Hong Kong Med J 2023 Jun;29(3):198–207 | Epub 6 Apr 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Preoperative considerations and benefits of neoadjuvant chemotherapy: insights from a 12-year review of the Hong Kong Breast Cancer Registry
Yolanda HY Chan, MB, BS, FHKAM (Surgery)1; Carol CH Kwok, MB, ChB, FHKAM (Radiology)2; Desiree MS Tse, MPH, BA3; HM Lee, MPhil, BSc3; PY Tam, MMedSc, BSc3; Polly SY Cheung, MB, BS, FHKAM (Surgery)3
1 Department of Surgery, Kwong Wah Hospital, Hong Kong SAR, China
2 Department of Oncology, Princess Margaret Hospital, Hong Kong SAR, China
3 Breast Cancer Research Centre, Hong Kong Breast Cancer Foundation, Hong Kong SAR, China
Corresponding author: Dr Polly SY Cheung (pollycheung@hkbcf.org)
Abstract
Introduction: Neoadjuvant chemotherapy (NAC)
was initially used for locally advanced or inoperable
breast cancers. Its extension to early disease has
facilitated breast-conserving surgery (BCS). This
study investigated the use of NAC in patients
registered with the Hong Kong Breast Cancer
Registry (HKBCR); it also assessed NAC effectiveness
according to rates of pathological complete response
(pCR) and BCS.
Methods: Records were retrieved from the HKBCR
regarding 13 435 women who had been diagnosed
with invasive breast cancer during the period of 2006
to 2017, including 1084 patients who received NAC.
Results: The proportion of patients treated with
NAC nearly doubled from 5.6% in 2006-2011 to 10.3%
in 2012-2017. The increase was most pronounced
among patients with stage II or III disease. In terms
of biological subtype, substantial increases in the
receipt of NAC were evident among patients with
triple-negative and human epidermal growth factor
receptor 2 (HER2)–positive (non-luminal) tumours.
The best rates of pCR were observed in patients
with HER2-positive (non-luminal) [46.0%] tumours,
followed by patients with luminal B (HER2-positive)
[29.4%] and triple-negative (29.3%) tumours. After
NAC, the rate of BCS was 53.9% in patients with clinical stage IIA disease, compared with 38.2% in
patients with pathological stage IIA disease who did
not receive NAC.
Conclusion: The use of NAC in Hong Kong increased
from 2006 to 2017. The findings regarding rates
of pCR and BCS indicate that NAC is an effective
treatment; it should be considered in patients with
stage ≥II disease, as well as patients with HER2-positive (non-luminal) or triple-negative breast cancers.
New knowledge added by this study
- The use of neoadjuvant chemotherapy (NAC) in Hong Kong increased from 2006 to 2017.
- Higher pathological complete response rates were detected in patients with human epidermal growth factor receptor 2–positive (non-luminal) and triple-negative tumours.
- After treatment with NAC, greater proportions of patients with clinical stage IIA or IIB disease underwent breast-conserving surgery.
- Alterations in breast cancer biomarkers after NAC suggest that reassessments of residual tumour would provide useful guidance regarding further adjuvant therapy.
- Under the care of a multidisciplinary team, patients with early breast cancer who have an appropriate indication should consider receiving NAC before surgery.
Introduction
Neoadjuvant chemotherapy (NAC)—chemotherapy
delivered before definitive breast cancer surgery—was first described in the late 1970s as treatment for
locally advanced (often inoperable) breast cancers;
it was intended to reduce tumour size and facilitate surgery.1 Subsequently, the use of NAC has been
extended to early operable breast cancers.2 3 4 5 This
approach offers the advantages of down-staging the
disease, potentially reducing the extent of surgery,
and allowing breast-conserving surgery (BCS); in the
current era of individualised treatment, it supports
evaluations of therapeutic efficacy.2 6
There is evidence that NAC is equivalent
to adjuvant chemotherapy in terms of preventing
breast cancer recurrence.6 It demonstrated equal
effectiveness in terms of disease-free survival and
overall survival in the National Surgical Adjuvant
Breast and Bowel Project B-18 trial.7 Furthermore,
a recent meta-analysis by the Early Breast
Cancer Trialists’ Collaborative Group showed no
significant differences between NAC and adjuvant
chemotherapy for distant recurrence, breast cancer
mortality, or death from any cause.8
Here, we hypothesised that the use of NAC
would change over time among patients with breast
cancer in Hong Kong, considering its increasing
acceptance as a treatment approach. Thus, the
objectives of this study were to investigate the use
of NAC over time in patients registered with the
Hong Kong Breast Cancer Registry (HKBCR), and
to assess the effectiveness of NAC among patients
with breast cancer in Hong Kong according to
rates of pathological complete response (pCR) and
BCS. This study also evaluated alterations in breast
cancer biomarkers, including oestrogen receptor
(ER), progesterone receptor (PR), human epidermal
growth factor receptor 2 (HER2), and Ki-67
proliferation index.
Methods
Records were retrieved from the HKBCR regarding
Hong Kong Chinese female patients who were diagnosed with invasive breast cancer in the
period of 2006 to 2017. Patients were excluded for
the following reasons: stage 0 or stage IV disease,
missing or unknown information regarding surgery,
and concurrent neoadjuvant endocrine treatment
or NAC received outside Hong Kong (which may
involve different clinical considerations).
Breast cancer was categorised into four
biological subtypes based on clinicopathological
criteria, in accordance with recommendations by the
St Gallen 2013 Consensus Guideline.9 A cut-off of
<14% reportedly has the strongest correlation with
the gene-expression definition of the luminal A-like
subtype; a cut-off of ≥14% is generally regarded as
the threshold for a high Ki-67 proliferation index.
Histological grade 3 was used as a surrogate indicator
of the luminal B-like subtype if Ki-67 information was
unavailable.10 Pathological complete response was
defined as no histological evidence of malignancies
(ypT0) or the presence of only in-situ residuals in
breast tissue (ypTis) and complete disappearance
of lymph node metastasis (ypN0) after surgery.11
The same definitions have been adopted by the MD
Anderson Cancer Center,12 as well as the Austrian
Breast & Colorectal Cancer Study Group.13
Ethics approval for this study has been
obtained from six relevant approving bodies. Written
informed consent for data collection was obtained
during patient recruitment into the HKBCR,
who were from 20 hospitals and 37 clinics (online supplementary Appendix). Patient demographics,
pre-chemotherapy and post-chemotherapy disease
staging, tumour characteristics, and prescribed
chemotherapeutic agents were evaluated. The
effectiveness of neoadjuvant chemotherapy was
assessed in terms of the rates of pCR and BCS.
Baseline tumour characteristics were analysed,
including size, nodal stage, histological grade, Ki-67
level, hormone receptor status, and HER2 status.
Descriptive statistics were used to summarise
demographic and clinical characteristics of patients.
Continuous variables are shown as mean, standard
deviation, and range; categorical variables are
reported as frequency and percentage. Means were
compared between groups using independent
samples t tests. The Pearson Chi squared test was
used to evaluate differences in pCR according to
biological subtype and surgical approach. Data were
analysed using SPSS (Windows version 22.0; IBM
Corp, Armonk [NY], United States). All P values
were derived from two-sided statistical tests, and P
values <0.05 were considered statistically significant.
Results
Patient selection
In total, 13 990 patients with invasive breast cancer were initially screened for inclusion. After the exclusion of 555 patients, 13 435 patients (13 625
breast cancer cases) were included in this study
(Fig 1). The NAC group comprised 1084 patients
(1097 breast cancer cases) and the non-NAC group
comprised 12 351 patients (12 528 breast cancer
cases).
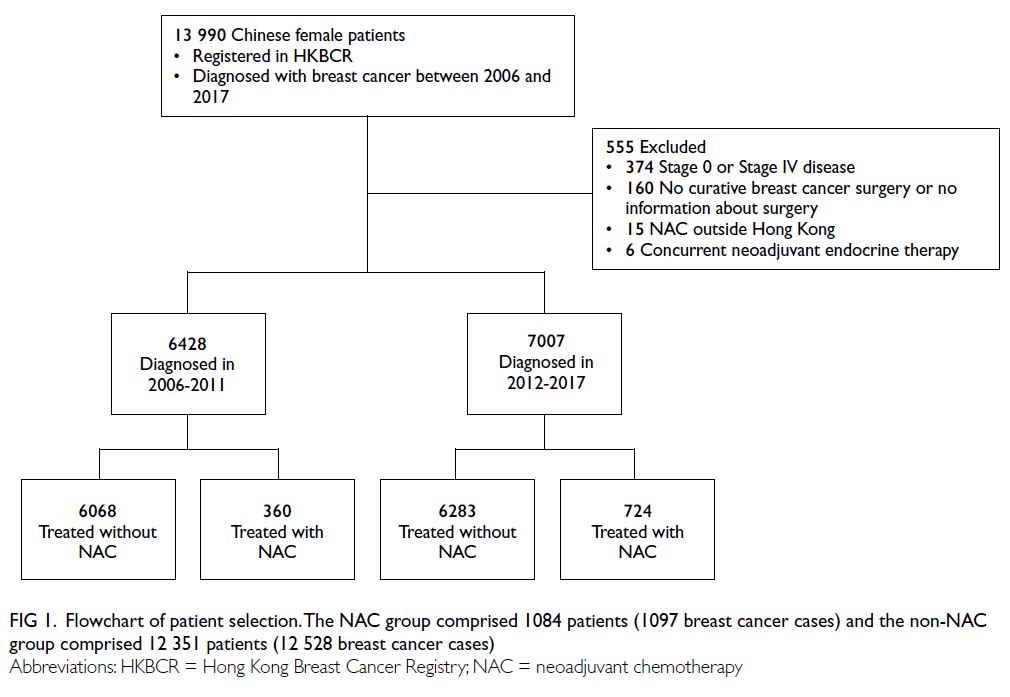
Figure 1. Flowchart of patient selection. The NAC group comprised 1084 patients (1097 breast cancer cases) and the non-NAC group comprised 12 351 patients (12 528 breast cancer cases)
Characteristics of patients who received
neoadjuvant chemotherapy
In the NAC group, the median age was 49.7 years
(interquartile range, 43.5-56.7; range, 21.9-81.6), and
half of the patients (53.8%) were premenopausal.
The median invasive clinical tumour size was
4.0 cm (range, 0.55-20.0). The patients’ clinical
characteristics (eg, age, biological subtype, clinical
tumour stage, nodal stage, and cancer stage) are
shown in Table 1.
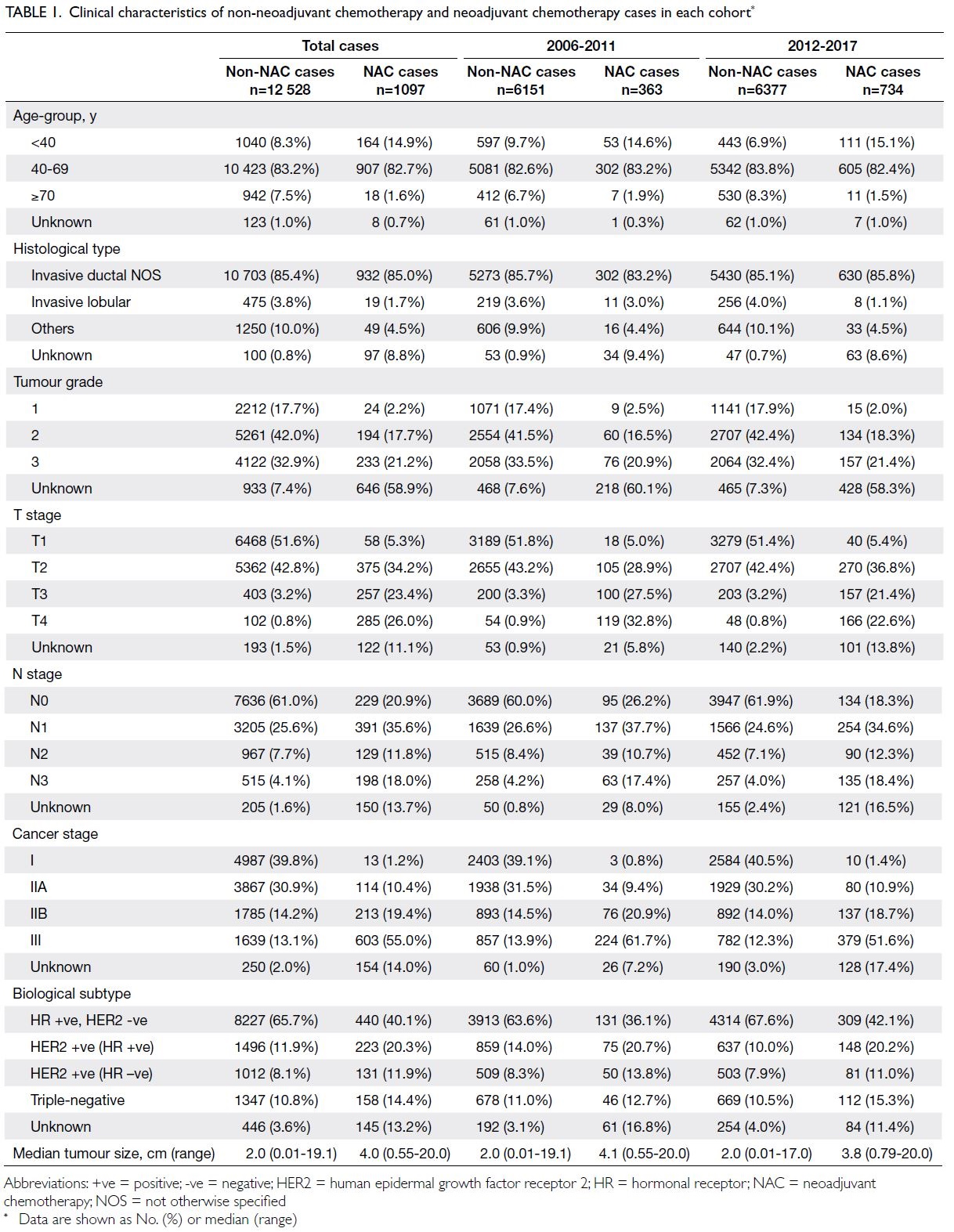
Table 1. Clinical characteristics of non-neoadjuvant chemotherapy and neoadjuvant chemotherapy cases in each cohort
Among the 13 625 breast cancer cases, 13.6%
of affected patients aged <40 years were treated
with NAC, compared with 8.0% and 1.9% of affected
patients aged 40-69 years and ≥70 years, respectively
(Table 1). The administration of NAC was positively
associated with cancer stage at diagnosis: the
proportion increased from 0.3% in patients with
stage I disease to 26.9% among patients with stage III
disease (Table 1). Furthermore, greater proportions
of patients with luminal B (HER2-positive), HER2-positive (non-luminal), or triple-negative subtypes
of breast cancer received NAC.
Use of neoadjuvant chemotherapy in two
temporal cohorts
For the assessment of changes in NAC adoption,
the 13 435 patients were divided into two groups
according to the year of diagnosis: periods of 2006-2011 and 2012-2017. The proportion of patients
treated with NAC nearly doubled from 5.6% in 2006-2011 to 10.3% in 2012-2017 (Table 1).
Further analysis indicated that the use of NAC
was significantly increased in patients with stages II
and III breast cancers, but not in patients with stage I
breast cancer. It was most pronounced among patients
with stages IIB (7.8% in 2006-2011 vs. 13.3% in 2012-2017) and III (20.7% vs. 32.6%) disease. An increase
in the use of NAC was also observed in patients with
all biological subtypes of breast cancer. In particular,
substantial increases were observed among patients
with triple-negative (6.4% vs. 14.3%), HER2-positive
(non-luminal) [8.9% vs. 13.9%], and luminal B (HER2-positive) [8.0% vs. 18.9%] tumours (Table 1).
Regimens of neoadjuvant chemotherapy
Among the 1084 patients who received NAC,
353 were diagnosed with HER2-positive (non-luminal)
cancer. Anti-HER2 agents were added
to chemotherapy in 73.7% of these patients, and
the proportions increased from 57.6% in 2006-2011 to 82.5% in 2012-2017; taxane-carboplatin-trastuzumab
was the most frequently used regimen. In contrast, for patients with HER2-negative tumours
or unknown HER2 status, NAC regimens most
commonly consisted of anthracyclines (doxorubicin
or epirubicin), administered in combination or
sequentially with taxanes (paclitaxel or docetaxel).
Responses to neoadjuvant chemotherapy
Rates of pathological complete response
Two hundred and twenty-one (20.1%) of 1097 breast
cancer cases treated with NAC achieved pCR in
the breast and axillary lymph nodes. Subsequent
analysis according to biological subtype revealed
that outcomes were optimal in patients with HER2-positive (ER-negative and PR-negative) tumours,
among which nearly half (46.0%) achieved pCR.
Pathological complete response rates in luminal
B (HER2-positive) and triple-negative subtypes
were 29.4% and 29.3%, respectively; these were
significantly higher than the rates in other hormone-positive
subtypes (all P<0.05; Fig 2).
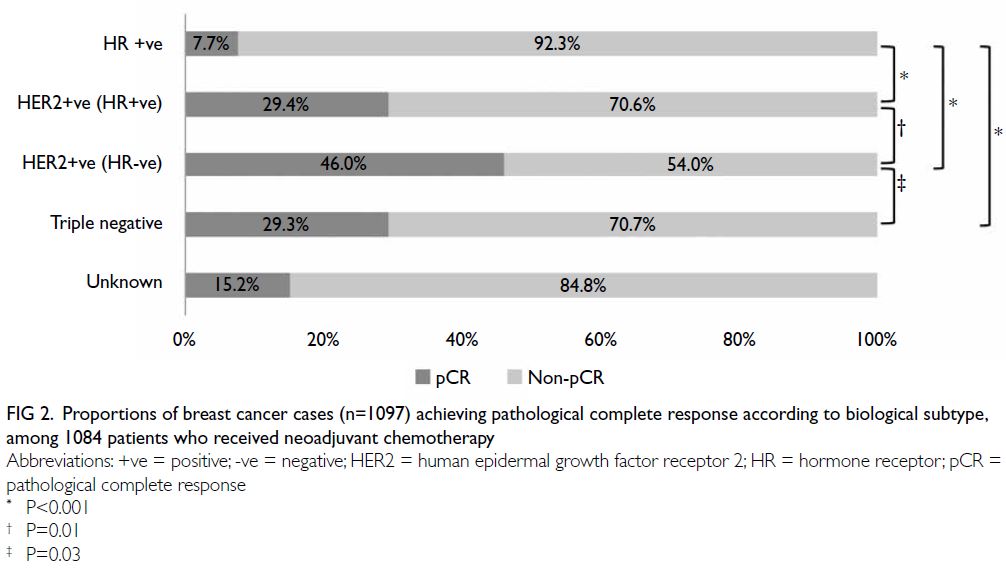
Figure 2. Proportions of breast cancer cases (n=1097) achieving pathological complete response according to biological subtype, among 1084 patients who received neoadjuvant chemotherapy
Factors significantly associated with pCR
included ER/PR negativity and HER2 positivity.
Within the HER2-positive population, pCR was more
common for hormone receptor–negative tumours
than for hormone receptor–positive tumours; it
was also more common in patients who received
trastuzumab. Other factors (eg, age, menopausal
status, clinical tumour and nodal stages, ER status,
and Ki-67 proliferation index) did not appear to
influence the achievement of pCR.
Rates of breast-conserving surgery
Figure 3 shows the proportions of patients treated
with NAC who subsequently underwent different types of breast surgery, categorised according to
clinical cancer stages. Patients with clinical stage IIA
disease were most likely to switch from mastectomy
to BCS after NAC; 53.9% underwent BCS after
NAC, compared with 38.2% of patients with stage
IIA disease who did not receive NAC. The second
highest proportion was observed among patients
with clinical stage IIB disease, 38.3% of whom
underwent BCS after NAC. Even among patients
with clinical stage III disease, 14.1% underwent BCS
after NAC. Significant differences in the rate of BCS
were also observed between the NAC and non-NAC
groups in patients with stages IIA (P=0.02) and IIB
(P=0.031) disease.
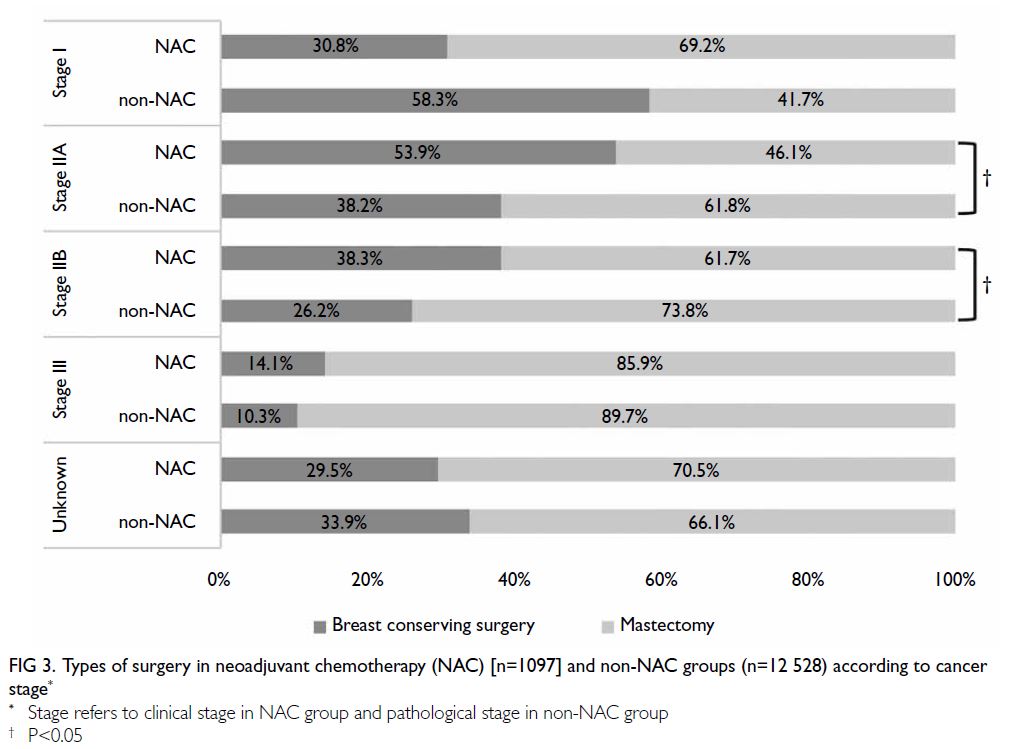
Figure 3. Types of surgery in neoadjuvant chemotherapy (NAC) [n=1097] and non-NAC groups (n=12 528) according to cancer stage
Alterations in breast cancer biomarkers
Biomarkers were compared between diagnostic core
biopsies and final surgical specimens. Excluding
the 221 patients who achieved pCR after NAC,
844 breast specimens with residual tumours were
evaluated after final surgery. Patients without data
regarding biomarkers in either pre-chemotherapy or
post-chemotherapy or both were excluded from this
analysis. Alterations in ER, PR, and HER2 statuses
after NAC are shown in Table 2. Most patients had
no change in their ER status, but 7.6% switched from
positive to negative or from negative to positive.
With respect to PR status, a shift occurred in 17.4%
of patients, and a shift in HER2 status was detected
in 10.9% of patients. More than one-fifth (21.3%)
of patients with residual tumours had a change
in at least one receptor status after NAC. Ki-67
proliferation index was also evaluated; among the
297 cases assessed, 131 (44.1%) showed alterations
after NAC.
Discussion
Use of neoadjuvant chemotherapy
During the early phase of the study period, a
multidisciplinary approach was not widely used for
breast cancer management; thus, most treatment
decisions were based on the discretion of the
attending surgeon or oncologist. Nevertheless, locally
advanced diseases and hormonal receptor–negative
tumours were generally the targets of NAC. Over
time, NAC has been increasingly accepted, as shown
in updates of various national and international guidelines (eg, National Comprehensive Cancer
Network guidelines14 and European Society of
Medical Oncology guidelines15). This inclination
clearly contributed to the substantial increase in
NAC use during the periods analysed in this study:
from 5.6% in 2006-2011 to 10.3% in 2012-2017.
The increased use of NAC was mainly attributed
to advancements in translational research, along
with new evidence from clinical trials that have led
to a better understanding of breast cancer biology
and the establishment of tumour biology–based
targeted treatments.16 After the expansion of its use
in adjuvant therapy, trastuzumab was first registered
for use as neoadjuvant therapy for breast cancer in
2006 under the Department of Health in Hong Kong.
Its entry into the Hospital Authority Drug Formulary
soon followed, and it was included in the safety net
enlistment by 2009. This timeframe suggests that
the drug has become accessible to a much broader
spectrum of patients under the care of public sector
hospitals in Hong Kong; it is also compatible with
the considerable increase in use of trastuzumab over
time. In our dataset, among patients with HER2-positive (non-luminal) tumours, the proportion of
patients using anti-HER2 regimens in neoadjuvant
therapy increased from 57.6% in 2006-2011 to 82.5%
in 2012-2017.
Pathological complete response
Neoadjuvant trials allow rapid assessment of drug
efficacy; they can accelerate the development and
approval of treatments for early breast cancer.
Pathological complete response has been proposed
as a surrogate endpoint for predictions of long-term
clinical benefit.17 Although it is difficult to compare
outcomes among trials and individual series because
of heterogeneity in terms of study design and patient
populations, the results of some meta-analyses have
suggested that the achievement of pCR after NAC is
a predictor of overall survival, disease-free survival,
and relapse-free survival.18
Our results are consistent with findings by
von Minckwitz et al11 and the Collaborative Trials
in Neoadjuvant Breast Cancer (CTNeoBC) meta-analysis,17 which concluded that frequency of
pCR was low in patients with low-grade, hormone
receptor–positive tumours, whereas it was much
higher among patients with more aggressive subtypes
(ie, triple-negative and HER2-positive [non-luminal]
tumours). Overall, these data suggest that the
underlying molecular subtypes influence the rates
of pathological responses. Further improvements
in the rate of pCR have been observed in cases of
HER2-positive (non-luminal) tumours treated with
dual anti-HER2 targeted agents, as well as cases of
triple-negative breast cancer treated with platinum
and immunotherapy. Moreover, trials have also
been done or in progress to evaluate the need for
additional chemotherapy in selected patients with
residual disease after NAC; the results of those trials
are expected to provide further insights regarding
treatments for further improving survival outcomes
in neoadjuvant setting.18 19 20
Standard prognostic indicators, such as
tumour size at the time of surgical resection or the
number of involved lymph nodes, are no longer
applicable in the neoadjuvant setting; systemic
therapy often down-stages the disease and may lead
to eradication. There is increasing evidence that the
tumour response to NAC can facilitate prognostic
predictions. In the multidisciplinary management
of breast cancer, the identification of prognostic
variables for patients receiving NAC can help to
determine whether additional therapy is warranted.
Given the strong support for an association between
prognosis and clinicopathological features in the
neoadjuvant setting, clinicians may be able to avoid
additional interventions after surgery (e.g., additional
chemotherapy) in patients who are otherwise
considered high risk at initial presentation since pCR
has been achieved. This is because although HER2-positive and triple negative breast cancers carry
poor prognosis, these tumours have higher pCR
rates after NAC, and pCR in HER2-positive (non-luminal)
and triple-negative tumours was associated
with excellent prognosis.11 17 21
Breast-conserving surgery
Quality of life–focused research has shown that
body image scores are significantly better among
patients who undergo BCS than among patients
who undergo mastectomy. Patients who undergo
BCS are less worried about their appearance, have
more freedom in their choice of clothing, feel less
upset about changes in their bodies, and feel more
accepted by their partners.22 These findings reinforce
the benefits of NAC for breast cancer in terms of
down-staging the disease, increasing resectability,
and enhancing BCS eligibility among patients who
would otherwise require mastectomy. Furthermore,
a systematic review of NAC for operable breast
cancer revealed that the mastectomy rate was lower
among patients who received NAC than among
patients who underwent surgery prior to adjuvant
chemotherapy (relative risk=0.71; 95% confidence
interval [CI]=0.67-0.75); the use of NAC did not
hinder local control (hazard ratio=1.12; 95% CI=0.92-1.37).23 Long-term follow-up analyses also showed
that preoperative chemotherapy increased rates of
BCS without increasing the rates of locoregional
recurrence.24 25 In a previous study in Hong Kong,
univariate analysis revealed that patients who
achieved pCR after NAC had a higher likelihood of
successful BCS (P=0.028). Pre-chemotherapy disease
staging (P=0.001) and tumour size (P=0.005) were
also important factors that influenced successful
conversion to BCS.5
However, a recent meta-analysis by the Early
Breast Cancer Trialists’ Collaborative Group showed
that, compared with adjuvant chemotherapy, NAC
was associated with more frequent local recurrence;
the 15-year rates of local recurrence were 21.4% for
NAC and 15.9% for adjuvant chemotherapy (rate
ratio=1.37; 95% CI=1.17-1.61; P=0.0001).8 Thus,
continued follow-up of patients registered in the
HKBCR and updates will provide important insights
with respect to NAC on long-term outcomes.
Alterations in breast cancer biomarkers
Neoadjuvant chemotherapy can cause changes in
ER, PR, and HER2 statuses, as well as the Ki-67
level, in patients with invasive breast cancer.26 27
A possible explanation for this phenomenon is
that chemosensitive cancer cells are destroyed
by chemotherapy, whereas chemoresistant cells
survive; such a change could alter the receptor
status. Furthermore, because ER, PR, and HER2
are highly interdependent, a change in one receptor
could lead to changes in the other receptors.28
A systematic review showed that the rates of ER
and/or PR discordance range from 2.5% to 51.7%;
among patients who received NAC combined with
trastuzumab, up to 43% exhibited a switch to HER2
negativity.29
Thus far, there are only limited data regarding
the prognostic value of changes in biomarkers after
NAC among patients with breast cancer.28 Several
groups have reported that a switch from negative to
positive status (for ER, PR, or HER2) is associated with
better overall survival.30 31 Additionally, outcomes
are better among patients with stable hormone
receptor status profiles than among patients with
altered profiles.32 Notably, Guarneri et al33 reported
that patients with loss of HER2 overexpression
tended to have a greater risk of relapse, compared
with patients who remained HER2-positive; in
contrast, a decrease in Ki-67 expression after NAC
was reportedly associated with better outcomes.34
Because of the above observations, biomarkers
and Ki-67 levels should be retested after NAC. Such
retesting is particularly important for tumours
that were ER/PR-negative and/or HER2-negative
before treatment because a shift to a positive status
would indicate a need for endocrine therapy and/or trastuzumab. The results of these changes may
influence clinical decisions regarding subsequent
treatment and help to identify patients with better
outcomes after NAC.28 35
Limitations
This study had several limitations. First, it was
a retrospective analysis and the earliest records
in the database were incomplete; the missing
information particularly affected breast cancer
biomarkers, and Ki-67 was not routinely tested in
Hong Kong public hospitals. Second, selection bias
may have been present because the receipt of NAC
was largely dependent on surgeon assessment and
patient preference. In recent years, the potential for
such bias has decreased because multidisciplinary
management of breast cancer is gradually
becoming the preferred approach. Considering the
complexities of treatment planning, monitoring,
and evaluation, decisions regarding preoperative
systemic therapy require input from surgeons,
oncologists, radiologists, and pathologists. Of note,
the comparison of rates of surgery types between
NAC and non-NAC groups can only be regarded as
approximation, as assignment of patients into these
two groups is not randomised; furthermore, clinical
stages may differ from pathological stages, thus they
may not be comparable.
Conclusion
Changes in the clinical management of breast cancer led to increased use of NAC in Hong Kong during the
period of 2006 to 2017. Neoadjuvant chemotherapy
was effective in tumour down-staging; one-fifth of
patients subsequently achieved pCR in the breast
and axillary lymph nodes. In particular, higher
rates of pCR were detected in HER2-positive (non-luminal) and triple-negative subtypes. After NAC,
greater proportions of patients with clinical stage
IIA or IIB disease underwent BCS. Currently, post-NAC adjustments to treatment are based on whether
pCR has been achieved. In the future, alterations in
breast cancer biomarkers after NAC may provide
useful guidance regarding further adjuvant therapy.
The indications for NAC have expanded from the
treatment of locally advanced breast cancers (to
facilitate surgery) to the down-staging of early
disease, thereby facilitating BCS. Under the care of
a multidisciplinary team, patients with early breast
cancer who have an appropriate indication should
consider receiving NAC before surgery. Further
studies are warranted to evaluate the benefits of
individual NAC regimens.
Author contributions
Concept or design: PSY Cheung.
Acquisition of data: DMS Tse, HM Lee, PY Tam.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: YHY Chan.
Critical revision of the manuscript for important intellectual content: PSY Cheung, CCH Kwok.
Acquisition of data: DMS Tse, HM Lee, PY Tam.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: YHY Chan.
Critical revision of the manuscript for important intellectual content: PSY Cheung, CCH Kwok.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors thank all patients who have joined the Hong Kong
Breast Cancer Registry (HKBCR), as well as the research staff
who have participated in data collection from 20 hospitals
and 37 clinics throughout the territory (online supplementary Appendix). The authors also acknowledge the following
steering committee members who provided guidance for
the development of the HKBCR: Dr Sharon Wing-wai Chan
(United Christian Hospital), Dr Wai-ka Hung (Pedder Clinic),
Dr Lawrence Pui-ki Li (Alpha Oncology Centre), and Dr
Chun-chung Yau (Hong Kong Sanatorium & Hospital).
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Ethics approval for this study has been obtained from the following six approving bodies:
1. The Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee, Hong Kong (Ref No.: CRE-2009.037)
2. Kowloon West Cluster Research Ethics Committee, Hospital Authority, Hong Kong (Ref No.: KW/EX/08-090)
3. Research Ethics Committee (Kowloon Central/ Kowloon East), Hospital Authority, Hong Kong (Ref No.: KC/KE-09-0013/ER-3)
4. Hong Kong East Cluster Research Ethics Committee, Hospital Authority, Hong Kong (Ref No.: HKEC-2010-004)
5. New Territories West Cluster Clinical & Research Ethics Committee, Hospital Authority, Hong Kong (Ref No.: (8) in NTWC/CREC/866/10)
6. Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster, Hong Kong (Ref No.: UW 09-378)
1. The Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee, Hong Kong (Ref No.: CRE-2009.037)
2. Kowloon West Cluster Research Ethics Committee, Hospital Authority, Hong Kong (Ref No.: KW/EX/08-090)
3. Research Ethics Committee (Kowloon Central/ Kowloon East), Hospital Authority, Hong Kong (Ref No.: KC/KE-09-0013/ER-3)
4. Hong Kong East Cluster Research Ethics Committee, Hospital Authority, Hong Kong (Ref No.: HKEC-2010-004)
5. New Territories West Cluster Clinical & Research Ethics Committee, Hospital Authority, Hong Kong (Ref No.: (8) in NTWC/CREC/866/10)
6. Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster, Hong Kong (Ref No.: UW 09-378)
Written consent was also obtained from all patients in the study who were recruited from the participating hospitals and clinics.
References
1. Rubens RD, Sexton S, Tong D, Winter PJ, Knight RK, Hayward JL. Combined chemotherapy and radiotherapy
for locally advanced breast cancer. Eur J Cancer (1965)
1980;16:351-6. Crossref
2. Thompson AM, Moulder-Thompson SL. Neoadjuvant treatment of breast cancer. Ann Oncol 2012;23 Suppl
10:x231-6. Crossref
3. Gampenrieder SP, Rinnerthaler G, Greil R. Neoadjuvant chemotherapy and targeted therapy in breast cancer: past,
present, and future. J Oncol 2013;2013:732047. Crossref
4. Vugts G, Maaskant-Braat AJ, Nieuwenhuijzen GA, Roumen RM, Luiten EJ, Voogd AC. Patterns of care in the
administration of neo-adjuvant chemotherapy for breast
cancer. a population-based study. Breast J 2016;22:316-21. Crossref
5. Man VC, Cheung PS. Neoadjuvant chemotherapy increases rates of breast-conserving surgery in early operable breast cancer. Hong Kong Med J 2017;23:251-7. Crossref
6. Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a metaanalysis. J Natl Cancer Inst 2005;97:188-94. Crossref
7. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant
Breast and Bowel Project Protocols B-18 and B-27. J Clin
Oncol 2008;26:778-85. Crossref
8. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus
adjuvant chemotherapy in early breast cancer: meta-analysis
of individual patient data from ten randomised
trials. Lancet Oncol 2018;19:27-39.
9. Goldhirsch A, Winer EP, Coates AS, et al. Personalizing the
treatment of women with early breast cancer: highlights
of the St Gallen International Expert Consensus on the
Primary Therapy of Early Breast Cancer 2013. Ann Oncol
2013;24:2206-23. Crossref
10. Curigliano G, Burstein HJ, Winer EP, et al. De-escalating
and escalating treatments for early-stage breast cancer:
the St. Gallen International Expert Consensus Conference
on the Primary Therapy of Early Breast Cancer 2017. Ann
Oncol 2017;28:1700-12. Crossref
11. von Minckwitz G, Untch M, Blohmer JU, et al. Definition
and impact of pathologic complete response on prognosis
after neoadjuvant chemotherapy in various intrinsic breast
cancer subtypes. J Clin Oncol 2012;30:1796-804. Crossref
12. Green MC, Buzdar AU, Smith T, et al. Weekly paclitaxel
improves pathologic complete remission in operable breast
cancer when compared with paclitaxel once every 3 weeks.
J Clin Oncol 2005;23:5983-92. Crossref
13. Steger GG, Greil R, Lang A, et al. Epirubicin and docetaxel with or without capecitabine as neoadjuvant treatment for early breast cancer: final results of a randomized phase III
study (ABCSG-24). Ann Oncol 2014;25:366-71. Crossref
14. National Comprehensive Cancer Network. NCCN Guidelines. Breast Cancer. Available from: https://www.nccn.org/professionals/physician_gls/. Accessed 25 Jan 2021.
15. European Society for Medical Oncology. ESMO clinical practice guidelines: breast cancer. Early breast cancer. Available from: https://www.esmo.org/guidelines/breast-cancer. Accessed 25 Jan 2021.
16. Dent S, Oyan B, Honig A, Mano M, Howell S. HER2-targeted therapy in breast cancer: a systematic review of
neoadjuvant trials. Cancer Treat Rev 2013;39:622-31. Crossref
17. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014;384:164-72. Crossref
18. Masuda N, Lee SJ, Ohtani S, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017;376:2147-59. Crossref
19. von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast
cancer. N Engl J Med 2019;380:617-28. Crossref
20. ClinicalTrials.gov, US National Library of Medicine. Platinum in treating patients with residual triple-negative breast cancer following neoadjuvant chemotherapy. Available from: https://clinicaltrials.gov/ct2/show/NCT02445391?term=NCT02445391&draw=2&rank=1. Accessed 17 Mar 2023.
21. Waljee JF, Newman LA. Neoadjuvant systemic therapy and the surgical management of breast cancer. Surg Clin North Am 2007;87:399-415, ix. Crossref
22. Ng ET, Russell ZA, Tran BX, et al. Comparing quality of life in breast cancer patients who underwent mastectomy versus breast-conserving surgery: a meta-analysis. Int J
Environ Res Public Health 2019;16:4970. Crossref
23. Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg 2007;94:1189-200. Crossref
24. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001;19:4224-37. Crossref
25. Zhou X, Li Y. Local recurrence after breast-conserving surgery and mastectomy following neoadjuvant chemotherapy for locally advanced breast cancer—a meta-analysis.
Breast Care (Basel) 2016;11:345-51. Crossref
26. Wu YT, Li X, Lu LJ, et al. Effect of neoadjuvant chemotherapy on the expression of hormone receptors and Ki-67 in Chinese breast cancer patients: a retrospective study of 525 patients. J Biomed Res 2017;32:191-7. Crossref
27. Shaaban AM, Provenzano E. Receptor status after neoadjuvant therapy of breast cancer: significance and implications. Pathobiology 2022;89:297-308. Crossref
28. Trifunovic J, Memisevic N, Nikolin B, Salma S, Dugandzija T, Vidovic V. Modulatory effect of neoadjuvant chemotherapy on the prognosis of patients with breast cancer. J BUON 2017;22:638-43.
29. van de Ven S, Smit VT, Dekker TJ, Nortier JW, Kroep JR. Discordances in ER, PR and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev 2011;37:422-30.Crossref
30. Tacca O, Penault-Llorca F, Abrial C, et al. Changes in and prognostic value of hormone receptor status in a series of operable breast cancer patients treated with neoadjuvant
chemotherapy. Oncologist 2007;12:636-43. Crossref
31. Hirata T, Shimizu C, Yonemori K, et al. Change in the hormone receptor status following administration of
neoadjuvant chemotherapy and its impact on the long-term
outcome in patients with primary breast cancer. Br J Cancer 2009;101:1529-36. Crossref
32. Yang L, Zhong X, Pu T, Qiu Y, Ye F, Bu H. Clinical significance and prognostic value of receptor conversion in
hormone receptor positive breast cancers after neoadjuvant
chemotherapy. World J Surg Oncol 2018;16:51. Crossref
33. Guarneri V, Dieci MV, Barbieri E, et al. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol 2013;24:2990-4. Crossref
34. Chen C, Zhang Y, Huang Z, Wu J, Huang W, Zhang G. Decrease in the Ki67 index during neoadjuvant chemotherapy predicts favorable relapse-free survival in patients with locally advanced breast cancer. Cancer Biol Med 2019;16:575-86. Crossref
35. Dede DS, Gumuskaya B, Guler G, Onat D, Altundag K, Ozisik Y. Evaluation of changes in biologic markers
ER, PR, HER 2 and Ki-67 index in breast cancer with administration of neoadjuvant dose dense doxorubicin, cyclophosphamide followed by paclitaxel chemotherapy. J BUON 2013;18:366-71.