© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Epistaxis, Pneumocystis jirovecii pneumonia and
aplastic anaemia: chicken or egg?
Karen KY Leung, MB, BS, MRCPCH1; CC Au, MB, BS, MRCPCH1; KL Hon, MB, BS, MD1; Mark MH Cheng, MB, BS, MRCPCH2; Jeff CP Wong, MB, BS, MRCPCH1; MK Shing, MB, ChB, MRCPCH1
1 Department of Paediatrics and Adolescent Medicine, Hong Kong Children’s Hospital, Hong Kong SAR, China
2 Department of Paediatrics and Adolescent Medicine, Princess Margaret Hospital, Hong Kong SAR, China
Corresponding author: Dr KL Hon (ehon@hotmail.com)
Introduction
Aplastic anaemia is a rare disease in young children
that arises from damage to the bone marrow and
resident haematopoietic stem cells. Affected patients
are susceptible to bacterial and invasive fungal
infections due to profound persistent neutropenia.1
Pneumocystis jirovecii pneumonia (PJP) is rarely
reported in patients with aplastic anaemia. We
review a case of early presentation of PJP associated
with very severe aplastic anaemia and the associated
literature.
Case report
A 31-month previously healthy boy with normal
growth and development presented with a 2-month
history of profound epistaxis, easy bruising and
petechial rash. Apart from a 1-day history of fever
prior to admission, he displayed no constitutional
symptoms. There was no history suggestive of
consumption of herbal or over-the-counter medicine
and no family history of haematological disease or
malignancy. He was an only child. Complete blood
picture revealed pancytopenia (haemoglobin level of
7 g/dL, white blood cell count of 0.3×109/L, platelet
count of 3×109/L, reticulocyte count of <0.2%) and
after further workup he was diagnosed with very
severe aplastic anaemia. Liver function tests were
deranged with raised alanine aminotransferase level
of 1687 IU/L, alkaline phosphatase level of 347 IU/L,
gamma-glutamyl transferase level of 128 IU/L, and
total bilirubin level of 11 μmol/L, but the levels of
ammonia and lactate were normal. Ultrasound of
the liver was suggestive of mild hepatic parenchymal
disease such as hepatitis.
Bone marrow trephine showed severe
deficiency of all cell lines, markedly hypocellular
marrow (overall cellularity <10%) and no abnormal
infiltrations. Immunophenotyping of the lymphoid
population revealed marked lymphopenia with a
reversed CD4:CD8 ratio at 0.3:1. His CD4 count
was very low. Within one week of presentation, he
developed antibiotic-resistant pneumonia. He was
commenced initially on piperacillin/tazobactam
but switched to meropenem and micafungin due to fever and progressive chest X-ray changes
of bilateral infiltrates (Fig 1). He had increased
respiratory distress and required support with high-flow
oxygen therapy in the paediatric intensive
care unit. Computed tomography scan of the chest
revealed bilateral extensive patchy changes, most
severe at both anterior segments of the upper lobe
and posterior segments of the lower lobes (Fig 2).
Prompt bronchoalveolar lavage was performed and
Grocott methenamine silver staining revealed the
pathogen to be P jirovecii (Fig 3). Bronchoalveolar
lavage culture also showed scanty growth of alpha-haemolytic
streptococcus (<1000 CFU/mL) with no
white cells seen on microscopy. He was treated with
a 21-day course of co-trimoxazole (120 mg/kg/day)
and oral prednisolone (2 mg/kg/day for 5 days,
1 mg/kg/day for 5 days, then 0.5 mg/kg/day for
11 days). The pneumonia gradually resolved and
his liver function tests normalised. Nonetheless he
remained pancytopenic.
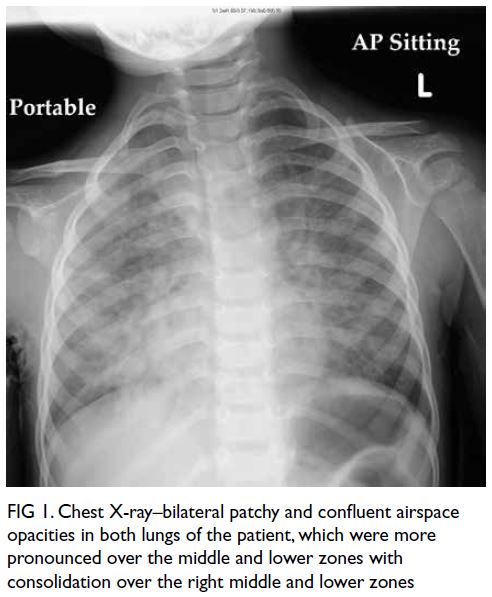
Figure 1. Chest X-ray–bilateral patchy and confluent airspace opacities in both lungs of the patient, which were more pronounced over the middle and lower zones with consolidation over the right middle and lower zones
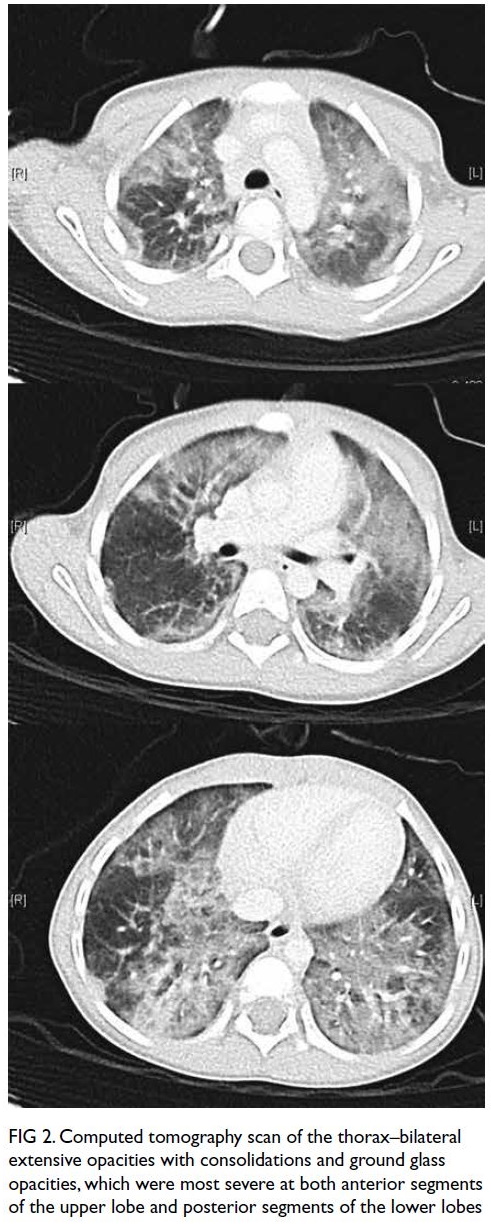
Figure 2. Computed tomography scan of the thorax–bilateral extensive opacities with consolidations and ground glass opacities, which were most severe at both anterior segments of the upper lobe and posterior segments of the lower lobes
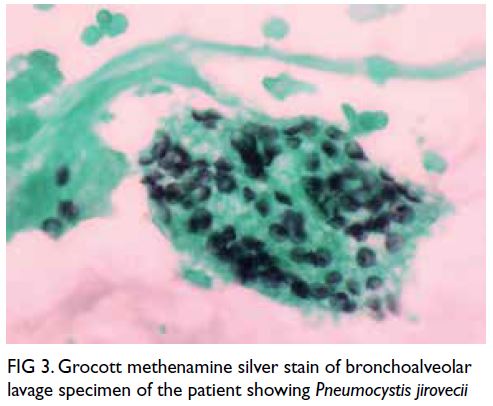
Figure 3. Grocott methenamine silver stain of bronchoalveolar lavage specimen of the patient showing Pneumocystis jirovecii
No test was suggestive of an inherited
marrow failure syndrome or recent infection.
Bronchoalveolar lavage for viral polymerase chain
reaction and culture, Legionella culture, and acid-fast
bacillus culture were also negative. No drug history
was identified that could account for his aplastic
anaemia. Autoimmune screening (immunoglobulin
A [IgA], IgG, IgM, complement component 3, complement component 4, antinuclear antibody, and anti-extractable nuclear antigen) was likewise insignificant.
The child was commenced on
immunosuppressive therapy with
methylprednisolone, cyclosporin A, and
lymphocyte immune globulin, antithymocyte
globulin after treatment of the PJP. He has been
on immunosuppressive therapy for >1 year and is
demonstrating a good response to treatment.
Discussion
Aplastic anaemia is a potentially life-threatening
clinical syndrome characterised by pancytopenia
with a hypocellular bone marrow in the absence of
abnormal infiltration or increased reticulin.2 It is a
rare disorder with an incidence of about two cases
per million population per year. Nonetheless the
incidence is two- to three-fold higher in Asia than
in Europe.3 4
Persistent neutropenia is the major risk
factor for the development of all types of infection
in patients with aplastic anaemia, while bacterial
and invasive fungal infections are the major causes
of mortality.1 2 Respiratory tract infection is one of
the common manifestations of infection in children
with aplastic anaemia, and pneumonia in particular
can be life-threatening.2 Detection of an infectious
agent may be possible in only approximately 12%
to 20% of cases, and diagnosis is mainly based on
clinical symptoms and radiological investigations.5 6
Previous reviews of respiratory infection in patients
with severe aplastic anaemia and very severe aplastic
anaemia revealed common causes such as invasive
fungal infection (Aspergillus and Zygomycetes)
and respiratory viral infections (influenza A and
B, respiratory syncytial virus, parainfluenza virus, adenovirus, and cytomegalovirus).2 5 7 Pneumocystis jirovecii pneumonia is rarely reported in patients
who have commenced immunosuppressive therapy,
and no case of PJP has been reported in three
large studies of patients prescribed antithymocyte
globulin and cyclosporin A.1 8
The risk of PJP in patients with aplastic anaemia
has not been defined in the literature, but the risk
should be low since the T cells are not defective.1
T cells (CD4+) are crucial for PJP clearance as they
coordinate inflammatory responses in the host by
recruiting and activating effector cells.9 Only three
cases of PJP in patients with Diamond–Blackfan
anaemia and one case in a patient with Fanconi’s
anaemia have been reported and all were receiving
high-dose corticosteroids.10 11 Our patient is possibly
the first reported case of PJP in a patient with aplastic
anaemia not yet started on any immunosuppressive
therapy. The development of PJP was probably
due to the immune deficiency of aplastic anaemia
rather than the aetiology, as evidenced by the
reversed CD4:CD8 ratio. Patients with aplastic
anaemia are usually not considered to be at risk of
PJP, hence prophylaxis is not routinely prescribed.12
Nonetheless PJP in aplastic anaemia should not
be overlooked as the mortality of PJP in human
immunodeficiency virus–seronegative patients is
significant (32%-50%).13 14 Our patient developed PJP
within a week of diagnosis, before he was started
on any immunosuppressive therapy. This raises a
broader question of whether PJP prophylaxis (eg,
co-trimoxazole, dapsone and pentamidine) should
be considered in patients with aplastic anaemia,
especially those who fulfil the criteria of very severe
aplastic anaemia.
The Red Book 2018 suggests that standard
precautions should be taken,15 while the Centers
for Disease Control and Prevention in the United
States16 and Health Protection Scotland17 both
recommend that patients with PJP should not share
a hospital room with other immunocompromised
patients. Although there have been clusters of PJP
cases reported in wards of immunocompromised
patients, and a study has also shown that airborne
person-to-person transmission of P jirovecii is
possible, we believe there is insufficient evidence to
warrant mandatory isolation.18 It is more important
to identify patients at risk of PJP and commence
prophylaxis.19
Conclusion
Pneumocystis jirovecii infection is probably due to immune deficiency in aplastic anaemia, not
the aetiology of aplastic anaemia. Clinical and
radiological pictures of PJP in a patient with aplastic
anaemia are not specific for P jirovecii. All such
patients with symptoms of lung infection resistant
to antibacterial and antifungal therapy should be examined for PJP. Our patient is possibly the first
reported case of PJP in a patient with aplastic anaemia
prior to commencement of immunosuppressive
therapy. Although standard guidelines do not
recommend PJP prophylaxis in patients with aplastic
anaemia, further studies should assess whether the
benefits of chemoprophylactic agents outweigh the
risks in those considered to have very severe aplastic
anaemia.
Author contributions
Concept or design: KKY Leung, KL Hon.
Acquisition of data: KKY Leung, KL Hon.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: KKY Leung, KL Hon.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: KKY Leung, KL Hon.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: KKY Leung, KL Hon.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, KL Hon was not involved in the peer review process. Other authors have disclosed no conflicts
of interest.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki, with informed consent provided for treatment,
procedures, and publication.
References
1. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol 2009;46:269-76. Crossref
2. Dezern AE, Brodsky RA. Clinical management of aplastic anemia. Expert Rev Hematol 2011;4:221-30. Crossref
3. Shimamura A, Nathan DG. Acquired aplastic anemia and pure red cell aplasia. In: Orkin SH, Nathan DG, Ginsburg D,
Look AT, Fisher DE, Lux SE, editors. Nathan and Oski’s
Hematology of Infancy and Childhood. Philadelphia (PA):
Elsevier; 2014: 275-306.
4. Montané E, Ibáñez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica 2008;93:518-23. Crossref
5. Pawelec K, Salamonowicz M, Panasiuk A, Matysiak M, Demkow U. Respiratory and systemic infections in children
with severe aplastic anemia on immunosuppressive
therapy. Adv Exp Med Biol 2013;788:417-25. Crossref
6. Rudan I, El Arifeen S, Bhutta ZA, et al. Setting research priorities to reduce global mortality from childhood
pneumonia by 2015. PLoS Med 2011;8:e1001099. Crossref
7. Quarello P, Saracco P, Giacchino M, et al. Epidemiology of infections in children with acquired aplastic anaemia:
a retrospective multicenter study in Italy. Eur J Haematol
2012;88:526-34. Crossref
8. Cordonnier C, Cesaro S, Maschmeyer G, et al. Pneumocystis jirovecii pneumonia: still a concern in patients with
haematological malignancies and stem cell transplant
recipients. J Antimicrob Chemother 2016;71:2379-85. Crossref
9. Otieno-Odhiambo P, Wasserman S, Hoving JC. The contribution of host cells to pneumocystis immunity: an
update. Pathogens 2019;8:52. Crossref
10. Huh WW, Gill J, Sheth S, Buchanan GR. Pneumocystis carinii pneumonia in patients with Diamond–Blackfan
anemia receiving high-dose corticosteroids. J Pediatr
Hematol Oncol 2002;24:410-2. Crossref
11. Pedersen FK, Hertz H, Lundsteen C, Platz P, Thomsen M. Indication of primary immune deficiency in Fanconi’s
anemia. Acta Paediatr Scand 1977;66:745-51. Crossref
12. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J
Haematol 2016;172:187-207.Crossref
13. Gerrard JG. Pneumocystis carinii pneumonia in HIV-negative immunocompromised adults. Med J Aust 1995;162:233-5. Crossref
14. Kovacs JA, Hiemenz JW, Macher AM, et al. Pneumocystis carinii pneumonia: a comparison between patients with the
acquired immunodeficiency syndrome and patients with
other immunodeficiencies. Ann Intern Med 1984;100:663-71. Crossref
15. American Academy of Pediatrics. Committee on Infectious Diseases. Red Book (2018): Report of the Committee on
Infectious Diseases. 31st edition. Kimberlin DW, Brady
MT, Jackson MA, editors. Elk Grove Village (IL): American
Academy of Pediatrics; 2018: 651-6.
16. Centers for Disease Control and Prevention, Infectious Diseases Society of America. Guidelines for the prevention and treatment of opportunistic infections in adults and
adolescents with HIV. 2009. Available from: https://www.idsociety.org/practice-guideline/prevention-and-treatment-of-opportunistic-infections-among-adults-and-adolescents/. Accessed 18 Oct 2020.
17. Health Protection Scotland. Information for staff on
Pneumocystis Pneumonia (PcP). 2020. Available from:
https://hpspubsrepo.blob.core.windows.net/hps-website/nss/2117/documents/1_pcp-staff-information-2020-01.pdf. Accessed 18 Oct 2020.
18. Vargas SL, Ponce CA, Gigliotti F, et al. Transmission of Pneumocystis carinii DNA from a patient with P. carinii pneumonia to immunocompetent contact health care workers. J Clin Microbiol 2000;38:1536-8. Crossref
19. Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for
diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and
solid malignancies, 2014. Intern Med J 2014;44:1350-63. Crossref

