN patients given IV-rtPA, median DTN times
were 67 and 75.5 minutes, respectively (P=0.007).
The percentage of patients with a DTN time within
60 minutes was higher in the PSN group than in
the non-PSN group (37.9% vs 21.6%; P=0.019).
No statistically significant differences in clinical
outcomes were observed.
Conclusion: Although the PSN protocol shortened DTC and DTN times, clinical outcomes did not significantly differ.
New knowledge added by this study
- This study validates findings from a previous study that pre-hospital stroke notification (PSN) improves key performance indicators among stroke patients in Hong Kong.
- It is unclear whether PSN improves overall clinical outcomes among stroke patients.
- Further research is warranted to assess whether PSN improves patient outcomes and other acute care parameters.
- Considering the resource-intensive nature of PSN, its cost-effectiveness requires additional investigation.
Introduction
In Hong Kong, approximately 3000 stroke-related
deaths occur annually; stroke is among the top
three reasons for hospital admission.1 Strokes lead
to prolonged hospital stays, and affected patients
are likely to require long-term residential care.2
Early accurate identification of acute ischaemic
stroke and initiation of reperfusion therapy have
been associated with significant improvements
in functional outcomes and a lower likelihood of
hospital mortality.3 4 Therefore, efforts to shorten any
steps within the stroke onset-to-treatment cascade
can enhance outcomes for these patients.
The 2019 update to the American Stroke
Association (ASA) 2018 guidelines for the
management of acute ischaemic stroke recommends
early stroke recognition and notification during initial
medical contact using validated screening tools in
suspected stroke patients.5 Pre-hospital notification
to the receiving hospital allows early resource
mobilisation prior to arrival of the suspected stroke
patient, ensuring timely management. In Hong
Kong, a recent study demonstrated improvements in
several major benchmarks for acute stroke care.6 In August 2021, a pre-hospital stroke notification (PSN)
protocol using the FASE protocol (facial drooping,
arm weakness, speech difficulties, and eye palsy) was
implemented across the Kowloon West Cluster, the
largest service cluster in Hong Kong, which serves
nearly 2 million residents.7 The inclusion of eye
palsy in FASE aims to detect often-missed cases of
posterior stroke8 9 and aid the identification of large
vessel occlusion (LVO).10 In this study, we aimed to
assess key performance indicators (KPIs) and clinical
outcomes before and after the implementation of
PSN.
Methods
Study design
This multicentre prospective cohort study with
historical controls involved four Accident and
Emergency Departments (AEDs) in the Kowloon
West Cluster, namely, Princess Margaret Hospital,
North Lantau Hospital, Caritas Medical Centre and
Yan Chai Hospital, and their respective neurology
divisions. Prior to implementation of the PSN
FASE protocol, there was no established emergency
medical services (EMS) ambulance protocol for pre-hospital
notification of suspected stroke patients.
The non-PSN FAST protocol (facial drooping, arm
weakness, speech difficulty, and time) was used
at the AED to screen suspected stroke patients. In
this study, all suspected stroke patients between
August 2021 and February 2022 were screened
using the PSN FASE protocol and included in the
PSN group; similar patients between August 2020
and February 2021 served as historical controls
in the non-PSN group. Data were collected from
each hospital’s neurology division and clinical data
system; accuracy was confirmed by two independent
authors. Suspected LVO was defined as the presence
of clinical signs and symptoms compatible with
internal carotid artery, middle cerebral artery,
or basilar artery infarcts, along with radiological
evidence from computed tomography (CT) brain
scans, as reviewed by a neurologist. Confirmed LVO
was defined as the presence of LVO on computed
tomography angiography (CTA).
Patients
The PSN FASE protocol was implemented during
initial contact by EMS personnel during ambulance
transfer. This protocol specifies that the patient
must be aged ≥18 years and exhibits acute stroke
symptoms of facial weakness, unilateral arm and/or leg weakness, speech disturbance, or eye palsy
within 4 hours. Protocol exclusion criteria included
symptoms with suspected trauma aetiology, Glasgow
Coma Scale score ≤8, systolic blood pressure
<100 mm Hg, previous medical history of seizure/epilepsy, or long-term chairbound or bedbound status. If a patient meets inclusion criteria with no exclusion criteria, EMS personnel activate the PSN
protocol by informing the closest AED to prepare
for the incoming stroke patient. In the present study,
patients transported with this protocol constituted
suspected stroke patients in the PSN group.
In contrast, the non-PSN FAST protocol is
activated by a physician in the AED. This protocol
requires the patient to display acute stroke
symptoms of facial asymmetry, limb weakness,
or speech disturbance, while meeting all of the
following criteria: (1) age ≥18 years; (2) onset
of stroke symptoms within 3.5 hours before the
request for intravenous recombinant tissue-type
plasminogen activator (IV-rtPA) administration;
(3) signs and symptoms compatible with acute
stroke; and (4) reasonable premorbid functional
status (at least not bedbound). Protocol exclusion
criteria included active internal bleeding, recent
severe head trauma or intracranial/spinal surgery
within the preceding 3 months, clinical presentation
suggestive of subarachnoid haemorrhage or aortic
dissection, acute stroke symptoms in the context
of infective endocarditis, intra-axial intracranial
neoplasm, coagulopathy (platelet count <100 × 109/L
or international normalised ratio >1.7), or ongoing
use of anticoagulant medication.
FASE protocol of pre-hospital stroke
notification
In the PSN FASE protocol, EMS personnel are trained
to screen potentially IV-rtPA–eligible stroke patients
and to notify the receiving AED about patients with
thrombolytic eligibility. An AED physician and a
nursing team are prepared for immediate assessment
upon patient arrival; an experienced on-duty stroke
nurse is notified prior to arrival. The AED physician
immediately determines whether the patient should
be considered for thrombolytic therapy. If the
thrombolytic therapy criteria are met, the patient
undergoes a plain CT brain scan and assessment by
an on-call neurologist for intravenous thrombolytic
therapy. If IV-rtPA treatment is approved by the
on-call neurologist, IV-rtPA is administered to the
patient; this administration was similar for both
historical and prospective groups.
Outcomes measurement
The primary outcome in this study was door-to-needle
(DTN) time, which the ASA recommends to
be within 60 minutes. The secondary outcomes were
onset-to-door (OTD) and door-to-CT (DTC) times.
The recommended DTC time is within 25 minutes,
but no specific recommendation exists for OTD
time.11 The National Institutes of Health Stroke Scale
(NIHSS) score at 24 hours post-rtPA and modified
Rankin Scale (mRS) score at 3 months post-rtPA
were also recorded. A good clinical outcome was defined as a reduction of ≥4 in NIHSS score at 24 hours post-rtPA or an mRS score of 0 to 1 at 3 months post-rtPA.
Statistical analysis
Baseline characteristics, KPIs, and clinical outcomes
were presented as count (%), mean ± standard
deviation, or median (interquartile range). The
Pearson Chi squared test, Fisher’s exact test,
Mann-Whitney U test, and independent t test were
used to compare the PSN and non-PSN groups.
Further comparisons between the two groups were
performed after one-to-one matching based on
hospital, sex, age-group (≤80 years and >80 years),
and NIHSS score at onset. Sensitivity, specificity,
accuracy, positive predictive value (PPV) and negative
predictive value (NPV), along with 95% confidence
intervals, were computed for the PSN group using
the FAS protocol (facial drooping, arm weakness,
and speech difficulties) with or without eye palsy, as
well as eye palsy alone. The PPVs of the protocols
were compared using relative predictive values in a
paired study design, as proposed by Moskowitz and
Pepe.12 Statistical analyses were performed using
SPSS software (Windows version 26.0; IBM Corp,
Armonk [NY], United States) and the DTComPair
package in R software (version 3.6.1). P values <0.05
were considered statistically significant.
Results
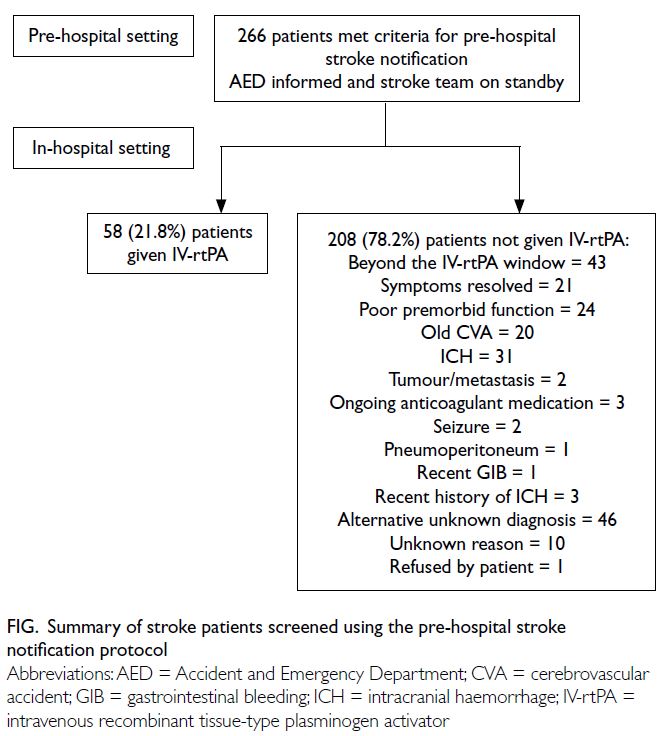
In total, 715 suspected stroke patients were
included, with 449 in the non-PSN group and 266
in the PSN group. Intravenous recombinant tissue-type
plasminogen activator was administered to
134 (29.8%) patients and 58 (21.8%) patients in the
non-PSN and PSN groups, respectively (P=0.019)
[Table 1]. Among the remaining 208 patients (78.2%)
not given IV-rtPA in the PSN group, 43 patients were
beyond the IV-rtPA window, and 46 patients had
alternative unknown diagnoses at AED attendance.
Twenty-one patients had symptoms that resolved or
improved by the time of AED attendance (Fig).
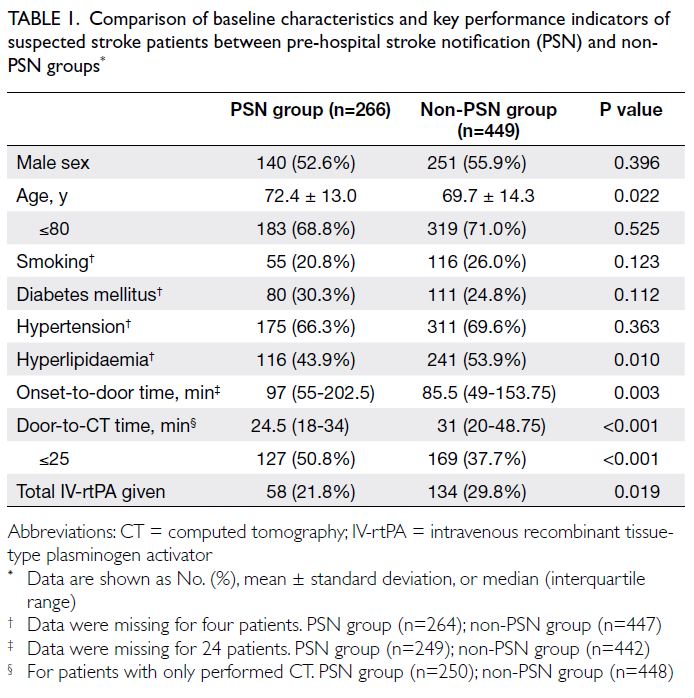
Table 1. Comparison of baseline characteristics and key performance indicators of suspected stroke patients between pre-hospital stroke notification (PSN) and non-PSN groups
Comparison in all suspected stroke patients
Demographic characteristics were compared between the non-PSN and PSN groups, as shown in Table 1. Age and hyperlipidaemia significantly
differed between the two groups. The median ages
were 69.7 years in the non-PSN group and 72.4
years in the PSN group (P=0.022). The percentages
of patients with hyperlipidaemia were 53.9% in
the non-PSN group and 43.9% in the PSN group
(P=0.010). Door-to-CT time was significantly
shorter in the PSN group than in the non-PSN group
(24.5 vs 31 minutes; P<0.001). The percentage of
patients achieving the DCT time goal of 25 minutes
was greater in the PSN group than in the non-PSN group (50.8% vs 37.7%; P<0.001). However, the median OTD time was longer in the PSN group than
in the non-PSN group (97 vs 85.5 minutes; P=0.003).
Comparison in patients given intravenous recombinant tissue-type plasminogen activator
Among stroke patients given IV-rtPA, sex,
hypertension, and hyperlipidaemia significantly
differed between the two groups, as illustrated in
Table 2. In the non-PSN group, 53.7% of patients
were men, compared with 69.0% in the PSN group
(P=0.049). Regarding key risk factors for ischaemic
stroke, the respective prevalences of hypertension
and hyperlipidaemia were 74.6% and 61.9% in the
non-PSN group, whereas they were 53.4% and 44.8%
in the PSN group. The NIHSS scores at symptom
onset were similar between the non-PSN and PSN
groups. The percentages of patients with suspected
LVO were also similar between the PSN and
non-PSN groups (40.4% vs 37.3%; P=0.759), as were
the percentages of patients with CTA-confirmed
LVO (52.6% vs 60.0%; P=1.000).
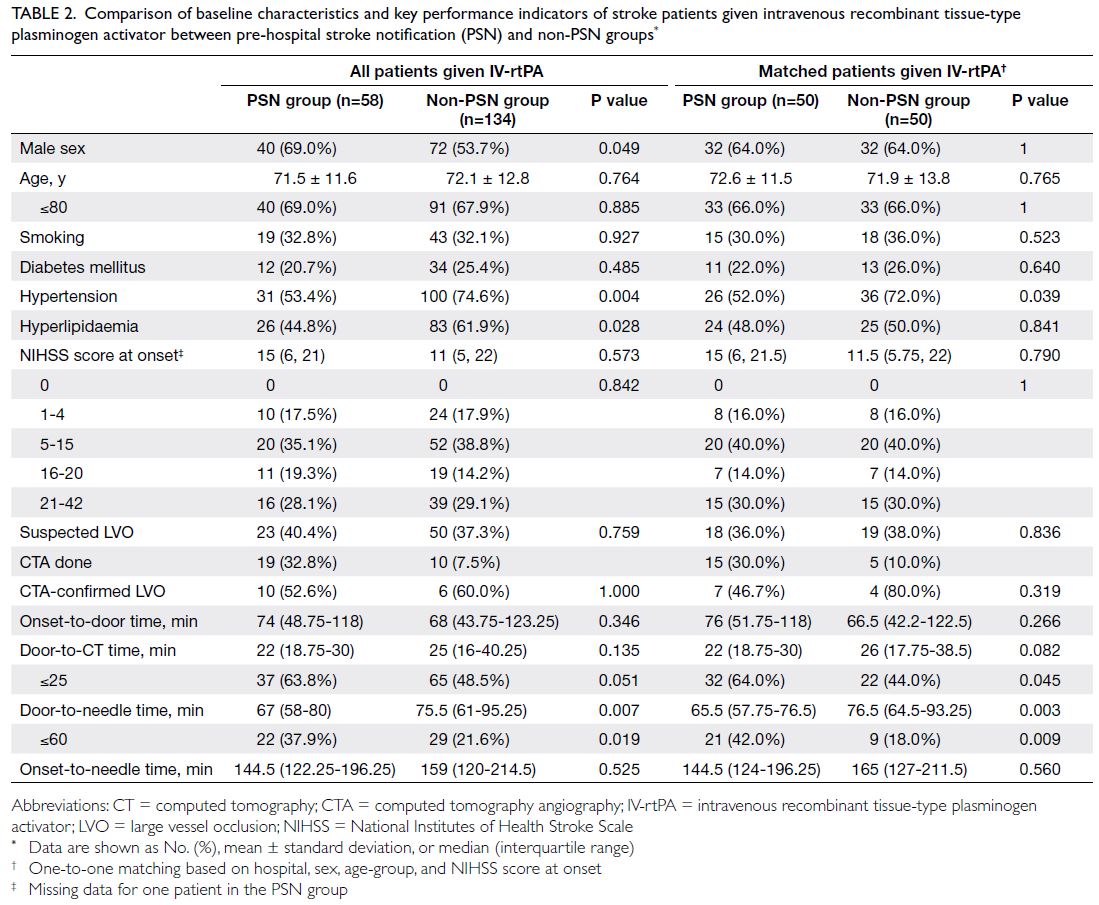
Table 2. Comparison of baseline characteristics and key performance indicators of stroke patients given intravenous recombinant tissue-type plasminogen activator between pre-hospital stroke notification (PSN) and non-PSN groups
The DTN time was shorter in the PSN group
than in the non-PSN group (67 vs 75.5 minutes;
P=0.007). Additionally, the percentage of patients
achieving the DTN time goal of 60 minutes was
greater in the PSN group (37.9% vs 21.6%; P=0.019).
However, there were no differences in median
DTC time and percentage of patients achieving
the DTC time goal of 25 minutes (Table 2). As
shown in Table 3, the percentages of patients with
good clinical outcomes after IV-rtPA were similar
between non-PSN and PSN groups, as indicated by
a reduction of ≥4 in NIHSS score at 24 hours (50.8%
vs 49.0%; P=0.829) and an mRS score of 0 to 1 at 90
days (43.3% vs 35.4%; P=0.342).
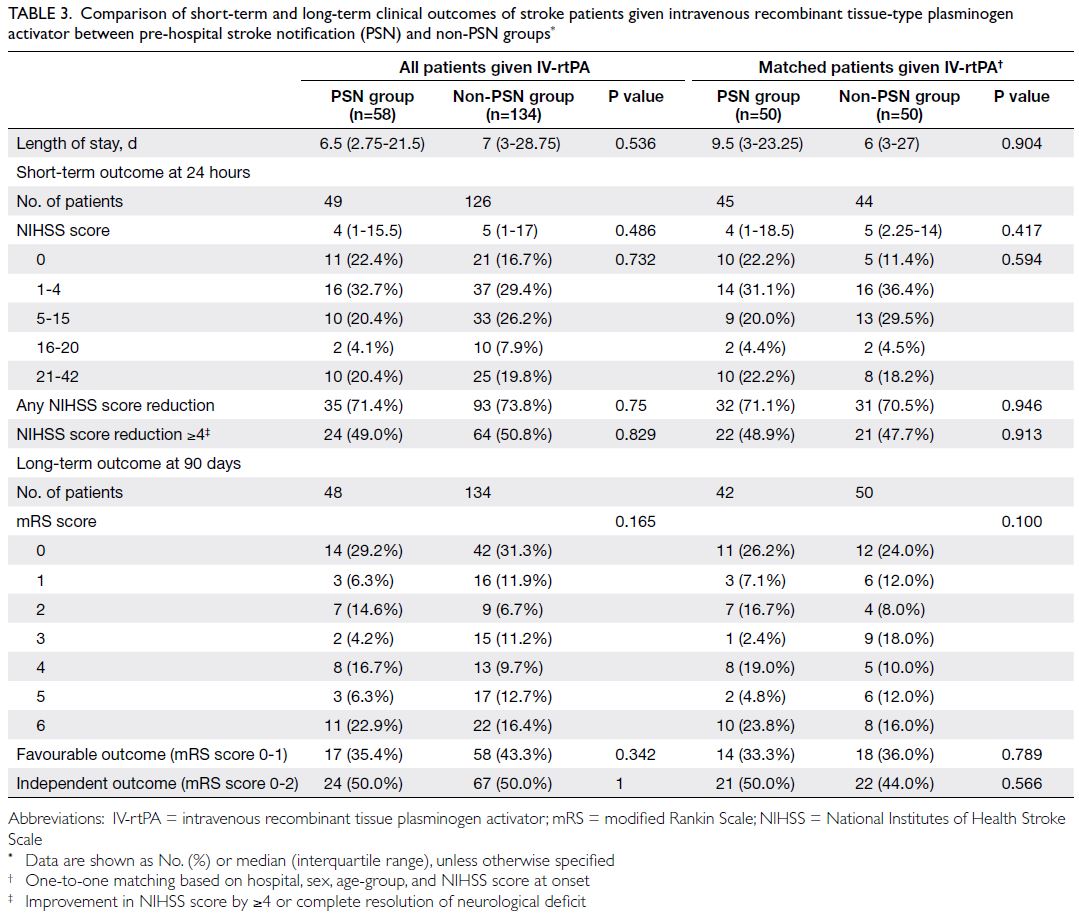
Table 3. Comparison of short-term and long-term clinical outcomes of stroke patients given intravenous recombinant tissue-type plasminogen activator between pre-hospital stroke notification (PSN) and non-PSN group
Matched comparison of patients given intravenous recombinant tissue-type plasminogen activator
The non-PSN and PSN groups were matched based
on hospital, sex, age-group, and NIHSS score at
onset. After matching, the percentage of patients
achieving the DTC time goal of 25 minutes was
greater in the PSN group than in the non-PSN group
(64.0% vs 44.0%; P=0.045). The median DTN time
was also shorter in the PSN group (65.5 vs 76.5
minutes; P=0.003). Moreover, the percentage of
patients achieving the DTN time goal of 60 minutes
was greater in the PSN group than in the non-PSN
group (42.0% vs 18.0%; P=0.009) [Table 2]. Finally, the
percentages of patients with good clinical outcomes
after IV-rtPA were similar between non-PSN and
PSN groups, as evidenced by a reduction of ≥4 in
NIHSS score at 24 hours (47.7% vs 48.9%; P=0.913)
and an mRS score of 0 to 1 at 90 days (36.0% vs 33.3%;
P=0.789) [Table 3].
Predictive value of eye palsy assessment in the pre-hospital stroke notification protocol
Among the 22 patients with eye palsy in the PSN
group, 18 patients had either facial drooping, arm
weakness or speech difficulties; seven patients were
administered IV-rtPA. In the PSN group, the PPVs
for using FAS, eye palsy alone, and FAS with eye palsy
to identify stroke patients eligible for IV-rtPA were
22.14%, 31.82%, and 38.89%, respectively (Table 4).
Compared with the PPV of FAS, the PPV of FAS with
eye palsy was significantly higher (P=0.046), whereas
the PPV of eye palsy alone did not significantly differ
(P=0.223).
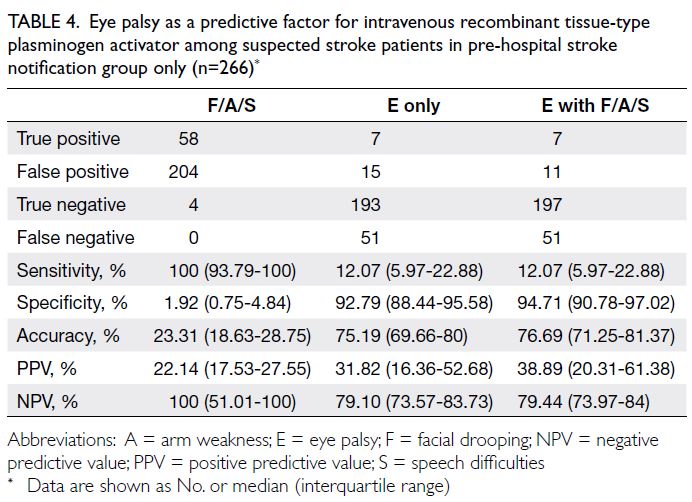
Table 4. Eye palsy as a predictive factor for intravenous recombinant tissue-type plasminogen activator among suspected stroke patients in pre-hospital stroke notification group only (n=266)
Discussion
The AHA and ASA recommend specific time goals for KPIs in stroke patients, such as OTD, DTC, and DTN times. Early recognition of stroke and
utilisation of PSN for these patients are emphasised
in the recent ASA guidelines as recommendations
that can facilitate achievement of these goals. The
recent adoption of a PSN protocol by the public
hospital system in Hong Kong is intended to improve
these KPIs and, ultimately, clinical outcomes among
stroke patients.
In the present study, the PSN FASE protocol
resulted in shorter DTC and DTN times, compared
with the non-PSN protocol. A shorter DTN is
associated with improved patient outcomes3 4 and
enables more patients to receive IV-rtPA within
the therapeutic window.13 However, the onset-to-needle
time did not differ between the two groups
(144.5 vs 159 minutes; P=0.525) [Table 2], which
may be explained by the longer OTD time in the
PSN group than in the non-PSN group (97 vs 85.5 minutes; P=0.003) [Table 1]. To control for potential
confounding factors, we matched the non-PSN and
PSN stroke patients based on multiple variables; the
results confirmed that DTC and DTN times were
shorter in the PSN group.
However, these improvements in KPIs did
not lead to statistically significant improvements in
clinical outcomes, as evidenced by a reduction of
≥4 in NIHSS score at 24 hours post-rtPA (50.8% vs
49.0%; P=0.829) and an mRS score of 0 to 1 at 90
days (43.3% vs 35.4%; P=0.342) [Table 3]. The results
of previous studies have suggested favourable mRS
score outcomes in 33% to 41% of stroke patients
given IV-rtPA12 14; the absence of favourable
neurological outcomes in the present study may be
attributed to the higher baseline level of neurological
improvement in the non-PSN group. Moreover, the
relatively small sample sizes in the PSN and non-PSN groups (58 vs 134; P=0.019) [Table 1] may explain
the lack of statistically significant clinical benefit in
this study; future studies with larger sample sizes
may provide further insights. The longer OTD time
in the PSN group compared with the non-PSN
group suggests that patients in the PSN group were
administered IV-rtPA later than patients in the
non-PSN group, potentially resulting in worse clinical
outcomes. Finally, the lack of statistically significant
improvements in clinical outcomes may be explained
by the higher NIHSS score at onset in the PSN group
(15 vs 11; P=0.573) [Table 2]; regardless of matching
to control for potential confounding factors, we did
not observe any statistically significant improvement
in clinical outcomes.
A higher percentage of stroke patients received
IV-rtPA in the non-PSN group compared with the
PSN group, which may differ from the findings in
some recent studies.6 15 This discrepancy may be
attributed to the learning curve associated with
the new FASE protocol in the PSN group; EMS
personnel may have engaged in ‘over-activation’ for
borderline suspected stroke patients during early
implementation. Additionally, because screening in
the PSN group was performed by EMS personnel,
it may have been less accurate than screening by
physicians (ie, in the non-PSN group). The longer
OTD time in the PSN group suggested that patients
in the PSN group presented to the AED later than
patients in the non-PSN group, increasing the
likelihood that they would miss the 4-hour window
for IV-rtPA administration.
The inclusion of eye palsy in the FASE protocol
is intended to identify potential cases of posterior
stroke8 9 and aid the identification of LVO.10 Although
we found that the FASE protocol had a higher PPV
(compared with the FAST protocol) for identifying
stroke patients eligible for IV-rtPA, we did not assess
whether the FASE protocol reliably identified patients
with posterior strokes. Future studies validating the
FASE protocol would provide additional insights.
Considering the role of conjugate eye deviation in
identifying LVO strokes,16 17 research exploring the
ability of the FASE protocol to identify these patients
would be valuable. Investigations of EMS personnel
accuracy in eye palsy recognition may also be useful.
Limitations
Possible limitations of this study include the
potential for experimenter bias, considering that
most investigators were also clinicians involved in
patient management. However, it may be difficult
to address this bias due to staffing constraints in
peripheral acute hospitals, where researchers also
serve as clinicians. Furthermore, the findings in this
study are consistent with the results of other studies
regarding pre-hospital notification protocols for
suspected stroke patients.
We also included the percentage of stroke
patients with CTA-confirmed LVO to provide a
more comprehensive analysis, considering that LVO
strokes have been linked to worse clinical outcomes
compared with non-LVO strokes.18 We observed no
statistically significant differences in the percentages
of suspected LVO and CTA-confirmed LVO strokes
between the two study groups. However, because
logistical considerations and resource limitations
hindered our ability to perform diagnostic CTA for
all patients, the true number of CTA-confirmed LVO
strokes may be underestimated. Finally, the relatively
small sample size may restrict our capacity to draw
definitive conclusions.
Conclusion
This study validated the previous finding that a PSN
protocol improves multiple stroke KPIs in Hong
Kong. It also improves the understanding of whether
a PSN protocol directly improves overall clinical
outcomes among stroke patients, an area with limited
evidence in current literature.19 The implementation
of a PSN protocol using the new FASE assessment
guideline shortened DTN and DTC times compared
with a non-PSN protocol. However, this study did not
reveal any statistically significant improvement in
overall clinical neurological outcomes between these
two protocols. Further research may be warranted to
assess whether PSN improves patient outcomes and
other acute care parameters.
Author contributions
Concept or design: KY Cheng, ELM Yu.
Acquisition of data: KY Cheng, T Yamamoto.
Analysis or interpretation of data: KY Cheng, ELM Yu.
Drafting of the manuscript: KY Cheng, ELM Yu.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: KY Cheng, T Yamamoto.
Analysis or interpretation of data: KY Cheng, ELM Yu.
Drafting of the manuscript: KY Cheng, ELM Yu.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank Mr Kin-wah Tam from North Lantau Hospital for data retrieval at the Hospital.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This research was approved by the Kowloon West Cluster
Research Ethics Committee of Hospital Authority, Hong
Kong [Ref No.: KW/EX-21-134(163-12)]. A waiver of patient consent was granted by the Committee since the data had been
collected prior to this research and the risk of identification
is minimal, and no new additional data was required for the
research.
References
1. Centre for Health Protection, Department of Health, Hong
Kong SAR Government. Physical activity: a major strategy
for stroke prevention. Non-communicable diseases watch.
October 2022. Available from: https://www.chp.gov.hk/files/pdf/ncd_watch_oct_2022.pdf. Accessed 28 Mar 2024.
2. Woo J, Ho SC, Goggins W, Chau PH, Lo SV. Stroke
incidence and mortality trends in Hong Kong: implications
for public health education efforts and health resource
utilisation. Hong Kong Med J 2014;20(3 Suppl 3):S24-9.
3. Saver JL, Fonarow GC, Smith EE, et al. Time to treatment
with intravenous tissue plasminogen activator and outcome
from acute ischemic stroke. JAMA 2013;309:2480-8. Crossref
4. Man S, Xian Y, Holmes DN, et al. Association between
thrombolytic door-to-needle time and 1-year mortality
and readmission in patients with acute ischemic stroke.
JAMA 2020;323:2170-84. Crossref
5. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018
guidelines for the early management of patients with acute
ischemic stroke: a guideline for healthcare professionals
from the American Heart Association/American Stroke
Association. Stroke 2018;49:e46-110. Crossref
6. Leung WC, Teo KC, Kwok WM, et al. Pre-hospital stroke
screening and notification of patients with reperfusion-eligible
acute ischaemic stroke using modified Face Arm
Speech Time test. Hong Kong Med J 2020;26:479-85. Crossref
7. Hospital Authority. Annual report 2011-2012. Available
from: https://www.ha.org.hk/ho/corpcomm/ar201112/html/eng/headoffice/kwc.html#:~:text=The%20Kowloon%20West%20Cluster%20(KWC,Tsuen%20Wan%20and%20Tung%20Chung . Accessed 28 Mar 2024.
8. Kleindorfer DO, Miller R, Moomaw CJ, et al. Designing a
message for public education regarding stroke: does FAST
capture enough stroke? Stroke 2017;38:2864-8. Crossref
9. Aroor S, Singh R, Goldstein LB. BE-FAST (Balance,
Eyes, Face, Arm, Speech, Time): reducing the proportion
of strokes missed using the FAST mnemonic. Stroke
2017;48:479-81. Crossref
10. Beume L, Hieber M, Kaller CP, et al. Large vessel occlusion
in acute stroke. Stroke 2018;49:2323-9. Crossref
11. Matsuo R, Yamaguchi Y, Matsushita T, et al. Association
between onset-to-door time and clinical outcomes after
ischemic stroke. Stroke 2017;48:3049-56. Crossref
12. Moskowitz CS, Pepe MS. Comparing the predictive values
of diagnostic tests: sample size and analysis for paired
study designs. Clin Trials 2006;3:272-9. Crossref
13. Wardlaw JM, Murray V, Berge E, et al. Recombinant
tissue plasminogen activator for acute ischaemic stroke:
an updated systematic review and meta-analysis. Lancet
2012;379:2364-72. Crossref
14. Emberson J, Lees KR, Lyden P, et al. Effect of treatment
delay, age, and stroke severity on the effects of intravenous
thrombolysis with alteplase for acute ischaemic stroke: a
meta-analysis of individual patient data from randomised
trials. Lancet 2014;384:1929-35. Crossref
15. Hsieh MJ, Tang SC, Chiang WC, et al. Effect of prehospital
notification on acute stroke care: a multicenter study.
Scand J Trauma Resusc Emerg Med 2016;24:57. Crossref
16. Ollikainen JP, Janhunen HV, Tynkkynen JA, et al. The
Finnish Prehospital Stroke Scale detects thrombectomy
and thrombolysis candidates—a propensity score–matched study. J Stroke Cerebrovasc Dis 2018;27:771-7. Crossref
17. Keenan KJ, Kircher C, McMullan JT. Prehospital prediction
of large vessel occlusion in suspected stroke patients. Curr
Atheroscler Rep 2018;20:34. Crossref
18. Malhotra K, Gornbein J, Saver JL. Ischemic strokes due
to large-vessel occlusions contribute disproportionately
to stroke-related dependence and death: a review. Front
Neurol 2017;8:651. Crossref
19. Sangari A, Akhoundzadeh K, Vahedian M, Sharifipour E.
Effect of pre-hospital notification on delays and
neurological outcomes in acute ischemic stroke. Australas
Emerg Care 2022;25:172-5. Crossref