Hong Kong Med J 2024 Apr;30(2):167–9 | Epub 17 Apr 2024
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Burkholderia pseudomallei pericarditis as a mimicker of tuberculous pericarditis: a case report
Frederick HC Lau, MB, BS; MF Lin, MB, BS, FHKAM (Surgery); WS Ng, MB, BS, FHKAM (Surgery)
Department of Cardiothoracic Surgery, Queen Elizabeth Hospital, Hong Kong SAR, China
Corresponding author: Dr Frederick HC Lau (hcf.lau@ha.org.hk)
Introduction
Melioidosis, also known as Whitmore’s disease,
occurs predominantly in tropical climates and
can cause pneumonia, soft tissue infection, brain
abscess, and septicaemia. Very few cases of
melioidosis pericarditis have been reported. The
most common cause of constrictive pericarditis
in Hong Kong is tuberculosis. We report a patient
with melioidosis constrictive pericarditis with
presentation mimicking tuberculous pericarditis
infection in whom we performed pericardiectomy.
Case presentation
A 61-year-old man who was a chronic smoker and
worked on a construction site presented with fever
and heart failure symptoms including dyspnoea,
orthopnoea, and ankle oedema. Laboratory results
showed elevated white blood cell count (21.7 × 109/L)
with neutrophils predominant (19 × 109/L) and raised
levels of C-reactive protein (303 mg/L) and liver
parenchymal enzymes (alanine transaminase level:
153 U/L, alkaline phosphate level: 125 U/L). Repeated
blood cultures were negative. Sputum for acid-fast
bacilli smear and culture and Mycobacterium
tuberculosis polymerase chain reaction were
negative. Computed tomography (CT) with contrast
showed bilateral multiple small lung nodules
(largest 1.3 cm) with mediastinal and right hilar
lymphadenopathy. Transthoracic echocardiogram
revealed bilateral pleural effusion with pericardial
effusion requiring pericardial drain insertion 2 days
after admission. Analysis of pericardial fluid showed
elevated adenosine deaminase (ADA) level (58 U/L)
and culture showed scanty growth of pseudomonal
species after 6 days. No Mycobacterium species was
isolated after 6 weeks of incubation. The patient
was commenced on empiric antituberculosis
treatment (isoniazid 300 mg daily, rifampicin 600 mg
daily, pyrazinamide 1500 mg daily, ethambutol
900 mg daily, and pyridoxine 20 mg daily) 3 weeks
after admission in view of the elevated ADA level,
persistent intermittent fever while on intravenous
piperacillin/tazobactam and due to the risk of
tuberculous pericarditis in Hong Kong. He was discharged and referred to our cardiothoracic centre
for elective pericardial biopsy.
While waiting for our outpatient clinic
assessment, the patient was admitted as an emergency
1 month after commencing antituberculosis
treatment with shortness of breath and frequent
episodes of atrial flutter. He was later transferred
to our cardiothoracic unit for further management.
Echocardiogram and CT scan of the thorax showed
a large heterogeneous pericardial collection with
adhesions and internal echogenicities of up to 3.5 cm
in diameter, located next to the left ventricle with
signs of compression and constriction (Fig 1). There
were also signs of septal bounce and exaggerated
respirophasic changes of tricuspid and mitral inflow.
Left and right ventricular systolic function was
reduced due to impaired bi-ventricular contractility
caused by pericardial adhesions and constriction,
confirmed by right heart catheterisation (high
right ventricular end-diastolic pressure–to–right
ventricular systolic pressure ratio, discordance
between left ventricular systolic pressure and right
ventricular systolic pressure during respiration).
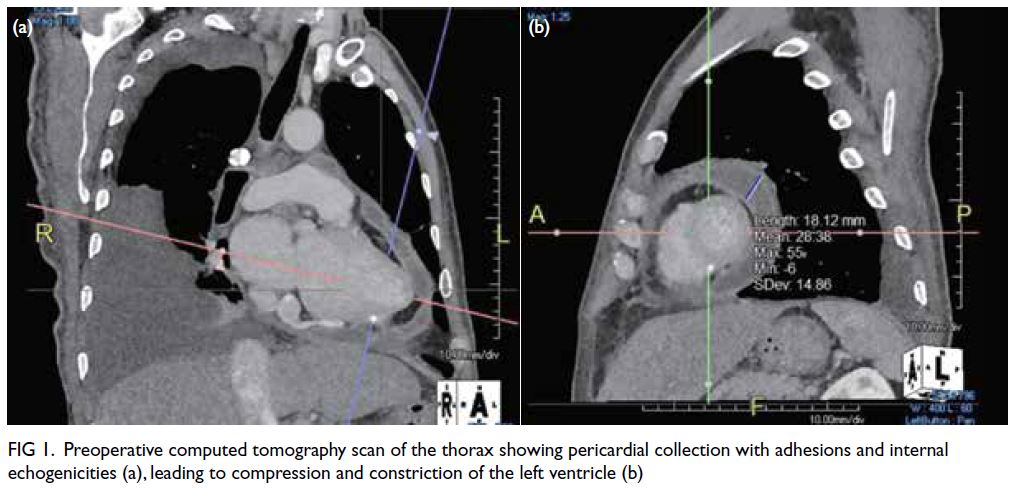
Figure 1. Preoperative computed tomography scan of the thorax showing pericardial collection with adhesions and internal echogenicities (a), leading to compression and constriction of the left ventricle (b)
Pericardiectomy was performed and the excised
pericardium sent for sectioning revealed suppurative
granulomatous inflammation (Fig 2). The Ziehl–Neelsen stain and Mycobacterium tuberculosis
polymerase chain reaction were negative. Pericardial
pus aspirate contained Burkholderia pseudomallei species.
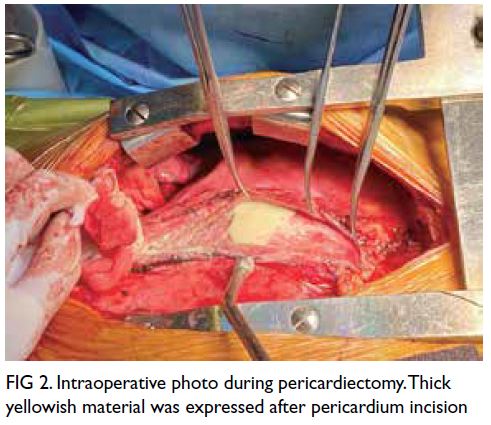
Figure 2. Intraoperative photo during pericardiectomy. Thick yellowish material was expressed after pericardium incision
Antituberculosis treatment was withheld and
melioidosis was treated with intravenous meropenem
for 4 weeks and oral co-trimoxazole with doxycycline
as maintenance therapy. The patient’s postoperative
course was uneventful. Postoperative CT of the
thorax at 3 months showed reduced pericardial
effusion, decreased lung consolidation, and reduced
pleural effusion.
Discussion
Melioidosis is caused by intracellular Gram-negative
saprophytic B pseudomallei. This bacterium was
formerly classified in the genus Pseudomonas
but is now separated from Pseudomonas and Stenotrophomonas. It is predominantly found in
contaminated water and soil and spread to humans
through direct contact with contaminated sources.
Melioidosis is a multiorgan infectious disease with
pneumonia as the most common presentation. A
progressive increase in the number of cases has been
observed in Hong Kong over the last 20 years, as
well as in Asia over the past decade.1 There was no
previous reported case of melioidosis pericarditis in
Hong Kong.1
Tuberculosis is caused by the acid-fast bacillus
M tuberculosis. It is airborne and primarily affects
the lungs, and is common in Southeast Asia. The
level of ADA, an enzyme in lymphocytes, increases in the presence of inflammatory effusions caused by
bacterial infection, granulomatous inflammation,
malignancy, and autoimmune disease. It is typically
higher in effusions caused by tuberculosis than
those caused by other conditions with a level
>40 U/L in lymphocyte-predominant effusions
having a sensitivity of 87% to 93% and a specificity
of 89% to 97% for tuberculosis.2 Nonetheless in
neutrophil-predominant effusions, ADA level may
lead to false-positive results since ADA is normally
elevated.2
Constrictive pericarditis is a disease
characterised by pericardial effusion with constrictive
pathology. The most common cause is idiopathic,
followed by tuberculosis, or post-irradiation
and post-pericardiotomy. It is characterised by
echocardiography findings of septal bounce, diastolic
shift of the interventricular septum, restrictive left
ventricular filling pattern, and cardiac catheterisation
finding of square root sign.3
The presentation, laboratory results and
imaging findings are very similar for melioidosis
and tuberculous pericarditis. Most patients have a
subacute-to-chronic disease course. The protein,
sugar and white cell count of pericardial fluid
may also be similar in both diseases. Compared
with patients with tuberculous pericarditis, those
with melioidosis pericarditis more often have a
neutrophil-predominant white blood cell count,4
as in our case. Histological findings can show
granulomatous inflammation with tuberculosis
showing a caseous type.5
It is important to differentiate between B pseudomallei and M tuberculosis and make a correct diagnosis since the treatment for melioidosis pericarditis and tuberculous pericarditis varies
markedly. A prolonged course of intravenous
ceftazidime followed by a combination of co-trimoxazole
and doxycycline is prescribed for
melioidosis while a prolonged course of rifampicin,
ethambutol, pyrazinamide and isoniazid is prescribed
for tuberculosis.3 An incorrect diagnosis not only
delayed treatment for melioidosis in our patient with
consequent worsening of his clinical condition, but
may also have caused more harm by exposing the
patient to the side-effects of tuberculosis treatment.
It is important to send pericardial fluid for culture
and to look meticulously for the two organisms.
Conclusion
The presentation, laboratory results and imaging
findings can be very similar for melioidosis and
tuberculous pericarditis. It is important to bear
this in mind during investigations for chronic
pericarditis and obtain pericardial culture. This will
ensure prompt diagnosis and timely commencement
of appropriate treatment.
Author contributions
All authors contributed to the concept or design, acquisition
of data, analysis or interpretation of data, drafting of the
manuscript, and critical revision of the manuscript for
important intellectual content. All authors had full access to
the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration
of Helsinki and provided informed consent for all treatments
and procedures, and consent for publication.
References
1. Lui G, Tam A, Tso YK, et al. Melioidosis in Hong Kong. Trop Med Infect Dis 2018;3:91. Crossref
2. Chau E, Sarkarati M, Spellberg B. Adenosine deaminase diagnostic testing in pericardial fluid. JAMA 2019;322:163-4. Crossref
3. Chow CY, Lun KS. Idiopathic effusive constrictive
pericarditis in an adolescent boy: a rare cause of heart
failure with diagnostic difficulty. Hong Kong J Paediatr
(new series) 2015;20:110-4.
4. Chetchotisakd P, Anunnatsiri S, Kiatchoosakun S,
Kularbkaew C. Melioidosis pericarditis mimicking
tuberculous pericarditis. Clin Infect Dis 2010;51:e46-9. Crossref
5. Wong KT, Puthucheary SD, Vadivelu J. The histopathology
of human melioidosis. Histopathology 1995;26:51-5. Crossref

