Hong Kong Med J 2023 Apr;29(2):121–31 | Epub 24 Feb 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Implementation of ovarian tissue cryopreservation in Hong Kong
Jacqueline PW Chung, MB, ChB, FHKAM (Obstetrics and Gynaecology)1,2; David YL Chan, BSc (Ulster), DPhil (Oxon)1,2; Y Song, BSc (Peking University), PhD (CUHK)3; Elaine YL Ng, BSc (CUHK), MPhil (CUHK)1; Tracy SM Law, MB, ChB, FHKAM (Obstetrics and Gynaecology)1; Karen Ng, MB, ChB, FHKAM (Obstetrics and Gynaecology)1; Maran BW Leung, BSc (CUHK), PhD (CUHK)3; S Wang, MB, BS, MSc1; HM Wan, BEng (Jinan University), MSc (Jinan University)1; Joshua JX Li, MB, ChB, FHKAM (Pathology)5; CC Wang, MB, BS, PhD (Surgical Sciences in Obstetrics and Gynaecology)1,2,6
1 Assisted Reproductive Technology Unit, Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong
2 Fertility Preservation Research Centre, Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong
3 Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong
4 Department of Obstetrics and Gynaecology, Union Hospital, Hong Kong
5 Department of Anatomical and Cellular Pathology, The Chinese University of Hong Kong, Hong Kong
6 Li Ka Shing Institute of Health Science, School of Biomedical Sciences; and Chinese University of Hong Kong–Sichuan University Joint Laboratory in Reproductive Medicine, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Prof Jacqueline PW Chung (jacquelinechung@cuhk.edu.hk)
Abstract
Introduction: Worldwide, >130 babies have been
born from ovarian tissue cryopreservation (OTC)
and ovarian tissue transplantation (OTT). Ovarian
tissue cryopreservation can improve quality of life
among young female cancer survivors. Here, we
assessed the feasibility of OTC and subsequent OTT
in Hong Kong via xenografts in nude mice.
Methods: This pilot study was conducted in a
university-affiliated tertiary hospital. Fifty-two
ovarian tissues were collected from 12 patients aged
29 to 41 years during ovarian surgery, then engrafted
into 34 nude mice. The efficacies of slow freezing
and vitrification were directly compared. In Phase I,
non-ovariectomised nude mice underwent ovarian
tissue engraftment. In Phase II, ovariectomised
nude mice underwent ovarian tissue engraftment,
followed by gonadotrophin administration to
promote folliculogenesis. Ovarian tissue viability
was assessed by gross anatomical, histological, and
immunohistochemical examinations before and
after OTC. Follicular density and morphological
integrity were also assessed.
Results: After OTC and OTT, grafted ovarian tissues remained viable in nude mice. Primordial
follicles were observed in thawed and grafted
ovarian tissues, indicating that the cryopreservation
and transplantation protocols were both effective.
The results were unaffected by gonadotrophin
stimulation.
Conclusion: This study demonstrated the feasibility
of OTC in Hong Kong as well as primordial follicle
viability after OTC and OTT in nude mice. Ovarian tissue cryopreservation is ideal for patients who
cannot undergo the ovarian stimulation necessary
for oocyte or embryo freezing as well as prepubertal
girls (all ineligible for oocyte freezing). Our findings
support the clinical implementation of OTC and
subsequent OTT in Hong Kong.
New knowledge added by this study
- This study assessed the viability of ovarian tissue cryopreservation and subsequent ovarian tissue transplantation in Hong Kong via xenografts in nude mice.
- Grafted ovarian tissues remained viable after transplantation, regardless of protocol (slow freezing or vitrification).
- Ovarian tissue cryopreservation is ideal for patients who cannot undergo the ovarian stimulation necessary for oocyte or embryo freezing, as well as prepubertal girls (all ineligible for oocyte freezing).
- These findings support the clinical implementation of ovarian tissue cryopreservation and subsequent ovarian tissue transplantation in Hong Kong.
- Further studies are needed to clarify optimal cryopreservation and engraftment protocols.
Introduction
A diagnosis of cancer is disheartening news for
every patient in terms of both disease and treatment.
Anticancer treatments for common cancers (eg,
chemotherapy and radiotherapy) are gonadotoxic and
detrimental to future fertility.1 Advances in medical
treatment have improved the 5-year survival rates of
some cancers to >80% in children and adolescents.2
However, most surviving patients experience illness-related
infertility.3 Infertility is also a concern for
patients with severe endometriosis, patients with
poor ovarian reserve, patients with benign medical
conditions requiring chemotherapy, and transgender
individuals undergoing gender-affirming surgery.4
Fortunately, advancements in fertility preservation
(FP) technologies offer these patients the opportunity
to have biological offspring in the future. In Hong
Kong, only 45.6% of clinicians,5 22.2% of medical
students,6 and 21.7% of the general public7 are
familiar with FP. Therefore, FP technologies require
greater attention in Hong Kong. Both clinicians and
the public should be aware where and how to seek
help when patients are diagnosed with cancer and
need to use FP technologies to preserve their fertility.
For patients who cannot undergo the ovarian
stimulation necessary for oocyte or embryo freezing
as well as prepubertal girls (all ineligible for oocyte
freezing), ovarian tissue cryopreservation (OTC)
and subsequent orthotopic or heterotopic ovarian tissue transplantation (OTT) are ideal options for
FP after recovery.8 Many European countries have
provided OTC for patients with various medical
reasons.4 Although OTC and OTT are widely
available in Belgium, Denmark, Spain, France,9
Japan, Singapore,10 the United States, India,
Australia, the Philippines, Korea,11 and some parts
of China,12 these FP technologies remain unavailable
in Hong Kong. Thus far, >130 babies worldwide have
been born via OTC and OTT,13 and the American
Society for Reproductive Medicine removed the
‘experimental’ designation for these technologies
in 2019.14 Ovarian tissue cryopreservation can
be performed via slow freezing or vitrification.
Slow freezing has been the standard treatment for
ovarian tissues,15 but there is increasing evidence
to support the use of vitrification.16 17 Nevertheless,
controversies remain concerning subsequent oocyte
viability and the preservation of morphological
integrity after ovarian tissues have been processed
using these two cryopreservation techniques.18
The development of OTC, which can
improve quality of life among young female cancer
survivors,19 is urgently needed in Hong Kong. Here,
we performed a pilot study to assess the feasibility
of OTC and subsequent OTT in Hong Kong via
xenografts in nude mice. In this study, we collected
ovarian tissues, established both slow freezing and
vitrification protocols, and evaluated tissue viability
and follicle preservation after OTC and OTT in a nude
mouse xenograft model. This mouse model provided
important insights that will support the clinical
implementation of OTC and OTT in Hong Kong.
Our primary outcome was ovarian tissue viability
after slow freezing or vitrification, as determined
by histological analysis and immunohistochemistry.
Our secondary outcomes were follicular density
and the morphological integrity of grafted ovarian
tissues.
Methods
This study was conducted between July 2019 and
December 2021 at the Prince of Wales Hospital, a
university-affiliated tertiary hospital in Hong Kong.
All participants received a detailed explanation of the
study, then provided written consent for inclusion.
All researchers involved in the animal experiments
were licensed by the Department of Health of the
Hong Kong SAR Government.
Ovarian tissue collection
Ovarian tissues were collected from women or
transgender individuals who underwent laparoscopic
or open, unilateral or bilateral, ovarian cystectomy
or salpingo-oophorectomy as treatment for benign
ovarian cysts or tumours. During the operation,
each patient underwent removal of a small section of ovarian tissue or the whole ovary; each specimen of
donated ovarian tissue was retrieved from a routine
surgical specimen or directly removed during surgery.
To prevent thermal injury during tissue removal,
cold scissors were used and diathermy was avoided.
The amount of donated tissue varied among patients,
depending on their age and clinical condition. For
example, larger volumes of ovarian tissue were often
collected from patients undergoing oophorectomy.
Each patient was assigned a unique identification
number linked to an encrypted file containing the
patient’s data and demographic information; during
analyses of tissue from each patient, the pathologist
and research staff who conducted histology and
immunohistochemistry analyses were blinded to the
contents of the encrypted files.
Ovarian tissue cryopreservation
Tissues were transported to the laboratory in a
standardised culture medium at 4°C and processed
within 30 minutes after collection. After removing
the medullary region, the ovarian tissue was frozen
in accordance with a controlled-rate slow freezing
machine protocol (Ovarian Tissue Cryopreservation
Scientific Roundup; Planer, UK)20 or a vitrification
manual (Ova Cryo Kit Type M, VT301S; Kitazato
Corporation, Japan).21 The cortical region was
cut into small fragments with a thickness of
approximately 1 mm. Some collected ovarian tissues
were very small and could not be sectioned for
parallel slow freezing and vitrification fresh tissue
controls; these small tissues were either frozen using
the standard slow freezing method or vitrification.22
Larger ovarian tissues (typically collected from
transgender individuals during oophorectomy) were
cut into smaller fragments prior to slow freezing and
vitrification, or prior to use as fresh tissue controls.
Before the xenograft procedure, fragments were
removed from fresh tissue, thawed slow-frozen
tissue, and thawed vitrified tissue; these fragments
were subsequently compared with grafted tissues to
identify any differences related to engraftment.
A subset of fresh ovarian tissue fragments was
fixed and subjected to histological analysis. When
a large amount of ovarian tissue was available from
a single patient, we compared cryopreservation
methods using tissue from that patient; we also
compared the cryopreserved tissue with fresh tissue.
Slow freezing
Slow freezing was performed in accordance with a
validated protocol.23 24 25 Collected ovarian cortices
were equilibrated at 4°C on a tilting shaker for
30 minutes in freezing solution (1.5 mol/L ethylene
glycol and 0.1 mol/L sucrose in G-MOPS PLUS;
Vitrolife, Sweden). After equilibration, the ovarian
tissue pieces were placed into 1.8-mL cryogenic vials
that had been pre-filled with 1 mL of freezing solution (two tissue pieces per vial). The cryogenic vials were
then placed into an automated, computer-controlled
freezing system (Kryo-360; Planer, UK).20 The slow
freezing protocol was performed in accordance with
the method described by Dolmans et al26 and the
Planer Ovarian Tissue Cryopreservation Scientific
Roundup.20
Vitrification
For vitrification of ovarian cortices, the Ovarian Tissue Vitrification Kit (Ova Cryo Kit Type M,
VT301S; Kitazato Corporation, Japan)21 was used.
The collected ovarian cortices were cut into 1 × 1
× 1 cm3 cubes using a surgical knife and a square
measuring device provided in the kit. Vitrification
was then performed in accordance with the kit
manufacturer’s protocol, and the ovarian tissues
were stored in liquid nitrogen.
Thawing of ovarian tissue for transplantation
Slow-frozen tissues were removed from liquid
nitrogen and exposed to room temperature air for
5 seconds, then placed in 37°C water for 2 minutes.
Subsequently, they were transferred to thawing
solution 1 (0.75 mol/L ethylene glycol and 0.25 mol/L
sucrose in G-MOPS PLUS) for 10 minutes, then to
thawing solution 2 (0.25 mol/L sucrose in G-MOPS
PLUS) for 10 minutes, and finally to a handling
medium (G-MOPS) for 10 minutes. Vitrified ovarian
tissue fragments were thawed using Ova Thawing
Kit Type M (V302S; Kitazato Corporation, Japan), in
accordance with the manufacturer’s instructions.21
Ovarian tissue transplantation into nude mice
The nude mouse xenograft model is ideal for
assessment of OTC and OTT outcomes. Ovarian
xenografts in immunodeficient nude mice can be
used to test follicular viability and development.
This approach can reveal whether freezing and
thawing cause damage to ovarian tissue; it can also
demonstrate the ability of cryopreserved tissue to
support the development of large antral follicles.27
Considering the higher rate of immune leakiness in
severe combined immunodeficient mice,28 we used
BALB/c athymic nude mice to validate our OTC
and OTT protocols before clinical implementation.
Thirty-four female BALB/c athymic nude mice (age,
4-6 weeks; Laboratory Animal Services Centre, The
Chinese University of Hong Kong) were used for
this study. To prevent fighting between engrafted
mice, only three mice were housed in individually
ventilated cages at 28°C under controlled sterile
conditions, with a 12-hour light/dark cycle and free
access to an autoclaved pelleted diet and water. Mice
were anaesthetised by intraperitoneal injection of
ketamine (75 mg/kg)/xylazine (10 mg/kg) (AlfaMedic Limited, Hong Kong; manufactured in Holland).
Ovarian tissues collected from patients were grafted
onto nude mice. During ovarian tissue engraftment,
the cortical surface was carefully oriented outward
and tightly attached to the subcutaneous tissue or
abdominal wall. There were two phases in our study,
as described in the following sections (Fig 1).
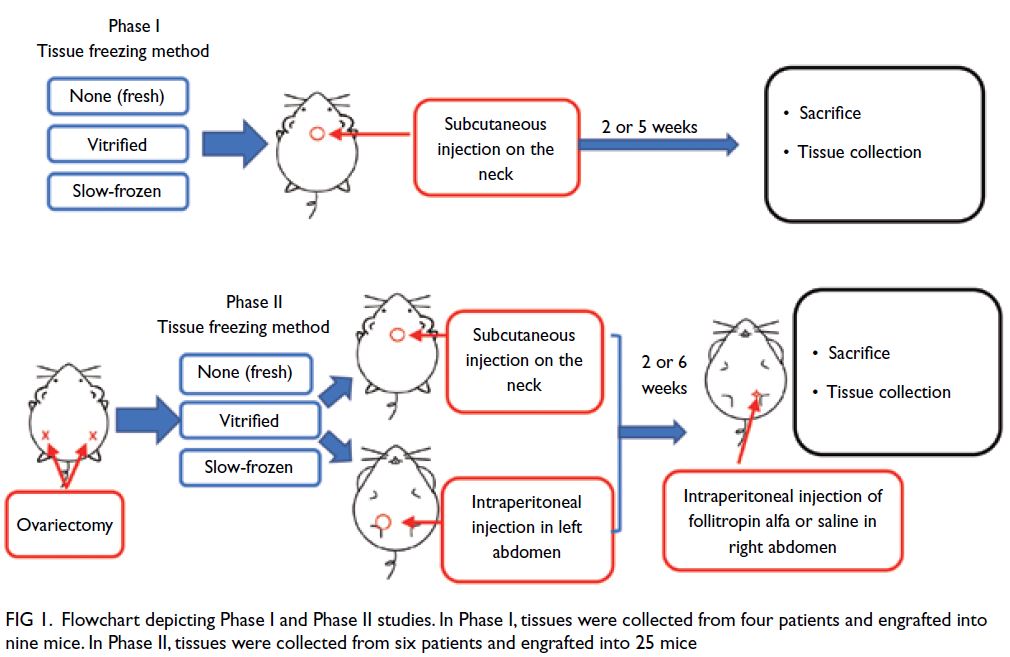
Figure 1. Flowchart depicting Phase I and Phase II studies. In Phase I, tissues were collected from four patients and engrafted into nine mice. In Phase II, tissues were collected from six patients and engrafted into 25 mice
Phase I: Analysis of ovarian tissue xenograft
viability in non-ovariectomised nude mice
To maintain endogenous hormone secretion and
avoid the risk of ovariectomy-related death, mice in
this phase were not subjected to ovariectomy. One
fresh, slow-frozen, or vitrified tissue of approximately
4 × 6 × 1 mm3 was engrafted into the subcutaneous
site on the neck of nine nude mice.29 The mice
were then sacrificed by intraperitoneal injection of
overdose of the anaesthetic. One mouse engrafted
with vitrified tissue, two engrafted with slow-frozen
tissues and one engrafted with fresh tissue were
sacrificed after 2 weeks. Two mice engrafted with
vitrified tissues, one engrafted with slow-frozen
tissue and two engrafted with fresh tissues were
sacrificed after 5 weeks.
Phase II: Analysis of ovarian tissue xenograft
viability, folliculogenesis, and ovulation in ovariectomised nude mice
To promote graft survival and growth, mice in this phase were subjected to ovariectomy. Fresh, slow-frozen,
or vitrified tissues of approximately 4 × 6 × 1 mm3 were either engrafted into the subcutaneous
site on the neck of ovariectomised nude mice, or used
for intraperitoneal engraftment in the left abdomen
of those mice.29 Mice in this phase were divided into a
saline group and a treatment group after 2 or 6 weeks
of engraftment. The presence of gonadotrophins
can optimise graft establishment and stimulate
follicle growth.30 To promote folliculogenesis, mice
in the treatment group underwent intraperitoneal
injection (in the right abdomen) of 1 IU (100 μL) of
follitropin alfa (GONAL-f; Merck Serono, Geneva,
Switzerland) every other day for 5 to 8 weeks after 2
or 6 weeks’ engraftment.30 During the same period,
mice in the saline group underwent intraperitoneal
injection (in the right abdomen) of an equal volume
of physiological saline every other day. Thirty-six
hours before the mice were sacrificed, both groups
of mice received a single dose of 10 international
units of human chorionic gonadotrophin (Sigma-Aldrich, St Louis [MO], US) by injection to promote
ovulation.
Grafted ovarian tissue viability
All grafted tissues were fixed in buffered formalin and embedded in paraffin wax, then sectioned and
stained for analysis.
Histological analysis
Microscopic observations up to 400 times the original
magnification (Leica DMIRB; Leica Microsystem,
Wetzlar, Germany) of fresh and thawed ovarian
tissues were performed after the tissues had been
stained with haematoxylin and eosin (H&E). All
follicles from the entire grafted tissue specimen
on every slide were counted; section thickness
and the presence/absence of a nucleolus were also
considered.
Immunohistochemical assessment of stromal
tissue viability
Stromal tissue viability was determined by assessing
the morphologies of stromal cells on H&E-stained
sections. Viability was defined as the presence
of spindle cells with consistent cellularity; an
intact nuclear membrane; the absence of pyknotic
figures, apoptosis, or necrosis; and the absence of
fibrosis or calcification. Viability was confirmed by
immunohistochemical analyses using antibodies to
cluster of differentiation 10 (CD10) and oestrogen
receptors. Anti-CD10 antibody (clone NCL-CD10-270; Novocastra, Newcastle upon Tyne, UK) was
used at a dilution of 1:50 with an incubation time of
30 minutes at a sustained temperature of 37°C. Anti–oestrogen receptor antibody (RM-9101; Thermo
Fisher Scientific, Fremont [CA], US) was used at a
dilution of 1:150 with an incubation time of 32 minutes
at a sustained temperature of 37°C. Antigen retrieval
was performed using ethylenediaminetetraacetic
acid and microwave. Antibody detection was
performed using Roche Diagnostics OptiView
DAB IHC Detection Kit (Thermo Fisher Scientific,
Waltham [MA], US). Immunohistochemical
staining (original magnification × 400) was semi-quantitative
and based on signal intensity (absent,
weak, moderate, and strong). The presence of at least
weak staining intensity in ovarian stromal tissue was
regarded as a positive result.
Follicular density and quality after freezing,
thawing, and transplantation
All follicles from the entire grafted tissue specimen
on every H&E-stained slide were counted on
multiple levels within thick sections (≥20 μm).
The digital images were annotated on QuPath,31
obtaining the two-dimensional area of the slide
and number of follicles. Follicular density was
calculated by established methods described
previously.32 33 Ovarian follicles were classified as primordial, primary, or secondary follicles according
to morphological assessment of H&E-stained
sections.33 Evaluation of grafted follicle quality was
based on basement membrane integrity, cellular
density, presence or absence of pyknotic bodies,
and oocyte integrity. Only morphologically normal (ie, viable) follicles were counted. The results of
gross anatomical examinations were confirmed by
histological assessments. Gross tissue integrity was
defined as the presence of a distinct vascularised
tissue fragment that exhibited firmness and perfusion.
Microscopic findings indicating viability were the
presence of an intact nuclear membrane and the
absence of necrosis, apoptosis, and pyknotic nuclei.
Results
In total, 52 ovarian tissues were collected from
12 patients aged 29 to 41 years. Ovarian tissues
from different patients were cut into several pieces
according to tissue size. These tissues were treated
by vitrification or slow freezing, then engrafted into
34 mice as shown in Figure 1. Tables 1 and 2 only
show data from patients with follicles to facilitate
readability. Although there were nine tissues from four patients (Patients 1, 6, 7, and 10) in Phase I,
Table 1 only shows data from the two patients with
follicles (Patients 1 and 7). In Phase II, there were
25 tissues from six patients (Patients 1, 5, 8, 9, 11,
and 12), but Table 2 only shows data from the four
patients with follicles. In total, 18 control tissues
were collected from 11 patients (Patients 1, 2, 4, 5,
6, 7, 8, 10, 11, 12, and 13). One patient (Patient 5)
provided sufficient ovarian tissue for a comparison
of cryopreservation methods using tissue from a
single patient. Additionally, we compared fresh and
slow-frozen tissues from Patient 5, and compared
fresh and vitrified tissues from Patients 1, 5, and 12.
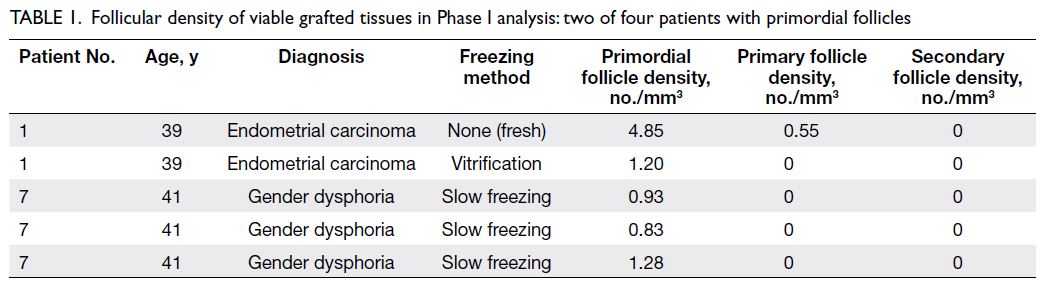
Table 1. Follicular density of viable grafted tissues in Phase I analysis: two of four patients with primordial follicles
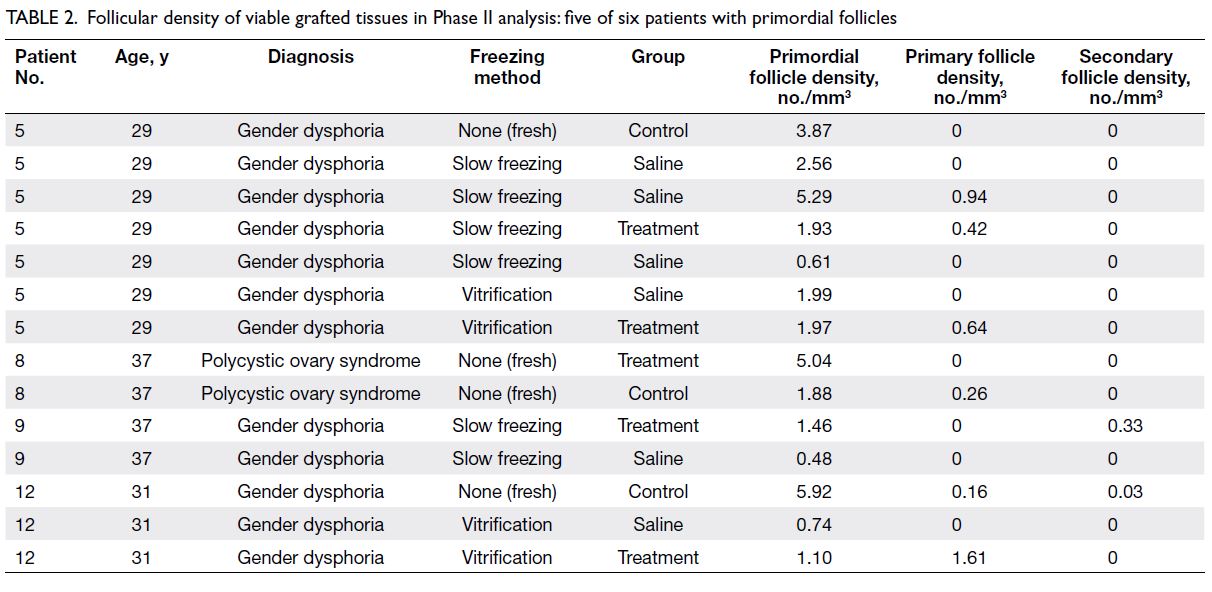
Table 2. Follicular density of viable grafted tissues in Phase II analysis: five of six patients with primordial follicles
Graft recovery rate and macroscopic
assessment
In Phase I, all xenografts were successfully
retrieved from the experimental mice. Macroscopic observations of fresh and thawed tissues did not show
substantial differences between cryopreservation
methods in terms of tissue integrity or morphology
(Fig 2a). Microscopic findings showed the presence of
viable nuclei and the absence of necrosis, apoptosis,
and pyknotic nuclei.
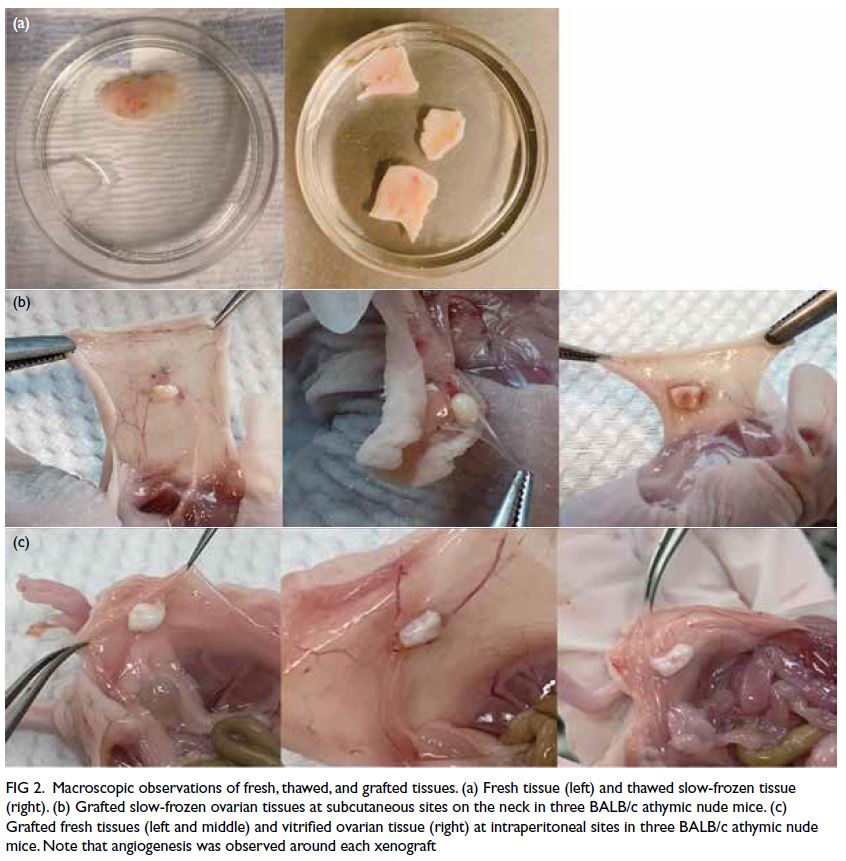
Figure 2. Macroscopic observations of fresh, thawed, and grafted tissues. (a) Fresh tissue (left) and thawed slow-frozen tissue (right). (b) Grafted slow-frozen ovarian tissues at subcutaneous sites on the neck in three BALB/c athymic nude mice. (c) Grafted fresh tissues (left and middle) and vitrified ovarian tissue (right) at intraperitoneal sites in three BALB/c athymic nude mice. Note that angiogenesis was observed around each xenograft
In Phase II, all xenografts were successfully
retrieved from the experimental mice, with
the exception of two calcified tissues. Most
subcutaneous sites contained soft tissue fragments
that were completely encased in membranes; the
graft–murine tissue interface was vascularised
(Fig 2b). Intraperitoneal sites contained soft tissue
fragments with small vessels visible on the graft
surface; the fragments were attached to surrounding
tissue, and some grafts were encased in abdominal
adipose tissue (Fig 2c).
Analysis of stromal tissue morphology
Immunohistochemical staining showed that all
retrieved grafts had maintained viability, with
the exception of two calcified tissues (Fig 3).
Haematoxylin and eosin staining, CD10 staining,
and oestrogen receptor staining showed no between-group
differences (fresh vs slow-frozen, fresh vs
vitrified, and slow-frozen vs vitrified). Moreover,
stromal tissue viability did not differ between the
treatment and saline groups in Phase II.
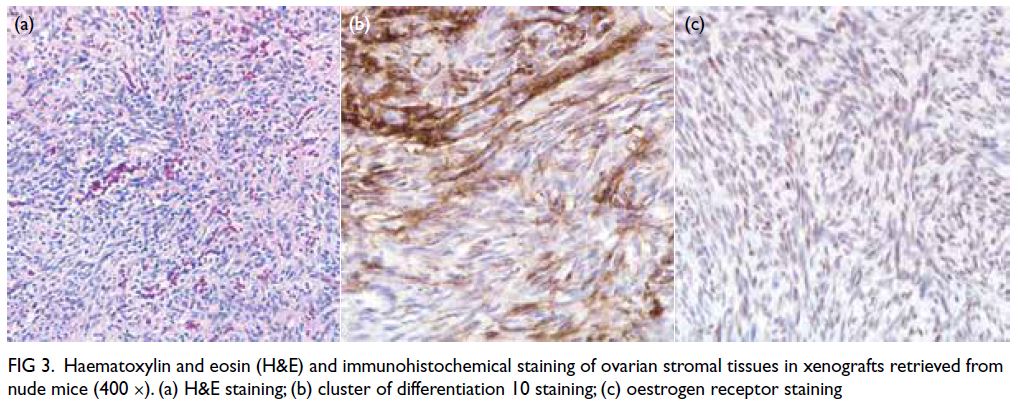
Figure 3. Haematoxylin and eosin (H&E) and immunohistochemical staining of ovarian stromal tissues in xenografts retrieved from nude mice (400 ×). (a) H&E staining; (b) cluster of differentiation 10 staining; (c) oestrogen receptor staining
Follicular histology and density
Retrieved grafts were embedded with paraffin and sectioned at a thickness of 4 or 30 μm. Microscopy
analysis revealed primordial, primary, and secondary
follicles (Fig 4). Tables 1 and 2 show the follicular
densities of retrieved grafts from Phases I and II,
respectively. Primordial follicles were observed in
fresh and cryopreserved grafts from the same patient
(Patient 5), regardless of cryopreservation method
(slow freezing or vitrification) or gonadotrophin
injection status.
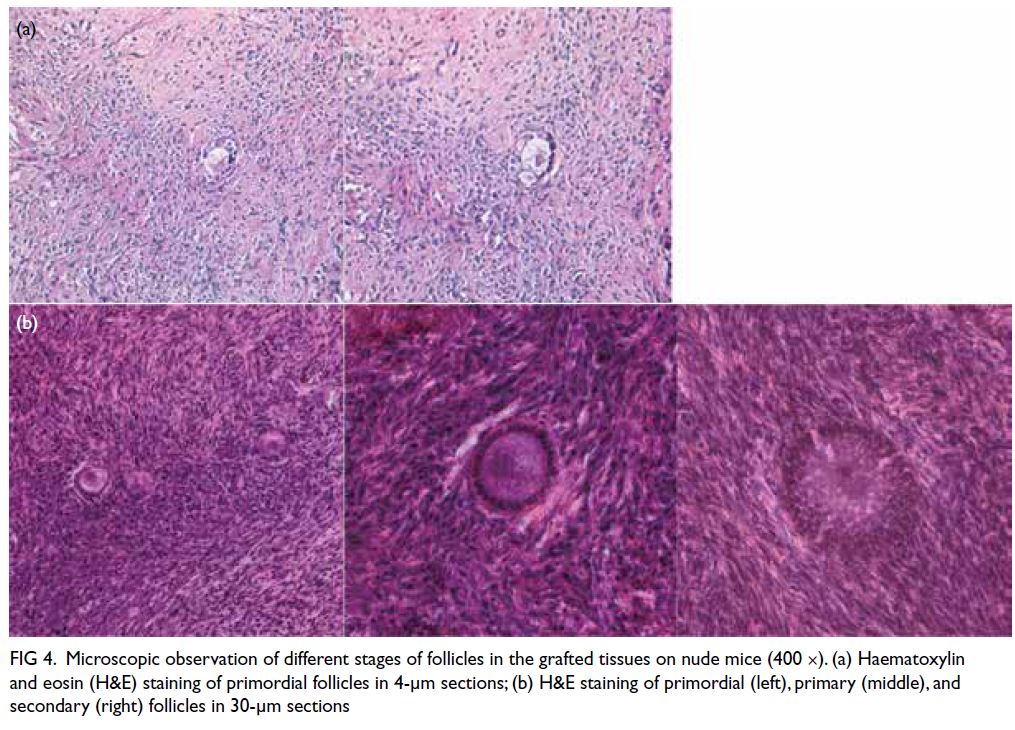
Figure 4. Microscopic observation of different stages of follicles in the grafted tissues on nude mice (400 ×). (a) Haematoxylin and eosin (H&E) staining of primordial follicles in 4-μm sections; (b) H&E staining of primordial (left), primary (middle), and secondary (right) follicles in 30-μm sections
Discussion
Summary of main findings
Our pilot study demonstrated the feasibility of OTC
with subsequent OTT in Hong Kong via xenografts
of fresh and cryopreserved ovarian tissues in nude
mice. Most grafted ovarian tissues remained viable
after engraftment, as demonstrated by CD10 and
oestrogen receptor staining results in stromal tissue,
along with the presence of viable nuclei and the
absence of necrosis, apoptosis, and pyknotic nuclei.
Regardless of cryopreservation method, primordial
follicles were observed in thawed ovarian tissues
after engraftment; thus, both cryopreservation
methods are feasible and effective. There were no
differences in folliculogenesis after gonadotrophin
injection. Overall, these findings validate our
protocol for surgical collection of ovarian tissue,
cryopreservation via slow freezing or vitrification,
and subsequent tissue engraftment into mice; this
protocol successfully generated primordial follicles
in the xenografts. To our knowledge, this type of protocol was not previously validated in Hong Kong.
The benefits of OTC and OTT are not limited
to gynaecology patients; they are also useful for
patients in other specialties4 (eg, medicine, oncology,
and paediatrics), including adolescents,34 as well as
women who cannot undergo ovarian stimulation. To
our knowledge, this is the first study to demonstrate
the feasibility of OTC and OTT in Hong Kong; our
findings support the clinical implementation of these
technologies at medical centres in Hong Kong.
Current condition and success rate of ovarian
tissue cryopreservation
Ovarian tissue cryopreservation has become an
accepted FP technology in many fertility centres
since the removal of its experimental designation.11 14 Notably, OTC allows the preservation of thousands of
primordial follicles in a single procedure; compared
with mature oocytes, preserved primordial follicles
are more resistant to cryodamage.35 This technology
is also appropriate for patients who cannot undergo
ovulation stimulation because they require urgent
chemotherapy or must avoid the enhancement of
a hormone-sensitive malignancy36; it is also the
only available FP technology for prepubertal girls.36
Furthermore, OTC allows natural conception;
several spontaneous pregnancies have been reported
after successful orthotopic autotransplantation.9 37 In
some instances, both fertility and gonadal function
are restored.34 According to a meta-analysis,38
endocrine function was restored in 63.9% of patients;
the combined rate of pregnancies and live births was 28.4%. Dolmans et al9 also reported that 26%
of women became pregnant and gave birth to one
or two infants after the transplantation of frozen-thawed
ovarian tissue; the live birth rate was 30.6%.
Barriers to clinical implementation of
ovarian tissue cryopreservation
Despite its advantages, there are multiple barriers to the clinical implementation of OTC. Effective use of this technology involves two surgical procedures:
the initial removal of ovarian tissue (prior to
cryopreservation) and a future transplantation
procedure, which may cause surgical and ethical
problems (particularly in prepubertal patients).39
The technology also requires expertise that is not
available in some parts of Asia. A Japanese group
reported a live birth in 201540; another successful
live birth was reported by a Chinese group in 2021,41 involving the cryopreserved ovarian tissue bank
established by the Beijing Obstetrics and Gynecology
Hospital.12 However, OTC is not widely available in
Hong Kong. Reproductive health centres in Hong
Kong may lack sufficient surgical expertise and/or
an optimal cryopreservation environment.11 Thus,
there is a need to reduce the obstacles to clinical
implementation of OTC. From our experience, in
terms of laboratory requirements, the protocol,
equipment, and consumables can be incorporated
into most assisted reproductive technology units.
However, practical education is needed regarding
OTC, including tissue management (eg, tissue
thinning during removal of the medulla) and
specific aspects of cryopreservation. Proper records
of success measures (eg, freeze-thaw outcomes
and graft survival rate) are essential; these data
should be carefully documented in laboratory
records. Additional equipment is also needed for
the clinical implementation of OTC as a routine
service because the harvesting surgery may be
performed on an urgent basis that differs from the
routine assisted reproductive technology laboratory
programme.11 While planning for this study, we
found that there have been inconsistencies in terms
of selection criteria, cryopreservation methods,
laboratory management of harvested tissue, and
the transplantation technique itself. Although we
found no differences in the morphological integrity
of ovarian tissue after cryopreservation via slow
freezing or vitrification, further studies with larger
numbers of patients are needed to confirm the
feasibility of follicular stimulation in vivo.
Current status of ovarian tissue
transplantation
Human OTT remains unavailable in Hong Kong.
Notably, our analysis of tissue engraftment was
conducted in a mouse model. After the clinical
implementation of OTC in Hong Kong, OTT
involving autotransplantation could be established
as a routine service. Ovarian autotransplantation
is performed when a patient has fully recovered
from disease, but this approach may carry a
small risk of reintroducing malignant cells in
patients with cancer.42 The results of some studies
have suggested that the risk of reintroducing
malignant cells could be minimised by meticulous
examination of representative biopsy samples via
histology, immunohistochemistry, and molecular
biology techniques.43 Moreover, optical coherence
tomography can be used to assess malignant cells in
thawed ovarian tissue before transplantation.44
Limitations
There were several limitations in this study. First, tissue engraftment was not conducted in humans
because of ethical concerns; thus, we analysed tissue engraftment in a nude mouse xenograft model,
which was the best available model. Second, some
ovarian tissues were collected from transgender
individuals who had undergone testosterone
replacement therapy, which might have affected
the hormonal milieu of the ovarian tissue.45 Third,
we only retrieved small fragments of ovarian tissue
(~1 cm) from random locations in the ovaries of
included patients; this may have led to sampling
error if the sampled cortical layers did not contain
primordial follicles. Fourth, the small number of
included patients hindered our ability to compare the
effects of cryopreservation methods on tissue from a
single patient. Moreover, the small sample size might
have reduced the strength of the findings. Finally,
there are no standardised protocols for freezing,
gonadotrophin stimulation, or transplantation in
nude mouse xenograft models. However, our study
demonstrated the feasibility of OTC in our centre.
Further randomised controlled trials are needed to
confirm our findings.
Future trends
We plan to conduct a randomised controlled trial
of the two cryopreservation methods used in
this study to determine which is best for clinical
implementation. From the experience of the Danish
group Rosendahl et al24 on OTC, they suggested
that before applying the technique to humans, each
laboratory should thoroughly test and validate the
OTC method. In the future, implantation of artificial
ovaries or the engraftment of human ovarian tissue
into mice may enable fertility restoration without the
potential reintroduction of malignant cells. These
approaches may be particularly useful in women
with a high risk of blood-borne leukaemia or cancers
with a high risk of ovarian metastasis, as well as
women who cannot undergo autotransplantation.46
Conclusion
Our study demonstrated the feasibility and viability of OTC with subsequent OTT in Hong Kong via
xenografts in nude mice. These findings support
the clinical implementation of OTC and subsequent
OTT in Hong Kong, particularly for prepubertal
young girls and for women who cannot undergo the
ovarian stimulation necessary for oocyte or embryo
freezing. Further studies are needed to clarify optimal
cryopreservation and engraftment protocols.
Author contributions
Concept or design: JPW Chung, DYL Chan.
Acquisition of data: All authors.
Analysis or interpretation of data: JPW Chung, DYL Chan, Y Song, MBW Leung, JJX Li, CC Wang.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: JPW Chung, DYL Chan, CC Wang.
Acquisition of data: All authors.
Analysis or interpretation of data: JPW Chung, DYL Chan, Y Song, MBW Leung, JJX Li, CC Wang.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: JPW Chung, DYL Chan, CC Wang.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, JPW Chung was not involved in the peer review process of the article. All other authors have no conflicts of interest to disclose.
Funding/support
This research was supported by Basecare Medical Device Co., Ltd. and the Theme-based Research Scheme funded by the
Research Grants Council of the Hong Kong SAR Government
(Ref No.: T13-602/21-N).
Ethics approval
The research was approved by the Institutional Review Board of the Joint Chinese University of Hong Kong–New
Territories East Cluster Clinical Research Ethics Committee
(Ref No.: 2019.356) and overseen by an independent data and
safety monitoring committee. The trial was registered with
the World Health Organization Primary Registry–Chinese
Clinical Trials Registry (Trial No.: ChiCTR2100041611). The
experimental animal protocol was approved by The Chinese
University of Hong Kong Animal Experimentation Ethics
Committee (Ref No.: 19-214-MIS). All participants received a
detailed explanation of the study and provided written consent
for inclusion. All researchers involved in animal experiments
were licensed by the Department of Health of the Hong Kong
SAR Government.
References
1. Maltaris T, Seufert R, Fischl F, et al. The effect of cancer treatment on female fertility and strategies for preserving fertility. Eur J Obstet Gynecol Reprod Biol 2007;130:148-55. Crossref
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. Crossref
3. Brydøy M, Fosså SD, Dahl O, Bjøro T. Gonadal dysfunction and fertility problems in cancer survivors. Acta Oncol 2007;46:480-9. Crossref
4. ESHRE Guideline Group on Female Fertility Preservation, Anderson RA, Amant F, et al. ESHRE guideline: female fertility preservation. Hum Reprod Open 2020;2020:hoaa052. Crossref
5. Chung JP, Lao TT, Li TC. Evaluation of the awareness of,
attitude to, and knowledge about fertility preservation in
cancer patients among clinical practitioners in Hong Kong.
Hong Kong Med J 2017;23:556-61. Crossref
6. Ng EY, Ip JK, Mak DR, Chan AY, Chung JP. Awareness of fertility preservation among Chinese medical students.
Hong Kong Med J 2020;26:184-91. Crossref
7. Yeung SY, Ng EY, Lao TT, Li TC, Chung JP. Fertility preservation in Hong Kong Chinese society: awareness, knowledge and acceptance. BMC Womens Health 2020;20:86. Crossref
8. Dolmans MM, Donnez J. Fertility preservation in women for medical and social reasons: oocytes vs ovarian tissue. Best Pract Res Clin Obstet Gynaecol 2021;70:63-80. Crossref
9. Dolmans MM, von Wolff M, Poirot C, et al. Transplantation
of cryopreserved ovarian tissue in a series of 285 women:
a review of five leading European centers. Fertil Steril 2021;115:1102-15. Crossref
10. Harzif AK, Santawi VP, Maidarti M, Wiweko B. Investigation of each society for fertility preservation in
Asia. Front Endocrinol (Lausanne) 2019;10:151. Crossref
11. Takae S, Lee JR, Mahajan N, et al. Fertility preservation for child and adolescent cancer patients in Asian countries. Front Endocrinol (Lausanne) 2019;10:655. Crossref
12. Jin F, Ruan X, Du J, et al. Analysis on the characteristics of
the patients and effects of ovarian tissue cryopreservation
in the first ovarian tissues cryopreservation bank in China
[in Chinese]. J Capital Univ Med Sci 2021;42:521-5.
13. Donnez J, Dolmans MM. Fertility preservation in women.
N Engl J Med 2017;377:1657-65. Crossref
14. Practice Committee of the American Society for
Reproductive Medicine. Fertility preservation in patients
undergoing gonadotoxic therapy or gonadectomy: a
committee opinion. Fertil Steril 2019;112:1022-33. Crossref
15. Rivas Leonel EC, Lucci CM, Amorim CA. Cryopreservation of human ovarian tissue: a review. Transfus Med Hemother 2019;46:173-81. Crossref
16. Marques LS, Fossati AA, Rodrigues RB, et al. Slow freezing versus vitrification for the cryopreservation of zebrafish (Danio rerio) ovarian tissue. Sci Rep 2019;9:15353. Crossref
17. Glujovsky D, Riestra B, Sueldo C, et al. Vitrification versus slow freezing for women undergoing oocyte cryopreservation. Cochrane Database Syst Rev 2014;2014:CD010047. Crossref
18. Bianchi V, Macchiarelli G, Borini A, et al. Fine morphological assessment of quality of human mature
oocytes after slow freezing or vitrification with a closed
device: a comparative analysis. Reprod Biol Endocrinol
2014;12:110. Crossref
19. Turan V, Oktay K. Sexual and fertility adverse effects associated with chemotherapy treatment in women. Expert Opin Drug Saf 2014;13:775-83. Crossref
20. Planer Ovarian Tissue Cryopreservation Scientific
Roundup. Ovarian Tissue Cryopreservation Scientific
Roundup. 2019. Available from: https://mail.planer.com/__80258426005B6A5E.nsf/0/C4C23666734672DF802584B1002F3777/$File/Ai091V1-Ovarian-Tissue-Cryopreservation-Scientific-Round-Up-28th-October-2019.pdf?OpenElement. Accessed 1 Mar 2022. Crossref
21. Kitazato Corporation. Ova Cryo Kit—Ovarian Tissue
Vitrification Kit. 2021. Available from: https://www.kitazato.co.jp/en/products/vitrification/ova-cryo-kit-type-m. Accessed 31 January 2023.
22. Huang L, Mo Y, Wang W, Li Y, Zhang Q, Yang D.
Cryopreservation of human ovarian tissue by solid–surface vitrification. Eur J Obstet Gynecol Reprod Biol 2008;139:193-8. Crossref
23. Jensen AK, Kristensen SG, Macklon KT, et al. Outcomes
of transplantations of cryopreserved ovarian tissue to 41
women in Denmark. Hum Reprod 2015;30:2838-45.Crossref
24. Rosendahl M, Greve T, Andersen CY. The safety of
transplanting cryopreserved ovarian tissue in cancer
patients: a review of the literature. J Assist Reprod Genet
2013;30:11-24. Crossref
25. Macklon KT, Jensen AK, Loft A, Ernst E, Andersen CY.
Treatment history and outcome of 24 deliveries worldwide
after autotransplantation of cryopreserved ovarian
tissue, including two new Danish deliveries years after
autotransplantation. J Assist Reprod Genet 2014;31:1557-64. Crossref
26. Dolmans MM, Luyckx V, Donnez J, Andersen CY,
Greve T. Risk of transferring malignant cells with
transplanted frozen–thawed ovarian tissue. Fertil Steril
2013;99:1514-22. Crossref
27. Nisolle M, Casanas-Roux F, Qu J, Motta P, Donnez J.
Histologic and ultrastructural evaluation of fresh and
frozen–thawed human ovarian xenografts in nude mice.
Fertil Steril 2000;74:122-9. Crossref
28. Bosma GC, Fried M, Custer RP, Carroll A, Gibson DM,
Bosma MJ. Evidence of functional lymphocytes in some
(leaky) scid mice. J Exp Med 1988;167:1016-33. Crossref
29. Dath C, Van Eyck AS, Dolmans MM, et al. Xenotransplantation of human ovarian tissue to nude mice: comparison between four grafting sites. Hum Reprod 2010;25:1734-43. Crossref
30. Dittrich R, Lotz L, Fehm T, et al. Xenotransplantation of cryopreserved human ovarian tissue—a systematic review of MII oocyte maturation and discussion of it as a realistic
option for restoring fertility after cancer treatment. Fertil
Steril 2015;103:1557-65. Crossref
31. Bankhead P, Loughrey MB, Fernández JA, et al. QuPath: open source software for digital pathology image analysis. Sci Rep 2017;7:16878. Crossref
32. Schmidt KL, Byskov AG, Nyboe Andersen A, Müller J, Yding Andersen C. Density and distribution of primordial follicles in single pieces of cortex from 21 patients and
in individual pieces of cortex from three entire human
ovaries. Hum Reprod 2003;18:1158-64. Crossref
33. Gougeon A, Chainy GB. Morphometric studies of small follicles in ovaries of women at different ages. J Reprod Fertil 1987;81:433-42. Crossref
34. Anderson RA, Mitchell RT, Kelsey TW, Spears N, Telfer EE,
Wallace WH. Cancer treatment and gonadal function:
experimental and established strategies for fertility
preservation in children and young adults. Lancet Diabetes
Endocrinol 2015;3:556-67. Crossref
35. Anderson RA, Wallace WH, Baird DT. Ovarian cryopreservation for fertility preservation: indications and outcomes. Reproduction 2008;136:681-9. Crossref
36. Jeruss JS, Woodruff TK. Preservation of fertility in patients with cancer. N Engl J Med 2009;360:902-11. Crossref
37. Anderson RA, Wallace WH, Telfer EE. Ovarian tissue cryopreservation for fertility preservation:
clinical and research perspectives. Hum Reprod Open
2017;2017:hox001. Crossref
38. Pacheco F, Oktay K. Current success and efficiency of autologous ovarian transplantation: a meta-analysis.
Reprod Sci 2017;24:1111-20. Crossref
39. Wallace WH, Kelsey TW, Anderson RA. Fertility preservation in pre-pubertal girls with cancer: the role of
ovarian tissue cryopreservation. Fertil Steril 2016;105:6-12. Crossref
40. Suzuki N, Yoshioka N, Takae S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod
2015;30:608-15. Crossref
41. Sun N, Li Z, Pang W, Wang L, Li W. Live birth after
transplantation of cryopreserved ovarian tissue with two-year
follow-up: report of the first Chinese case [in Chinese].
Chin J Reprod Contraception 2021;41:1026-30.
42. Peek R, Eijkenboom LL, Braat DD, Beerendonk CC.
Complete purging of Ewing sarcoma metastases from
human ovarian cortex tissue fragments by inhibiting the
mTORC1 signaling pathway. J Clin Med 2021;10:4362. Crossref
43. Lotz L, Dittrich R, Hoffmann I, Beckmann MW. Ovarian
tissue transplantation: experience from Germany and
worldwide efficacy. Clin Med Insights Reprod Health
2019;13:1179558119867357. Crossref
44. Peters IT, Stegehuis PL, Peek R, et al. Noninvasive detection
of metastases and follicle density in ovarian tissue using
full-field optical coherence tomography. Clin Cancer Res
2016;22:5506-13. Crossref
45. Irwig MS. Testosterone therapy for transgender men. Lancet Diabetes Endocrinol 2017;5:301-11. Crossref
46. Telfer EE, Andersen CY. In vitro growth and maturation of primordial follicles and immature oocytes. Fertil Steril 2021;115:1116-25. Crossref

