Hong Kong Med J 2023 Apr;29(2):162–4 | Epub 20 Mar 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Adult-onset Still’s disease after mRNA COVID-19 vaccination presenting with severe myocarditis with acute heart failure and cardiogenic shock: a case report
Andy KC Kan, MB, BS; Winnie WY Yeung, FHKCP, FHKAM (Medicine); CS Lau, MD (Hons), FRCP (Lond, Glasg, Edin); Philip H Li *, FRCP (Glasg), FHKAM (Medicine)
Division of Rheumatology and Clinical Immunology, Department of Medicine, Queen Mary Hospital, The University of Hong Kong, Hong Kong SAR, China
Corresponding author: Dr Philip H Li (liphilip@hku.hk)
Case report
We report the first case of adult-onset Still’s disease
(AOSD) following messenger ribonucleic acid
(mRNA) coronavirus disease 2019 (COVID-19)
vaccination presenting with severe myocarditis
with acute heart failure and cardiogenic shock. A
72-year-old Chinese female, with history of subtotal
thyroidectomy, hypertension, dyslipidaemia, and
osteoporosis, developed gradual onset of fever,
dyspnoea, sore throat, generalised arthralgia,
malaise, and poor appetite 15 days after receiving
the first dose of BNT162b2 mRNA COVID-19
vaccine, and was admitted 7 days after symptom
onset in September 2021. Physical examination
revealed fever, bilateral ankle oedema, and elevated
jugular venous pressure. Polymerase chain reaction
test for COVID-19 was negative on admission and
throughout hospitalisation. Initial workup found
increased C-reactive protein level of 182.3 mg/L
(reference range, <7.6), severely elevated cardiac
troponin I level of 7789 ng/L (reference range, <40)
and N-terminal pro B-type natriuretic peptide
level of 26 688 ng/L (reference range, <300),
as well as deranged liver function. Chest X-ray
showed progressive bilateral pulmonary infiltrates.
Electrocardiography revealed fast atrial fibrillation
with no other abnormalities including QRS or
ST changes. Echocardiography showed severely
reduced left ventricular ejection fraction of 20% with
normal right ventricular function and no features
of cardiomyopathy. Computed tomography of the
thorax and upper abdomen did not show any features
of malignancy, lymphadenopathy, interstitial lung
disease or hepatosplenomegaly. Acute heart failure
with reduced ejection fraction was diagnosed.
The patient was prescribed empirical intravenous
antibiotics and underwent septic workup with
unremarkable results.
In view of the severe acute heart failure,
the patient was transferred to the cardiac care
unit of our hospital for further management on day 26 after vaccination. Significant investigation
results are shown in the Table. Extensive viral
panel tests (including enterovirus, influenza, and
cytomegalovirus) were all negative. Shortly after
transfer, the patient developed acute pulmonary
oedema requiring 2 L/min oxygen and was treated
with amiodarone, apixaban, and furosemide. On
day 28 after vaccination, she developed cardiogenic
shock requiring intensive care admission and was
given noradrenaline and high-flow oxygen. Her
atrial fibrillation persisted, and cardioversion was
performed with consequent restoration of sinus
rhythm.
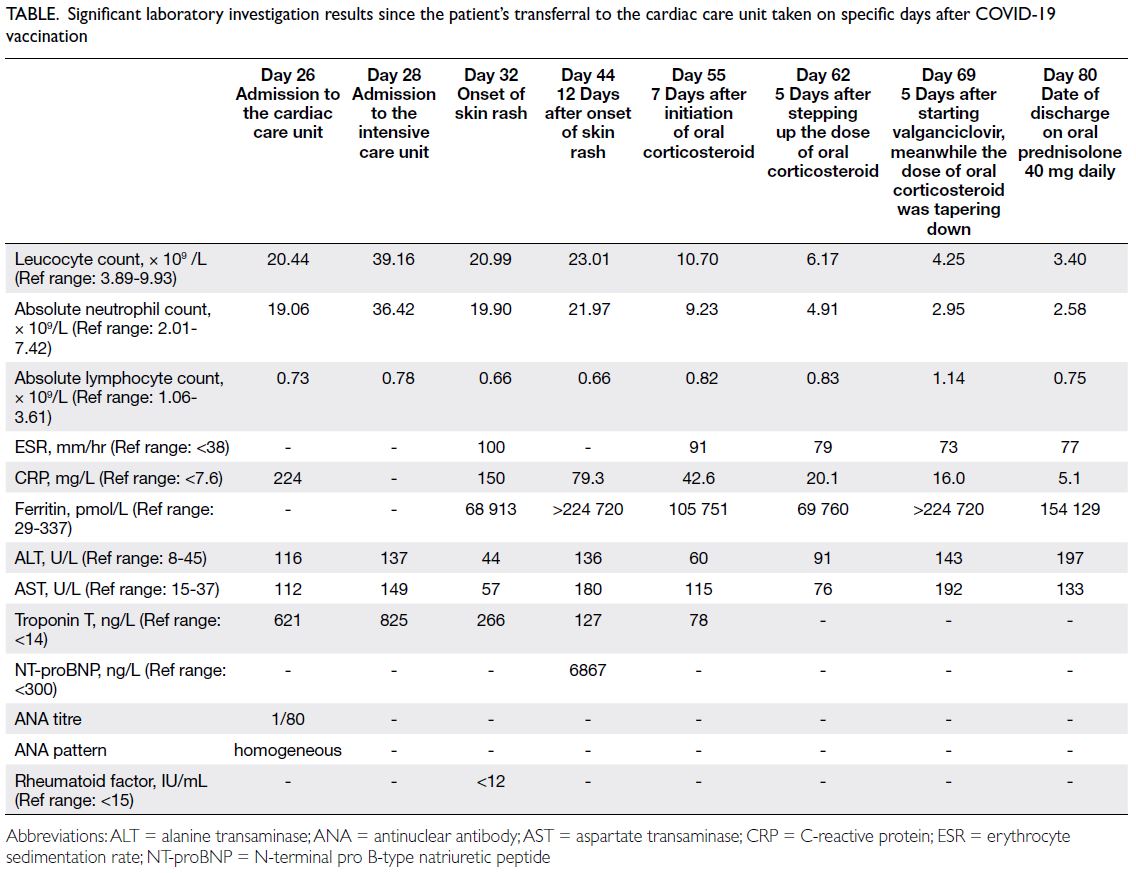
Table. Significant laboratory investigation results since the patient’s transferral to the cardiac care unit taken on specific days after COVID-19 vaccination
The patient’s cardiac function gradually
improved as evidenced by recovering left ventricular
ejection fraction upon echocardiography.
After stabilisation, coronary angiogram with
endomyocardial biopsy was performed on day
34 after vaccination. There was no evidence of
significant coronary artery disease; histology
showed mononuclear infiltrate and viral studies were
negative. Nonetheless the patient had persistent
quotidian fever of >39°C and arthralgia (bilateral
shoulders, wrists, and proximal interphalangeal
joints). She also developed a diffuse erythematous
maculopapular rash over the trunk and limbs, with
skin biopsy revealing interface dermatitis. She
was transferred to the rheumatology unit. Blood
test results showed neutrophilic leucocytosis
(leucocyte count: 20.99×109/L), hyperferritinaemia
level of 68 913 pmol/L (reference range, 29-337),
deranged liver function, and elevated inflammatory
markers (erythrocyte sedimentation rate and
C-reactive protein); autoimmune panel was
unremarkable except for an antinuclear antibody
titre of 1:80 (Table). Extensive septic workup after
microbiologist consultation, including viral panel
serology, was negative. Cardiac contrast magnetic
resonance imaging on day 44 after vaccination
showed diffuse myocardial oedema, consistent
with myocarditis. Subsequent contrast positron emission tomography–computed tomography showed reactive lymphadenopathy but no features
of malignancy.
The diagnosis of AOSD was made based on
the Yamaguchi criteria and the Fautrel criteria.1
The patient was treated with oral prednisolone
30 mg daily. Subsequent investigations revealed
cytomegalovirus pp65 antigenaemia, hence
valganciclovir was prescribed. Treatment was well-tolerated.
Her fever gradually subsided, and leucocyte
count and C-reactive protein level were normalised
although serum ferritin, erythrocyte sedimentation
rate and liver enzymes remained elevated. Three
months after discharge, the patient was clinically
well with prednisolone tapered down to 5 mg daily.
Echocardiographic reassessment demonstrated full
recovery with left ventricular ejection fraction of
60%.
Discussion
The pathogenesis of AOSD is hypothesised to be multifactorial and autoinflammatory, contributed
by genetic predisposition, environmental triggers,
and eventual immune dysregulation.1 Infections can
trigger AOSD, as pathogen-associated molecular
patterns of pathogenic organisms or damage-associated
molecular patterns from damaged host
cells activate the innate immune system through
pattern recognition receptors such as toll-like
receptors on neutrophils and macrophages. In the
presence of dysregulation, cytokine storm with
overproduction of interleukin (IL)-6, IL-8, IL-17,
tumour necrosis factor-alpha, IL-1β, and IL-18 can
lead to the hyperinflammatory state of AOSD.1 The
mRNA COVID-19 vaccines also stimulate innate
immune responses since the mRNA can act as both
an immunogen encoding the viral antigen and an
adjuvant that directly stimulates pattern recognition
receptors, thus mimicking an infection.2 Immune
response after vaccination, such as production of
cytokines, may trigger AOSD in a similar manner to
infections.
To the best of our knowledge, there have not
been reports of post–COVID-19 vaccine AOSD
presenting with severe myocarditis with acute heart
failure and cardiogenic shock. This case highlights
the possibility of such an atypical presentation of
post–COVID-19 vaccine AOSD, and the importance
of monitoring for signs and symptoms of systemic
inflammatory diseases in post–COVID-19 vaccine
myocarditis patients (especially if the signs and
symptoms are persistent). A high index of suspicion
should be maintained and, if indicated, extensive
workup carried out to establish a diagnosis so that
appropriate treatment (such as corticosteroids or
other immunosuppressants) can be offered.
Myocarditis is a rarely reported complication of
AOSD.1 Nonetheless all reports of post–COVID-19
vaccination AOSD to date, including our case, had
some features of myocarditis.3 4 5 Further studies are
warranted to determine whether myocarditis is more
likely to occur in post–COVID-19 vaccine AOSD,
and whether post–COVID-19 vaccine myocarditis
and post–COVID-19 vaccine AOSD are part of a
spectrum of diseases.
Physicians should be reminded that both
mRNA COVID-19 vaccine–related myocarditis
and AOSD are exceedingly rare. As illustrated
in this report, a comprehensive evaluation to
exclude more common causes of heart failure and
myocarditis should be performed first. Vaccine-related
myocarditis and AOSD should be a diagnosis
of exclusion, and all efforts should be made to not
miss other possible aetiologies. It is important to
emphasise that given the extreme rarity of such
adverse reactions, the overall benefits of COVID-19
vaccines still far outweigh the risks for the general
population.3 4 5
In conclusion, although exceedingly rare,
severe myocarditis with acute heart failure and
cardiogenic shock is a possible initial presentation
of AOSD after mRNA COVID-19 vaccination.
After exclusion of more common aetiologies,
it is important to consider AOSD as one of the differential diagnoses in the presence of compatible
features following COVID-19 vaccination, such that
appropriate and timely workup and treatment can be
offered.
Author contributions
Concept or design: All authors.
Acquisition of data: AKC Kan, WWY Yeung.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: AKC Kan.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: AKC Kan, WWY Yeung.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: AKC Kan.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki and has provided informed consent for all procedures and publication.
References
1. Gerfaud-Valentin M, Jamilloux Y, Iwaz J, Sève P. Adult-onset Still’s disease. Autoimmun Rev 2014;13:708-22. Crossref
2. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 2021;21:195-7. Crossref
3. Leone F, Cerasuolo PG, Bosello SL, et al. Adult-onset Still’s disease following COVID-19 vaccination. Lancet
Rheumatol 2021;3:e678-80. Crossref
4. Sharabi A, Shiber S, Molad Y. Adult-onset Still’s disease following mRNA COVID-19 vaccination. Clin Immunol 2021;233:108878. Crossref
5. Magliulo D, Narayan S, Ue F, Boulougoura A, Badlissi F. Adult-onset Still’s disease after mRNA COVID-19 vaccine. Lancet Rheumatol 2021;3:e680-2. Crossref

