Hong Kong Med J 2023 Oct;29(5):396–403 | Epub 4 Oct 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Risks and impacts of thromboembolism in patients with pancreatic cancer
Landon L Chan, MB, ChB1 #; KY Lam, LMCHK, FHKAM (Medicine)2 #; Daisy CM Lam, MB, BS, FHKAM (Radiology)1; YM Lau, MB, BS, FHKAM (Medicine)1; L Li, MB, BS, FHKAM (Medicine)1; Kelvin KC Ng, MB, BS, PhD3; Raymond SY Tang, MD4; Stephen L Chan, MD, FHKAM (Medicine)1,5
1 Sir Yue-kong Pao Centre for Cancer, Department of Clinical Oncology, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China
2 Department of Medicine, United Christian Hospital, Hong Kong SAR, China
3 Department of Surgery, The Chinese University of Hong Kong, Hong Kong SAR, China
4 Institute of Digestive Disease, Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong SAR, China
5 State Key Laboratory of Translational Oncology, The Chinese University of Hong Kong, Hong Kong SAR, China
# Equal contribution
Corresponding author: Prof Stephen L Chan (chanlam_stephen@cuhk.edu.hk)
Abstract
Introduction: Patients with pancreatic cancer have
a high risk of thromboembolism (TE), which may
increase mortality. Most relevant studies have been
conducted in Western populations. We investigated
risk factors for TE in a predominantly Chinese
population of patients with pancreatic cancer, along
with effects of TE on overall survival.
Methods: This retrospective cohort study included
patients diagnosed with exocrine pancreatic cancer
in Prince of Wales Hospital in Hong Kong between
2010 and 2015. Data regarding patient demographics,
World Health Organization performance status,
stage, treatment, TE-related information, and time
of death (if applicable) were retrieved from electronic
medical records. Univariate and multivariable
logistic regression analyses were performed to
identify risk factors for TE. Survival analyses were
performed using Kaplan-Meier analysis and Cox
proportional hazards regression.
Results: In total, 365 patients were included in the
study. The overall incidence of TE (14.8%) was lower
than in Western populations. In univariate logistic
regression analysis, stage IV disease and non-head
pancreatic cancer were significantly associated with
TE (both P=0.01). Multivariable logistic regression
analysis showed that stage IV disease was a significant
risk factor (odds ratio=1.08, 95% confidence interval
[CI]=1.00-1.17; P=0.046). Median overall survival
did not significantly differ between patients with and
without TE (4.88 months vs 7.80 months, hazard
ratio=1.08, 95% CI=0.80-1.49; P=0.58) and between patients with TE who received anticoagulation
treatment or not (5.63 months vs 4.77 months,
hazard ratio=0.72, 95% CI=0.40-1.29; P=0.27).
Conclusion: The incidence of TE was low in our
Chinese cohort. Stage IV disease increased the risk
of TE. Overall survival was not affected by TE or its
treatment.
New knowledge added by this study
- The incidence of thromboembolic events in patients with pancreatic cancer was lower in our Chinese cohort than in previous studies involving Western populations.
- Stage IV disease was associated with a greater risk of thromboembolism.
- In patients with pancreatic cancer, overall survival was not affected by thromboembolism or its treatment.
- Differences in the incidence and treatment outcomes of thromboembolism between Western and Chinese populations of patients with pancreatic cancer are highlighted.
- Low-molecular-weight heparin and direct oral anticoagulants are valid options for the treatment of thromboembolism in patients with pancreatic cancer. Treatment decisions should include patient preference, bleeding risk, patient renal function, and life expectancy.
- Patients with poor general condition (eg, World Health Organization performance status score of 3 to 4) or life expectancy <3 months should not receive anticoagulation treatment for thromboembolism.
Introduction
The association between malignancy and
thromboembolism (TE) was first described more
than 100 years ago as ‘migratory thrombophlebitis’,
commonly found in patients with visceral cancer.1
Indeed, TE is a common complication in patients
with cancer and the second most common cause of
death among such patients.2
Although the association between TE and
pancreatic cancer is well established, its effects
on overall survival remain unclear. The results of
studies conducted in Western countries generally
support the notion that TE is associated with
worse overall survival.3 4 For example, a recent
large retrospective study in France demonstrated a
statistically significant decrease in overall survival of
2.9 months among patients with TE, compared with
patients who did not exhibit TE.3 In contrast, studies
involving Asian populations tend to show similar
overall survival in patients with and without TE.5 6 7
Furthermore, among the published retrospective
studies concerning the incidence of TE in Asian
patients with pancreatic cancer, very few data have
focused on the impact of TE in Chinese patients with
pancreatic cancer.
In this study, we aimed to investigate the incidence of TE among patients with pancreatic cancer in our centre, where >99% of patients are
Chinese; explore risk factors associated with the
development of TE; and assess the prognostic impact
of TE.
Methods
Design
This retrospective study included patients with a
histological diagnosis of exocrine pancreatic cancer
who were treated at the Department of Clinical
Oncology of Prince of Wales Hospital in Hong
Kong between 2010 and 2015; eligible patients were
identified by a review of electronic medical records.
If histological findings were unavailable because of
the clinician’s decision to omit biopsy evaluation,
patients were identified using clinical diagnoses
based on radiological findings and substantial
elevation of the level of serum marker carbohydrate
antigen 19-9 (CA 19-9) (ie, >500 IU/mL).
Patients were excluded if they had an atypical
clinical presentation (eg, normal CA 19-9 level) or
histological findings of non-exocrine pancreatic
malignancies, such as neuroendocrine tumour or
metastatic disease.
Study procedures
The following data were extracted from each
patient’s electronic and physical medical records:
(1) demographics (sex and age); (2) World Health
Organization (WHO) performance status score (0:
able to perform normal activities without restriction;
1: ambulatory and able to perform light work with
limitations on strenuous activities; 2: ambulatory
[>50% of waking hours] and capable of self-care but
unable to perform any work activities; 3: symptomatic
and in a chair or bed for >50% of the day but not
bedridden; 4: completely disabled [bedridden] and
unable to perform any self-care); (3) disease stage
(according to the seventh edition of the American
Joint Committee on Cancer tumour-node-metastasis
staging system); (4) site of disease (head, neck,
body, or tail); (5) CA 19-9 level at diagnosis; and
(6) initial treatment (surgery, chemoradiotherapy,
chemotherapy, or supportive care). Any occurrences
of TE (venous, arterial, or both) were recorded from
the time of diagnosis until death or last follow-up;
the site of thrombosis (lung, lower limb, multiple,
or other) and type of anticoagulation treatment
were also recorded. After data entry, all patient data
were verified by two authors (LL Chan and KY Lam)
under the supervision of the corresponding author
(SL Chan). Each patient’s survival status was last
updated on 31 October 2019.
Statistical analyses
Patient factors (eg, age, sex, WHO performance status, and initial treatment), tumour-related factors
(eg, histological diagnosis status, CA 19-9 level at
diagnosis, stage, and site) and TE-related factors
(eg, type and site) were summarised as numbers and
percentages for categorical variables, and as medians
and interquartile ranges for continuous variables.
The Wilcoxon rank-sum test and Chi squared test
were used to identify variables associated with
the development of TE. Variables that displayed
statistical significance in univariate analysis were
included in multivariable analysis. Age and sex were
included in multivariable analysis as adjustment
variables because they are known risk factors for
the development of TE in patients with cancer,
as well as standard clinical variables commonly
included in such analyses.8 9 10 Kaplan-Meier survival
analysis and Cox proportional hazards regression
analysis were performed to evaluate the relationship
between overall survival and TE. P values <0.05 were
considered statistically significant. All analyses were
performed with R version 3.5.1.11
Results
Study population
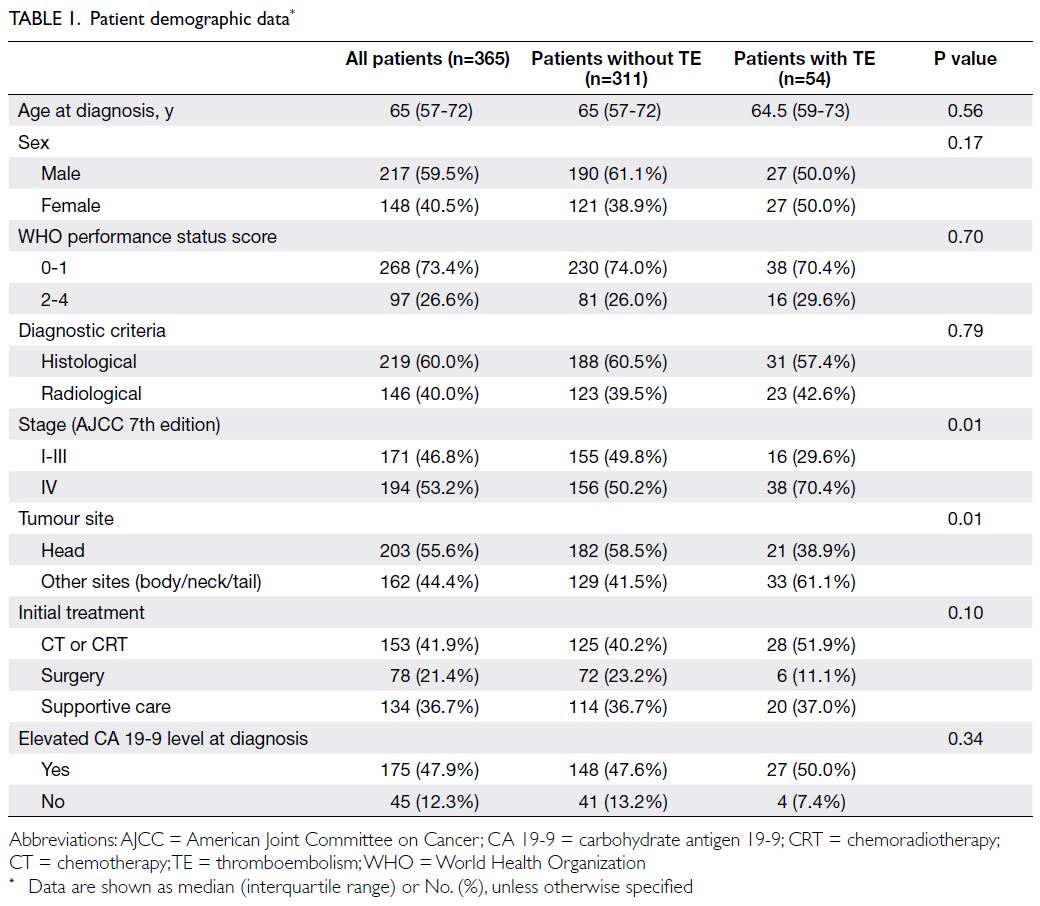
In total, 365 patients (217 [59.5%] men and 148 [40.5%] women; median age, 65 years [interquartile
range=57-72]) were included in the study; baseline
characteristics are summarised in Table 1. Of these
patients, 268 (73.4%) had WHO performance
status score of 0 to 1, whereas 97 (26.6%) scored
2 to 4. Furthermore, 219 patients (60.0%) had a
histologically confirmed diagnosis; the remaining
146 patients (40.0%) were diagnosed by radiological
and serological modalities. In terms of tumour
staging, 171 patients (46.8%) had stage I to III
disease; 194 patients (53.2%) had stage IV disease.
The tumour location was at the pancreatic head in
203 patients (55.6%) and other sites (neck, body,
or tail) in 162 patients (44.4%). Initial treatment
was surgery in 78 patients (21.4%), chemotherapy
or chemoradiotherapy in 153 patients (41.9%), and
supportive care in 134 patients (36.7%). Additional
details are provided in Table 1.
Risk of thromboembolism
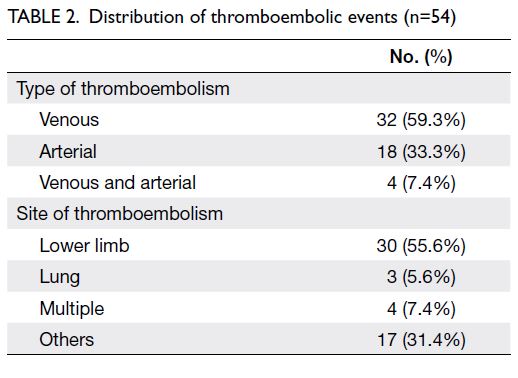
Among the 54 patients (14.8%) who developed TE,
32 (59.3%) had venous TE, 18 (33.3%) had arterial
TE, and four (7.4%) had both. Lower limbs were the most common sites of thrombosis, with 55.6% of all
thromboembolic events. Furthermore, three patients
(5.6%) had pulmonary embolism. These findings are
summarised in Table 2.
Predictors and prognosis of thromboembolism
In univariate analysis, non-head pancreatic cancer
(P=0.01) and stage IV disease (P=0.01) were
significantly associated with TE. Other factors such
as age at diagnosis, sex, WHO performance status,
elevated CA 19-9 level at diagnosis, and initial
treatment were not significantly associated with TE
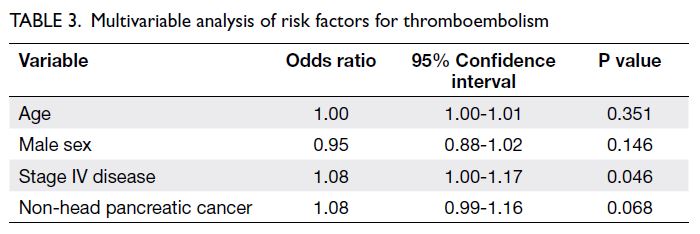
(Table 1). Multivariable analysis showed that stage IV
disease was a significant risk factor (odds ratio=1.08,
95% confidence interval [CI]=1.00-1.17; P=0.046)
[Table 3]. Median overall survival times in patients
with and without TE were 4.88 months and 7.80
months, respectively (Fig 1); the difference between
groups was not statistically significant (hazard ratio
=1.08, 95% CI=0.80-1.49; P=0.58). Among patients
with TE, median overall survival was not affected by
anticoagulation treatment (no anticoagulation=4.77
months vs anticoagulation=5.63 months, hazard
ratio=0.72, 95% CI=0.40-1.29; P=0.27) [Fig 2].
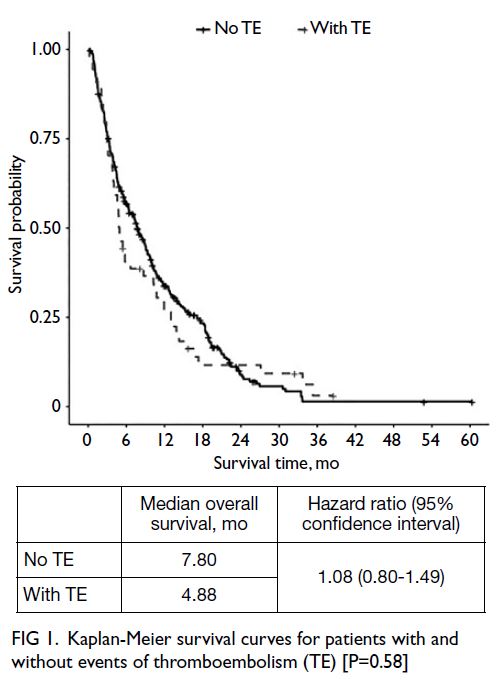
Fig 1. Kaplan-Meier survival curves for patients with and without events of thromboembolism (TE) [P=0.58]
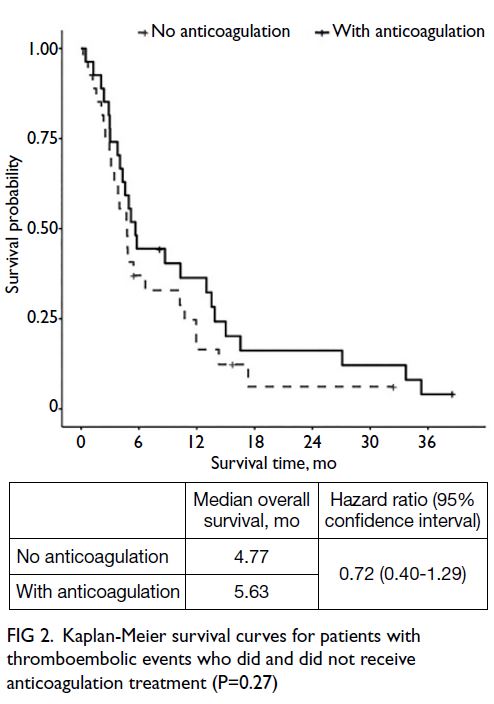
Fig 2. Kaplan-Meier survival curves for patients with thromboembolic events who did and did not receive anticoagulation treatment (P=0.27)
Discussion
In the present study, approximately 15% of patients
with pancreatic cancer developed TE. Lower limbs
were the most frequent sites of TE, and venous TE was the most common type. In univariate analysis,
both the site (non-head) and stage (IV) of disease
were significantly associated with TE; multivariable
analysis revealed that stage IV disease was a
significant risk factor for TE.
There is considerable evidence of an association
between pancreatic cancer and TE. In the first case
series describing the relationship between TE and
cancer, the incidence of TE was 60% in patients
with pancreatic cancer, whereas it was 15% to 30%
among patients with other malignancies.12 Several
pathological processes have been implicated
in this association.13 First, pancreatic cancer is
characterised by high expression levels of tissue
factor, which triggers the extrinsic coagulation
pathway leading to thrombin formation. Second,
the release of tumour-associated microvesicles
promotes hypercoagulability and activates platelet
aggregation. Third, the establishment of neutrophil
extracellular traps secondary to neutrophil activation
generates a matrix for platelet and tumour-associated
microvesicle adhesion, resulting in blood
clot formation.
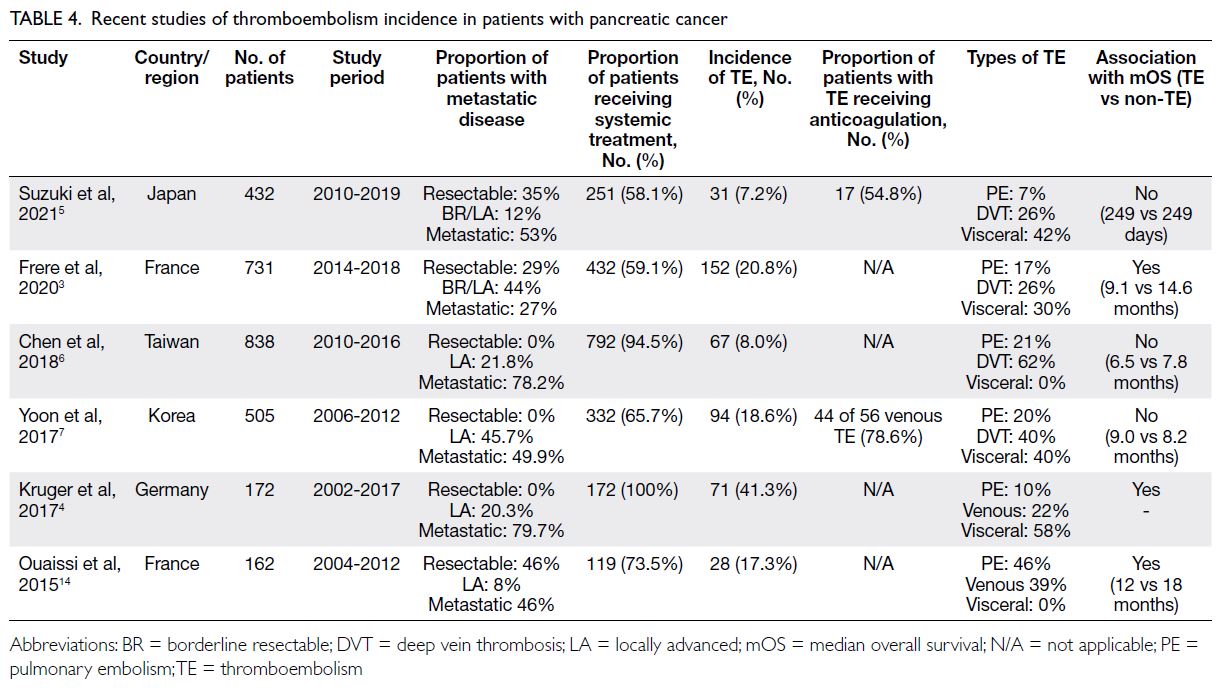
Thromboembolism incidence of around 15%
in our cohort is similar to that reported in other
studies of Asian populations5 6 7 but lower than that in
most Western populations (Table 4).3 4 14 The figures
ranged from 20% to 40% in Western populations and 8% to 18% in Asian populations. Consistent with
the findings in other studies of Asian populations,
we observed no difference in overall survival
between patients with and without TE. However,
the literature suggests that, in Western populations,
overall survival is affected by TE (Table 4).
Taken together, these findings support the
hypothesis that TE incidences and outcomes are
influenced by genetic and environmental differences
between Western and Asian populations. For
example, genetic variants in the clotting cascade
(eg, factor V Leiden and thrombin gene G20210A)
reportedly increase the risk of TE.15 These variants
are much more prevalent in Western populations
than in Asian populations.16 The resulting relative
hypercoagulability may be one of the main reasons
for the higher background incidence of TE in
Western populations than in Asian populations.17
Another factor that may contribute to the difference
in TE incidence between the two populations is
obesity, an established risk factor for TE that is more
common in Western populations.18
With respect to TE and pancreatic cancer
prognosis, survival appears to be inherently longer
in Western populations than in Asian populations
(Table 4). Considering the aggressive nature of
pancreatic cancer, it is possible that patients with
shorter survival (eg, patients in Asian populations) do not live long enough to benefit from treatment
of TE, whereas patients with longer survival (eg,
patients in Western populations) experience a
survival benefit from treatment of TE. Indeed, in a
recent systematic review regarding the treatment
outcomes of FOLFIRINOX and gemcitabine plus
nab-paclitaxel in patients with pancreatic cancer,
Lee et al19 showed that, compared with Asian
populations, Western populations experienced a
greater survival benefit from FOLFIRINOX (ie,
standard treatment for metastatic pancreatic cancer)
but a smaller survival benefit from gemcitabine
plus nab-paclitaxel (which was not available to our
patients during the present study). Therefore, a
reasonable assumption is that anticoagulation can
prolong survival in Western populations among
patients treated with FOLFIRINOX. Further studies
are needed to determine whether any subgroup of
Asian patients with pancreatic cancer can benefit
from the treatment of TE.
In univariate analysis, both non-head pancreatic
cancer and metastatic disease were associated with
the development of TE. However, in multivariable
analysis, the association with non-head pancreatic
cancer disappeared; metastatic disease was the sole
risk factor for TE. This is not surprising—non-head
pancreatic cancer is often detected at a late stage
because clinical symptoms (eg, biliary obstruction)
do not occur until the tumour becomes quite large.
Therefore, the association of TE with non-head
pancreatic cancer is mainly related to the advanced
stage of disease. This finding is also consistent with
the results of previous studies in which non-head
pancreatic cancer was frequently detected at a later
stage of disease.3 20
In the present study, we found that metastatic
disease was a risk factor for TE, which is consistent
with the results of previous studies.20 21 22 23 The
underlying pathophysiological mechanisms involve
multiple factors. For example, an advanced stage
of disease is often associated with a higher tumour
burden and bulky metastases, which can compress
blood vessels and inhibit blood flow. Higher tumour
burden can also affect WHO performance status,
resulting in decreased mobility and bedridden status.
During the present study, most of our patients
received low-molecular-weight heparin (LMWH)
as treatment for cancer-associated TE, based on
the results of the 2003 CLOT (Comparison of Low-molecular-weight heparin versus Oral anticoagulant
Therapy for the Prevention of Recurrent Venous
Thromboembolism in Patients with Cancer) trial
in which LMWH demonstrated superior efficacy in
preventing recurrent TE compared with coumarins
(eg, warfarin) while maintaining a similar risk of
bleeding.24 Recent studies have shown that direct
oral anticoagulants (DOACs) such as edoxaban25
and apixaban26 are non-inferior to LMWH as secondary prophylaxis for TE with similar safety
profiles. Accordingly, both LMWH and DOACs are
valid options for the treatment of TE in patients with
pancreatic cancer. This approach is consistent with
the latest National Comprehensive Cancer Network
2021 guidelines.27 Although LMWH and DOACs
demonstrate similar efficacy in preventing recurrent
TE, other factors to consider in drug selection
include baseline renal function, patient preference,
ease of administration, risk of bleeding (eg, by
tumour infiltration into the upper gastrointestinal
tract), and availability of antidotes that can reverse
anticoagulation.
Considering the overall poor prognosis of
pancreatic cancer and the lack of an overall survival
benefit associated with anticoagulation treatment
of TE, factors such as quality of life should be
considered when deciding whether to initiate
or discontinue anticoagulation treatment. It is
important to have clear discussions with patients
regarding the risks and benefits of anticoagulation,
particularly during the management of aggressive
malignancies such as pancreatic cancer, where
the life expectancy is often only months or weeks.
Anticoagulation treatment, such as LMWH, may
cause subcutaneous injection–related discomfort
and carries an increased risk of bleeding, but the
therapeutic effects of anticoagulation may relieve
symptoms of TE (eg, calf swelling and dyspnoea).
In a retrospective study of 128 patients with cancer-associated
venous TE, Napolitano et al28 analysed the
effects of anticoagulation on quality of life using the
EORTC-C30 questionnaire; they found that long-term
LMWH was not associated with worse quality
of life. However, patients approaching the end of life
often prefer to minimise their medication intake.29 In
our clinic, we tend not to administer anticoagulation
treatment if a patient’s life expectancy is <3 months
or whose WHO status score is 3 to 4. This approach
is consistent with the patient populations in recent
clinical trials comparing the efficacies of DOACs
and LMWH in the treatment of cancer-associated
TE; patients with poor WHO performance status
and short life expectancy were excluded from those
trials.25 26
Limitations
This study had a few limitations. First, its
retrospective nature may have permitted bias related
to missing data and the possibility of asymptomatic
TE. However, TE tends to be symptomatic in
patients with cancer; thus, it is unlikely that events
were missed. Additionally, analyses of symptomatic
TE are more relevant to real-world clinical practice.
Second, the overall survival time of patients in the
present study was worse than the survival times
reported in randomised clinical trials of patients with
metastatic pancreatic cancer.30 31 This discrepancy may have occurred because our study cohort was
representative of real-world patients who more
frequently have reduced liver function and worse
WHO performance status. It may also be related
to the absence of more effective chemotherapy (eg,
nab-paclitaxel) during the study period.
Conclusion
In conclusion, this study demonstrated that the
incidence of TE was around 15% in Chinese patients
with pancreatic cancer. Notably, the presence of TE
was not associated with worse overall survival, and
metastatic disease increased the risk of TE.
Author contributions
Concept or design: LL Chan, KY Lam, SL Chan.
Acquisition of data: All authors.
Analysis or interpretation of data: LL Chan, DCM Lam, KY Lam, SL Chan.
Drafting of the manuscript: LL Chan, SL Chan.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: LL Chan, DCM Lam, KY Lam, SL Chan.
Drafting of the manuscript: LL Chan, SL Chan.
Critical revision of the manuscript for important intellectual content: All authors.
Conflicts of interest
The authors have no conflicts of interest to disclose.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study protocol was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (Ref No.: 2016.730).
Informed patient consent was waived by the Committee due
to the retrospective nature of the research.
References
1. Metharom P, Falasca M, Berndt MC. The history of
Armand Trousseau and cancer-associated thrombosis.
Cancers (Basel) 2019;11:158. Crossref
2. Khorana AA. Cancer and thrombosis: implications of
published guidelines for clinical practice. Ann Oncol
2009;20:1619-30. Crossref
3. Frere C, Bournet B, Gourgou S, et al. Incidence of venous
thromboembolism in patients with newly diagnosed
pancreatic cancer and factors associated with outcomes.
Gastroenterology 2020;158:1346-58.e4. Crossref
4. Kruger S, Haas M, Burkl C, et al. Incidence, outcome and
risk stratification tools for venous thromboembolism in
advanced pancreatic cancer—a retrospective cohort study.
Thromb Res 2017;157:9-15. Crossref
5. Suzuki T, Hori R, Takeuchi K, et al. Venous thromboembolism
in Japanese patients with pancreatic cancer. Clin Appl
Thromb Hemost 2021;27:10760296211051766. Crossref
6. Chen JS, Hung CY, Chang H, et al. Venous
thromboembolism in Asian patients with pancreatic
cancer following palliative chemotherapy: low incidence
but a negative prognosticator for those with early onset. Cancers (Basel) 2018;10:501. Crossref
7. Yoon SY, Lee MY, Yun J, et al. The incidence of venous
thromboembolism is not low in Korean patients with
advanced pancreatic cancer [corrected]. Blood Res
2018;53:227-32. Crossref
8. Khorana AA, Francis CW, Culakova E, Kuderer NM,
Lyman GH. Frequency, risk factors, and trends for venous
thromboembolism among hospitalized cancer patients.
Cancer 2007;110:2339-46. Crossref
9. Eichinger S. Cancer associated thrombosis: risk factors and
outcomes. Thromb Res 2016;140 Suppl 1:S12-7. Crossref
10. Abdol Razak NB, Jones G, Bhandari M, Berndt MC,
Metharom P. Cancer-associated thrombosis: an overview
of mechanisms, risk factors, and treatment. Cancers
(Basel) 2018;10:380. Crossref
11. R Core Team. R: A Language and Environment for Statistical
Computing. R Foundation for Statistical Computing; 2018.
12. Sproul EE. Carcinoma and venous thrombosis: the
frequency of association of carcinoma in the body or tail
of the pancreas with multiple venous thrombosis. Am J
Cancer. 1938;34:566-85.
13. Campello E, Ilich A, Simioni P, Key NS. The relationship
between pancreatic cancer and hypercoagulability: a
comprehensive review on epidemiological and biological
issues. Br J Cancer 2019;121:359-71. Crossref
14. Ouaissi M, Frasconi C, Mege D, et al. Impact of venous
thromboembolism on the natural history of pancreatic
adenocarcinoma. Hepatobiliary Pancreat Dis Int
2015;14:436-42. Crossref
15. Blom JW, Doggen CJ, Osanto S, Rosendaal FR.
Malignancies, prothrombotic mutations, and the risk of
venous thrombosis. JAMA 2005;293:715-22. Crossref
16. Jun ZJ, Ping T, Lei Y, Li L, Ming SY, Jing W. Prevalence
of factor V Leiden and prothrombin G20210A mutations
in Chinese patients with deep venous thrombosis and
pulmonary embolism. Clin Lab Haematol 2006;28:111-6. Crossref
17. Wang KL, Yap ES, Goto S, Zhang S, Siu CW, Chiang CE.
The diagnosis and treatment of venous thromboembolism
in Asian patients. Thromb J 2018;16:4. Crossref
18. Yang G, De Staercke C, Hooper WC. The effects of obesity
on venous thromboembolism: a review. Open J Prev Med
2012;2:499-509. Crossref
19. Lee YS, Lee JC, Kim JH, Kim J, Hwang JH. Pharmacoethnicity
of FOLFIRINOX versus gemcitabine plus nab-paclitaxel
in metastatic pancreatic cancer: a systematic review and
meta-analysis. Sci Rep 2021;11:20152. Crossref
20. Lee JC, Ro YS, Cho J, et al. Characteristics of venous
thromboembolism in pancreatic adenocarcinoma in East
Asian ethnics: a large population-based observational
study. Medicine (Baltimore) 2016;95:e3472. Crossref
21. Dickmann B, Ahlbrecht J, Ay C, et al. Regional lymph
node metastases are a strong risk factor for venous
thromboembolism: results from the Vienna Cancer and
Thrombosis Study. Haematologica 2013;98:1309-14. Crossref
22. Khorana AA, Kuderer NM, Culakova E, Lyman GH,
Francis CW. Development and validation of a predictive
model for chemotherapy-associated thrombosis. Blood
2008;111:4902-7. Crossref
23. Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al.
Hospitalisation for venous thromboembolism in cancer
patients and the general population: a population-based
cohort study in Denmark, 1997-2006. Br J Cancer 2010;103:947-53. Crossref
24. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight
heparin versus a coumarin for the prevention of recurrent
venous thromboembolism in patients with cancer. N Engl J
Med 2003;349:146-53. Crossref
25. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the
treatment of cancer-associated venous thromboembolism.
N Engl J Med 2018;378:615-24. Crossref
26. Agnelli G, Becattini C, Meyer G, et al. Apixaban for the
treatment of venous thromboembolism associated with
cancer. N Engl J Med 2020;382:1599-607. Crossref
27. Streiff MB, Holmstrom B, Angelini D, et al. Cancer-associated
venous thromboembolic disease, version
2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:1181-201. Crossref
28. Napolitano M, Mansueto MF, Raso S, Siragusa S. Quality of
life in patients with cancer under prolonged anticoagulation
for high-risk deep vein thrombosis: a long-term follow-up.
Clin Appl Thromb Hemost 2020;26:1076029620918290. Crossref
29. Huisman BA, Geijteman EC, Arevalo JJ, et al. Use of
antithrombotics at the end of life: an in-depth chart review
study. BMC Palliat Care 2021;20:110. Crossref
30. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in
pancreatic cancer with nab-paclitaxel plus gemcitabine. N
Engl J Med 2013;369:1691-703. Crossref
31. Vaccaro V, Sperduti I, Milella M. FOLFIRINOX versus
gemcitabine for metastatic pancreatic cancer. N Engl J Med
2011;365:768-9; author reply 769. Crossref