© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Ten-year refractive and visual outcomes of
intraocular lens implantation in infants with congenital cataract
Joyce JT Chan, FRCOphth; Emily S Wong, FCOphthHK; Carol PS Lam, FCOphthHK; Jason C Yam, FRCSEd
Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Hong Kong Eye Hospital, Hong Kong
Corresponding author: Dr JC Yam (yamcheuksing@cuhk.edu.hk)
Abstract
Introduction: There is no consensus regarding
optimal target refraction after intraocular lens
implantation in infants. This study aimed to clarify
relationships of initial postoperative refraction with
long-term refractive and visual outcomes.
Methods: This retrospective review included 14
infants (22 eyes) who underwent unilateral or
bilateral cataract extraction and primary intraocular
lens implantation before the age of 1 year. All infants
had ≥10 years of follow-up.
Results: All eyes exhibited myopic shift over a mean
follow-up period of 15.9 ± 2.8 years. The greatest
myopic shift occurred in the first postoperative
year (mean=-5.39 ± +3.50 dioptres [D]), but
smaller amounts continued beyond the tenth
year (mean=-2.64 ± +2.02 D between 10 years
postoperatively and last follow-up). Total myopic
shift at 10 years ranged from -21.88 to -3.75 D
(mean=-11.62 ± +5.14 D). Younger age at operation
was correlated with larger myopic shifts at 1 year
(P=0.025) and 10 years (P=0.006) postoperatively.
Immediate postoperative refraction was a predictor
of spherical equivalent refraction at 1 year
(P=0.015) but not at 10 years (P=0.116). Immediate postoperative refraction was negatively correlated
with final best-corrected visual acuity (BCVA)
(P=0.018). Immediate postoperative refraction of
≥+7.00 D was correlated with worse final BCVA
(P=0.029).
Conclusion: Considerable variation in myopic
shift hinders the prediction of long-term refractive
outcomes in individual patients. When selecting
target refraction in infants, low to moderate
hyperopia (<+7.00 D) should be considered to
balance the avoidance of high myopia in adulthood
with the risk of worse long-term visual acuity related
to high postoperative hyperopia.
New knowledge added by this study
- The greatest myopic shift occurred in the first year after cataract surgery, but smaller shifts continued beyond the tenth year. Overall, 50% of eyes exhibited myopic shift >-2.00 dioptres between the tenth postoperative year and last follow-up.
- Considerable variation in refractive change after intraocular lens implantation in infants aged <1 year hinders the prediction of long-term refractive outcomes in individual patients. Immediate postoperative refraction was not correlated with spherical equivalent refraction at 10 years postoperatively.
- Immediate postoperative refraction of ≥+7.00 dioptres was correlated with worse final visual acuity.
- When selecting target refraction in infants, low to moderate hyperopia (<+7.00 dioptres) should be considered to balance the avoidance of high myopia in adulthood with the risk of worse long-term visual acuity related to high postoperative hyperopia.
Introduction
Appropriate optical correction after cataract
extraction in infants is important for efforts to
avoid amblyopia. Primary intraocular lens (IOL)
implantation allows constant in situ optical correction
during the critical years of visual development,
while avoiding the expenses and compliance issues
associated with contact lenses.1 Disadvantages
include increased rates of surgical complications and re-operations,2 as well as the inability to modify IOL power during ocular growth. A recent report by the
American Academy of Ophthalmology suggested
that IOL implantation can be safely conducted in
children aged >6 months.3 However, because of the
unpredictable nature of ocular growth, it remains
challenging to select a target refraction in infants
that allows achievement of optimal long-term visual
and refractive outcomes.
Surgeons target various initial hyperopia
values, ranging from +5.00 dioptres (D) to +10.50 D,4 5 6 7 8 9
to compensate for the rapid myopic shift that
occurs during infancy. However, prediction of the
myopic shift remains difficult; significant hyperopia
in infants requires stringent optical correction to
prevent amblyopia, and some studies have linked
high initial hyperopia to worse visual acuity.10 11 This
retrospective study aimed to clarify the relationships
of initial postoperative refraction with 10-year
spherical equivalent refraction (SER) and long-term
best-corrected visual acuity (BCVA) after IOL
implantation in infants.
Methods
Inclusion and exclusion criteria
This retrospective study included patients who
underwent unilateral or bilateral congenital cataract
extraction and primary IOL implantation before
the age of 1 year between 1997 and 2009 at a single
secondary and tertiary referral eye centre. Only
patients with ≥10 years of follow-up were included.
Eyes with associated ocular co-morbidities (eg,
persistent foetal vasculature and glaucoma) were
excluded.
Surgical technique and follow-up
The patients’ baseline characteristics (eg, age, axial
length [determined by applanation A-scan biometry],
and keratometry) were recorded. Intraocular lens
powers were calculated using the Sanders–Retzlaff–Kraff II formula. The operating surgeon selected
the target refraction and IOL power, considering
the patient’s age (all cases) and refractive error in
the fellow eye (unilateral cases). All operations
were performed using similar techniques, including
the creation of a 3.0-mm scleral tunnel, anterior
continuous curvilinear capsulorhexis, and lens
removal by automated irrigation and aspiration.
Heparin-surface-modified polymethyl methacrylate
IOLs or acrylic foldable IOLs were implanted. The
IOL was placed in the capsular bag or in the sulcus.
All wounds were sutured. In some cases, primary
posterior curvilinear capsulorhexis and anterior
vitrectomy were performed. Because of reports
that a significant number of eyes in young infants
required secondary posterior capsule opening
despite primary posterior capsulotomy,12 13 this
procedure was omitted in some eyes to increase the
likelihood of achieving capsular IOL implantation.
Postoperatively, all eyes were treated with intensive
topical steroid and antibiotic medication. Patients
were assessed on postoperative day 1, week 1, week
2, and week 4; they were then assessed every 3 to
6 months. When clinically significant posterior
capsular opacification developed, secondary
posterior capsulotomy was performed promptly. Glasses were used for postoperative optical
correction; in some cases, contact lenses were
also used. Amblyopia treatment by patching was
performed as necessary.
Outcome measures and statistical analysis
Spherical equivalent refraction at 2 weeks
postoperatively was regarded as immediate
postoperative refraction. Serial refractions at each
year of postoperative follow-up were recorded, and
SERs were calculated as the algebraic sum of the
sphere and half the cylindrical power. Postoperative
axial length was measured using non-contact optical
biometry, which was less invasive than applanation
biometry.
Statistical analysis was performed using
Microsoft Excel and SPSS (Windows version 21.0;
IBM Corp, Armonk [NY], United States). Best-corrected
visual acuities were converted to logarithm
of the minimum angle of resolution (logMAR)
values for statistical analysis. Correlations between
continuous variables were assessed by Spearman
correlation. Differences between groups were
analysed by the Mann–Whitney U test. Preoperative
axial length and keratometry were compared with
values at the last follow-up using the paired-samples Wilcoxon signed-rank test. The independent-sample
Kruskal–Wallis test was used to compare
10-year SER and BCVA values among groups with
immediate postoperative refraction ≤+3.50 D, +3.50
to +7.00 D, and ≥+7.00 D. Partial correlation analysis
was performed to detect correlations of immediate
postoperative refraction with spherical refraction
at 1 year and 10 years after adjustment for age at
operation. Multiple linear regression was performed
for multivariate analysis of statistically significant
factors identified during univariate analysis. P values
<0.05 were considered statistically significant.
Results
Twenty-two eyes of 14 patients were included in this
study. One eye in one patient with bilateral cataract
was excluded because it was surgically treated after
the patient reached 1 year of age. One eye in another
patient with bilateral cataract was excluded because
it exhibited secondary glaucoma. During surgery,
heparin-surface-modified polymethyl methacrylate
IOLs were implanted in three eyes, whereas acrylic
foldable IOLs were implanted in 19 eyes. The IOL
was placed in the capsular bag in 18 eyes and in the
sulcus in four eyes. Additionally, primary posterior
curvilinear capsulorhexis and anterior vitrectomy
were performed in 13 eyes. For postoperative
optical correction, all 14 patients wore glasses; four
patients (including two with unilateral cataract) also wore contact lenses. Thirteen patients underwent
amblyopia treatment by patching.
The Table summarises the baseline
characteristics, refractive outcomes, and visual
outcomes of eyes included in this study. All 22 eyes
exhibited myopic shift, ranging from -21.88 to -3.75 D
at 10 years. Figures 1 and 2 show the amounts of
myopic shift and SER, respectively, at 1 to 10 years
postoperatively and at last follow-up. The greatest
myopic shift occurred in the first postoperative
year, but smaller shifts continued beyond the tenth
year. Ninety percent of eyes exhibited myopic shift
>-2.00 D between the third postoperative year and
last follow-up (mean myopic shift: -6.40 ± +3.29 D;
range, -12.00 to -1.63 D). These proportions were
82% between the sixth postoperative year and last
follow-up (mean myopic shift: -4.14 ± +2.35 D;
range, -9.38 to -1.13 D), and 50% between the tenth
postoperative year and last follow-up (mean myopic
shift: -2.64 ± +2.02 D; range, -0.125 to -6.75 D).
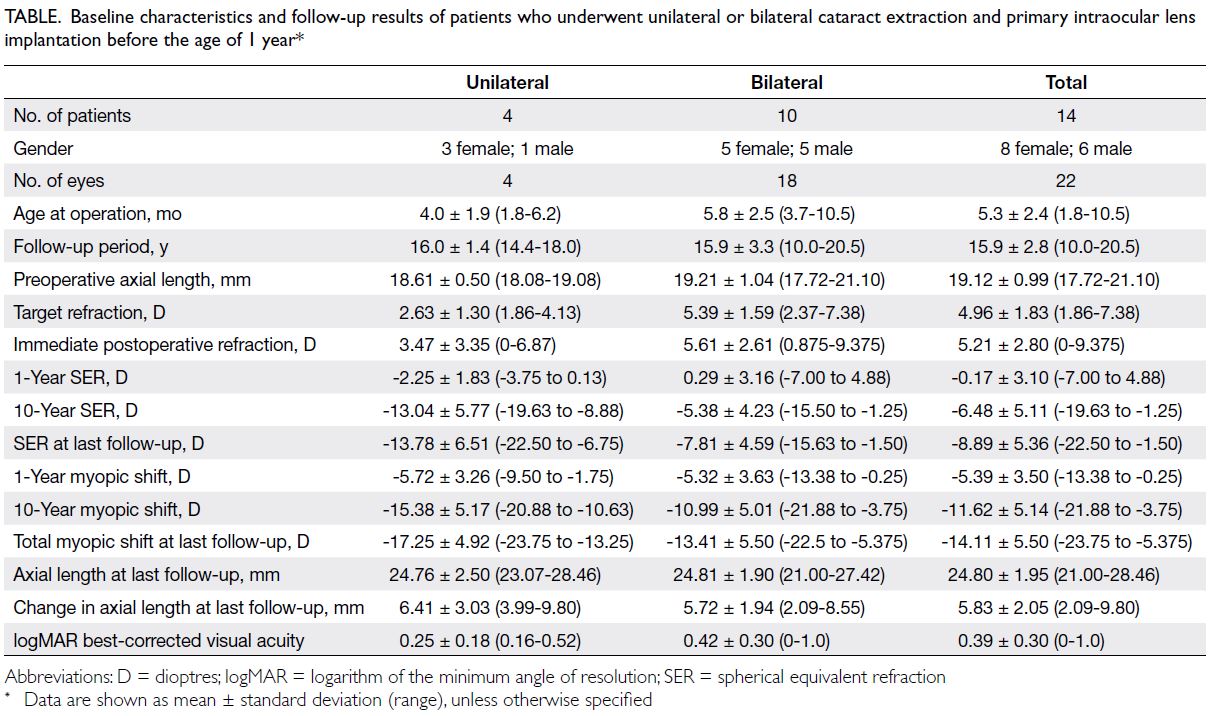
Table. Baseline characteristics and follow-up results of patients who underwent unilateral or bilateral cataract extraction and primary intraocular lens implantation before the age of 1 year
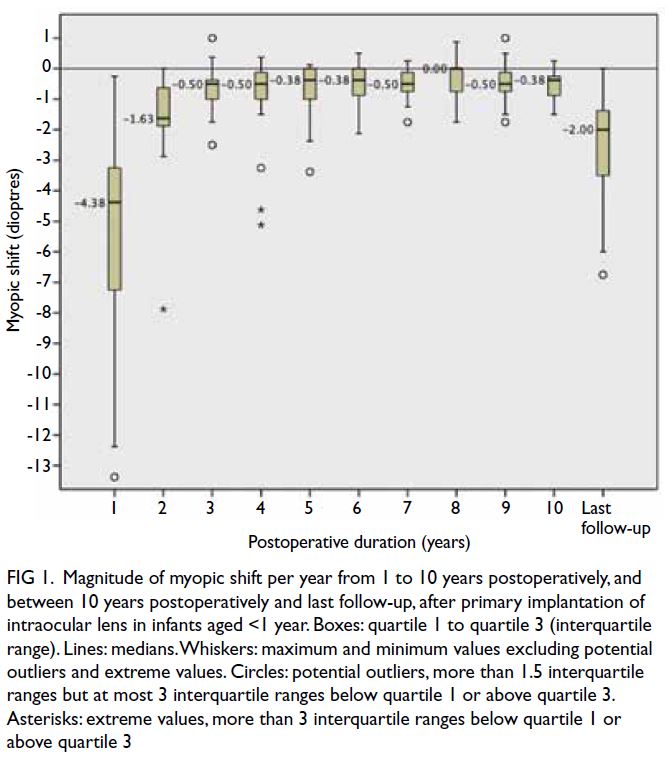
Figure 1. Magnitude of myopic shift per year from 1 to 10 years postoperatively, and between 10 years postoperatively and last follow-up, after primary implantation of intraocular lens in infants aged <1 year. Boxes: quartile 1 to quartile 3 (interquartile range). Lines: medians. Whiskers: maximum and minimum values excluding potential outliers and extreme values. Circles: potential outliers, more than 1.5 interquartile ranges but at most 3 interquartile ranges below quartile 1 or above quartile 3. Asterisks: extreme values, more than 3 interquartile ranges below quartile 1 or above quartile 3
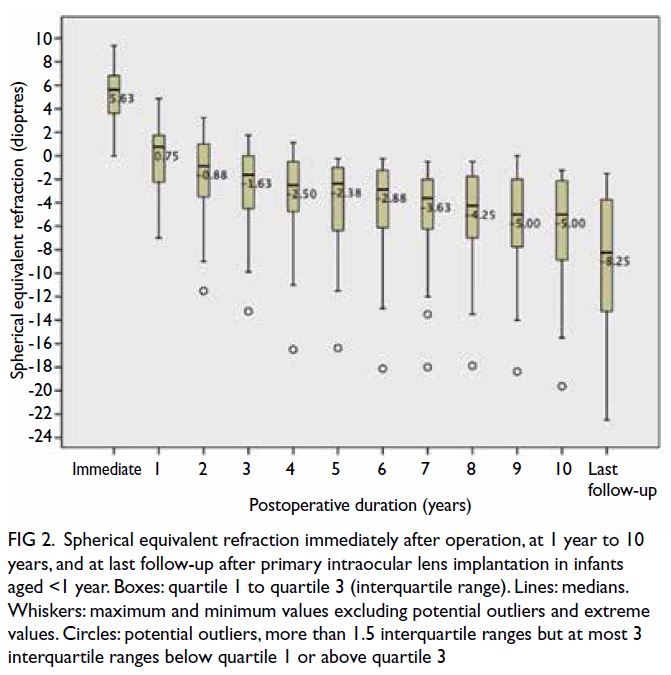
Figure 2. Spherical equivalent refraction immediately after operation, at 1 year to 10 years, and at last follow-up after primary intraocular lens implantation in infants aged <1 year. Boxes: quartile 1 to quartile 3 (interquartile range). Lines: medians. Whiskers: maximum and minimum values excluding potential outliers and extreme values. Circles: potential outliers, more than 1.5 interquartile ranges but at most 3 interquartile ranges below quartile 1 or above quartile 3
Factors affecting myopic shift at 1 year and at
10 years
In univariate analysis, a larger myopic shift at 1 year postoperatively was correlated with younger age
at operation (R2=0.585, P=0.004), more hyperopic
immediate postoperative refraction (R2=-0.533,
P=0.011), and a need for secondary posterior
capsulotomy (U=20, z=-2.066, P=0.04). One-year myopic shift was not correlated with initial axial
length (R2=0.038, P=0.878), and it did not differ
between unilateral (median=-5.81 D) and bilateral
cases (median=-4.38 D) [U=41.5, z=0.469, P=0.652].
Multiple linear regression was performed for
statistically significant factors identified during
univariate analysis. Only age at operation remained
statistically significant (P=0.025); immediate
postoperative refraction (P=0.191) and a need for
secondary posterior capsulotomy (P=0.781) were
not significant in multivariate analysis.
The total amount of myopic shift at 10 years
postoperatively was correlated with age at operation
(R2=0.579, P=0.006), but it was not correlated with
immediate postoperative refraction (R2=-0.339,
P=0.133) or initial axial length (R2=0.291, P=0.241).
There was no difference in the amount of myopic
shift at 10 years between unilateral (median=-14.62
D) and bilateral cases (median=-10.50 D) [U=40.5,
z=1.357, P=0.185] or between eyes that required
secondary posterior capsulotomy (median=-11.25
D) and eyes that did not (median = -6.19 D) [U=24,
z=-1.645, P=0.112].
Factors affecting spherical equivalent refraction at 1 year and at 10 years
Spherical equivalent refraction at 1 year did
not significantly differ between unilateral
(median=-2.69 D) and bilateral cases
(median=+1.13 D) [U=59, z=1.959, P=0.053]
or between eyes that required secondary
capsulotomy (median=+0.31 D) and eyes that
did not (median=+1.42 D) [U=32.5, z=-1.143,
P=0.261]. Partial correlation analysis showed that
after adjustment for age at operation, immediate
postoperative refraction (R2=0.522, P=0.015) was a
statistically significant predictor of SER at 1 year.
In contrast, SER at 10 years postoperatively
was significantly more myopic in unilateral
cases (median=-10.63 D) than in bilateral cases
(median=-4.81 D) [U=49.5, z=2.264, P=0.017]. This
finding may be related to surgeon preference for
less hyperopic target refractions in unilateral cases,
which can match the refraction of the fellow eye
and potentially prevent significant postoperative
anisometropia. Indeed, after adjustment for age,
immediate postoperative SER was significantly
less hyperopic in unilateral cases than in bilateral
cases (P=0.025). A need for secondary posterior
capsulotomy (U=28, z=-1.325, P=0.205) was not
correlated with SER at 10 years. After adjustment
for laterality, both age at operation (P=0.066) and
immediate postoperative refraction (P=0.116) were
not statistically significant predictors of SER at 10
years. There was no significant difference in 10-year
SER among eyes with immediate postoperative
refraction ≤+3.50 D, +3.50 to +7.00 D, and ≥+7.00 D
(P=0.439), as shown in Figure 3.
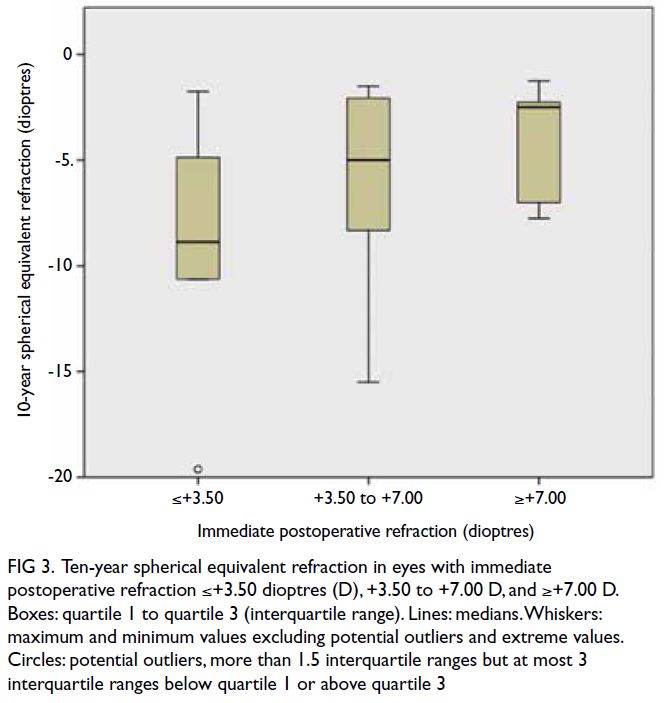
Figure 3. Ten-year spherical equivalent refraction in eyes with immediate postoperative refraction ≤+3.50 dioptres (D), +3.50 to +7.00 D, and ≥+7.00 D. Boxes: quartile 1 to quartile 3 (interquartile range). Lines: medians. Whiskers: maximum and minimum values excluding potential outliers and extreme values. Circles: potential outliers, more than 1.5 interquartile ranges but at most 3 interquartile ranges below quartile 1 or above quartile 3
Subgroup analysis was performed for bilateral
cases only. Multiple regression analysis showed
that at 1 year, both age at operation (P=0.014) and
immediate postoperative refraction (P=0.024)
remained significant predictors of SER after
unilateral cases had been excluded. At 10 years
postoperatively, age at operation was a significant
predictor of SER (P=0.015), whereas immediate
postoperative refraction was not (P=0.135).
Axial length and keratometry
Mean preoperative axial length was 19.12 mm,
whereas mean axial length at 10 years was 24.82 mm.
There were no differences in initial axial length
(U=32, z=0.894, P=0.421) or total axial length change
(U=22, z=-0.224, P=0.875) between unilateral and
bilateral cases. Final axial length was significantly
greater than preoperative axial length (z=3.823,
P<0.0005). Total axial length change was strongly
correlated with total myopic shift (R2=-0.791,
P<0.0005). There was no difference between
preoperative and final keratometry values (z=0.081,
P=0.936). The total change in the mean keratometry
value was not correlated with total myopic shift
(R2=-0.168, P=0.490).
Final best-corrected visual acuity
At the last follow-up, 11 eyes (50%) had a final BCVA
of 0.18 logMAR or better, six eyes (27%) had moderate
amblyopia with BCVA of 0.3 to 0.6 logMAR, and five
eyes (23%) had severe amblyopia with BCVA of 0.7
to 1.0 logMAR. There was a statistically significant
correlation between immediate postoperative
refraction and final BCVA (R2=0.440, P=0.041).
Best-corrected visual acuity was worse in eyes that
required secondary capsulotomy (U=74.5, z=1.995,
P=0.049). Multiple regression revealed that a need for
secondary capsulotomy was no longer a significant
predictor for BCVA (P=0.299), whereas immediate
postoperative refraction remained a significant
predictor for BCVA (P=0.018). Best-corrected
visual acuity was significantly worse in eyes with
immediate postoperative refraction of +7.00 D or
higher than in eyes with lower levels of immediate
postoperative hyperopia (P=0.029) [Fig 4]. There
were no significant correlations of final BCVA with
age at operation (R2=-0.041, P=0.856), SER at 10
years (R2=0.011, P=0.963), SER at last follow-up
(R2=-0.122, P=0.589), or laterality (U=48.5, z=-1.087,
P=0.300).
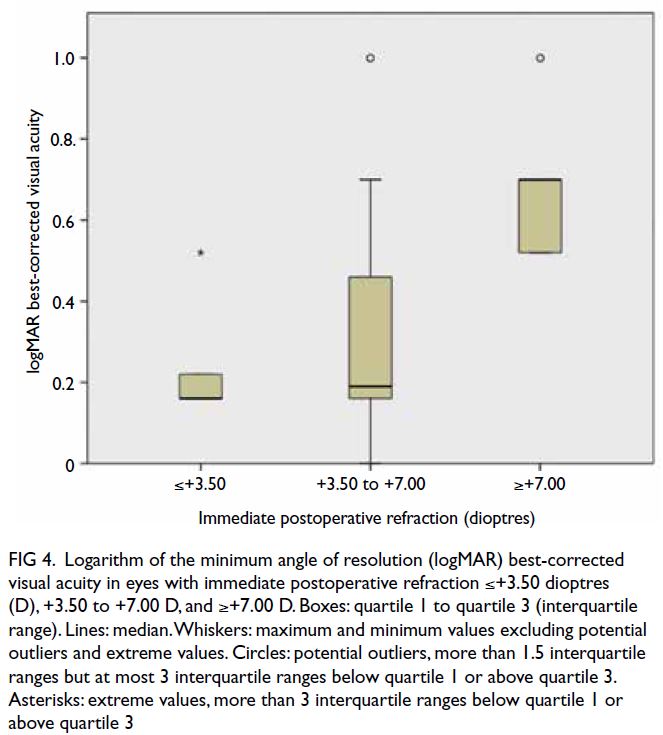
Figure 4. Logarithm of the minimum angle of resolution (logMAR) best-corrected visual acuity in eyes with immediate postoperative refraction ≤+3.50 dioptres (D), +3.50 to +7.00 D, and ≥+7.00 D. Boxes: quartile 1 to quartile 3 (interquartile range). Lines: median. Whiskers: maximum and minimum values excluding potential outliers and extreme values. Circles: potential outliers, more than 1.5 interquartile ranges but at most 3 interquartile ranges below quartile 1 or above quartile 3. Asterisks: extreme values, more than 3 interquartile ranges below quartile 1 or above quartile 3
Complications and re-operations
Seventeen eyes underwent 21 re-operations in total, 17 of which were secondary posterior capsulotomies.
All nine eyes that did not undergo primary posterior
capsulorhexis and anterior vitrectomy required
secondary capsulotomy; one of the nine eyes required
secondary capsulotomy twice. Seven of 13 eyes with primary posterior capsulorhexis and anterior
vitrectomy required secondary capsulotomy. Three
eyes underwent injection of intracameral tissue
plasminogen activator, one eye underwent dissection
of fibrinous membrane, and one eye required
removal of anterior capsular phimosis. Notably,
anterior capsular phimosis did not develop in any
other eyes. One eye developed secondary glaucoma
and was excluded from this study.
Discussion
Two important goals in the management of congenital
cataract include achievement of good long-term
visual acuity and minimisation of refractive error
in adulthood. This study focused on long-term
outcomes after primary IOL implantation in infants,
all of whom had ≥10 years of follow-up. Myopic
shift was present in all eyes, and its magnitude
considerably varied. Immediate postoperative
refraction was not a statistically significant predictor
of SER at 10 years. Moreover, there was a statistically
significant negative correlation between immediate
postoperative refraction and final BCVA. Finally,
immediate postoperative refraction of +7.00 D or
higher was correlated with worse final BCVA.
Refractive change in a growing eye
Refractive change in a normal growing eye involves a
complex interaction among axial length elongation,
corneal curvature flattening, and the reduction of
crystalline lens power.14 Additional effects on ocular
growth (eg, related to the presence or laterality
of congenital cataract, age at corrective surgery,
initial axial length, postoperative visual input, and
compliance with postoperative amblyopia therapy)
remain uncertain.15 The presence of an intraocular
lens magnifies myopic shift in a growing eye—the intraocular lens exhibits constant power and
moves anteriorly away from the retina during ocular
growth, hindering the extrapolation of data from
phakic eyes.5 Figure 5 shows the mean SER during
the first decade of life in pseudophakic eyes from
patients in the present study, compared with normal
eyes from the ongoing population-based Hong Kong
Children Eye Study.16 At 10 years after corrective
surgery, the mean SER was -6.48 D in pseudophakic
eyes, whereas it was -0.72 D in normal eyes of age-matched
children. The mean axial length at 10 years
was 24.82 mm in pseudophakic eyes, whereas it was
23.79 mm in normal eyes of age-matched children.16
These data imply the presence of a greater myopic
shift and greater increase in axial length among
pseudophakic eyes which continues beyond the first
2 years of life; notably, these increases are relative
to the mean growth of normal eyes in Hong Kong
children, who exhibit a higher prevalence of myopia
compared with other populations.16
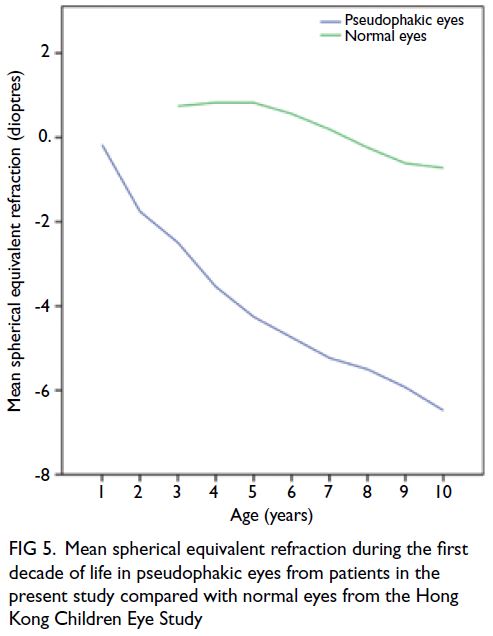
Figure 5. Mean spherical equivalent refraction during the first decade of life in pseudophakic eyes from patients in the present study compared with normal eyes from the Hong Kong Children Eye Study
Refractive change after primary intraocular
lens implantation in infants
Several other studies of myopic shift have revealed
considerable refractive change after primary IOL
implantation in infants aged <1 year. At 5 years
postoperatively, the Infant Aphakia Treatment
Study revealed a mean myopic shift of -8.97 D for
infants surgically treated at the age of 1 month and
-7.22 D for infants surgically treated at the age of
6 months,9 whereas Negalur et al17 found a median
myopic shift of -8.43 D after the same duration of
follow-up in infants operated before the age of
6 months. Fan et al18 reported a mean myopic shift
of -7.11 D at 3 years postoperatively in infants
operated before the age of 1 year; Lu et al19 reported
a mean myopic shift of -6.46 D at 2 years in 22 eyes,
as well as a mean myopic shift of -8.67 D at 6 years
in three eyes, among infants operated between the
age of 6 and 12 months. In our study, which had a
longer follow-up period, the mean 10-year myopic
shift was -11.62 ± 5.14 D and myopic progression
continued beyond 10 years postoperatively. These
findings highlight the importance of using long-term
data to guide management decisions, including the
selection of target refraction and the determination
of appropriate timing for enhancement procedures
(eg, IOL exchange).
Our results showed that myopic shift was
greatest in the first postoperative year and was correlated with age at operation, which is consistent
with findings in the literature.9 10 17 18 19 20 21 Because age
at operation is most frequently associated with the
magnitude of refractive change, many surgeons
prefer to adjust initial hyperopia according to age.
McClatchey et al22 recommended targets of +5.00
to +8.00 D in infants aged <1 year, whereas Valera
Cornejo et al4 selected targets of +7.00 to +9.00 D in
infants of the same age-group. The results of the Infant
Aphakia Treatment Study suggested that, to achieve
emmetropia at 5 years, immediate postoperative
hyperopia should be +10.50 D from 4 to 6 weeks of
age and +8.50 D from 7 weeks to 6 months of age.9
However, our results showed considerable variation
in myopic shift at 10 years (range, -21.88 to -3.75 D);
after adjustment for age, immediate postoperative
refraction was not a statistically significant predictor
of SER at 10 years. Other studies have shown that
initial refraction and IOL undercorrection were not
significantly associated with the magnitude or rate
of myopic shift9 18 22 23; they also revealed large and
unpredictable variations in refractive outcomes
after IOL implantation in young infants.10 20 21 22 24 25
At the 3-year follow-up, refractive change ranged
from +2.00 to -15.50 D in a study by Gouws et al26
and from -0.47 to -10.69 D in a study by Fan et al.18
Although we observed a trend towards more myopic
10-year refractions in groups with lower initial
postoperative hyperopia, there were no significant
differences because of substantial variability in the
data (Fig 3). The Infant Aphakia Treatment Study
showed that the actual and expected amounts
of myopic shift differed in a large percentage of
patients; 50% of patients exhibited differences of
+3.00 to +14.00 D from expected values.9 Therefore,
age-adjusted suggested targets only compensate for
the mean expected myopic shift; large interpatient
variability will often result in unanticipated long-term
outcomes for individual patients. Correlation
analysis in our study revealed that age at operation
only explained 58% of the variance in myopic shift at
10 years. This correlation is presumably influenced by
other factors that contribute to myopic progression,
such as genetics, ethnicity, outdoor exposure,
education level, and extent of near work.27
Long-term best-corrected visual acuity
The achievement of optimal long-term BCVA
is another important goal of surgical treatment
for congenital cataract. In our study, immediate
postoperative refraction of ≥+7.00 D was correlated
with worse BCVA. Similarly, in a study of infants
who underwent surgery between the ages of 2 and
21 months, with ≥4 years of follow-up, Magli et al10
found that BCVA was higher in infants with initial
spherical refraction between +1.00 and +3.00 D than
in infants with initial spherical refraction >+3.00 D.
In a study that included older children who underwent surgery at or before the age of 8.5 years,
Lowery et al11 found that low early postoperative
hyperopia (+1.75 to +5.00 D) yielded better longterm
BCVA, compared with refractions <+1.75
or >+5.00 D in unilateral cases; no difference was
observed in bilateral cases. Another study of older
children (surgically treated between the ages of
2 and 6 years) revealed no difference in BCVA
between initial postoperative refractive errors of
near emmetropia versus undercorrection of +2.00
to +5.50 D23; no patients had initial refraction values
>+5.50 D. High initial postoperative hyperopia
requires good compliance with refractive correction;
in infants, such hyperopia also requires amblyopia
treatment because younger children are at higher
risk of developing amblyopia. Hyperopia is more
amblyogenic than myopia because young children
have higher demands for near vision28; moreover,
hyperopia causes defocusing in both distance and
near vision, particularly among patients who exhibit
pseudophakia related to accommodation loss.
Studies have shown variable compliance with optical
correction and amblyopia treatment after congenital
cataract surgery19 29; the use of high-plus spectacles
is associated with various optical aberrations.
Additionally, contact lenses are suboptimal
because one of the original aims of intraocular lens
implantation is to avoid the need for contact lens.
Myopia is comparatively less amblyogenic because
it allows retention of near vision, particularly if
the amount of myopia remains low until later
in childhood when visual development is more
mature.30 Therefore, parental motivation and the
likelihood of compliance should be included in
decisions regarding postoperative refraction. Ideally,
high myopia in adulthood should be minimised.
However, this goal should be balanced with the risks
of amblyopia and long-term poor vision. Therefore,
the selection of high hyperopia (>+7.00 D) as an
initial postoperative target refraction should be
avoided when possible.
Strengths and limitations
A major strength of our study was the long follow-up period. Additionally, it only included infants who
underwent IOL implantation before the age of 1 year
because the refractive change in this group exhibits
the greatest variability and is most challenging to
predict.5
There were some limitations in this study. First,
it used a retrospective design and included a small
number of patients. Second, there was no objective
monitoring of compliance with optical correction or
amblyopia treatment. Third, few unilateral cases were
included, which may have hindered the detection
of larger myopic shifts in post–IOL implantation
in unilateral cases. Notably, some previous studies
revealed larger myopic shifts after IOL implantation in such cases.4 17 21 22
In conclusion, the large and variable refractive
change after IOL implantation in infants aged <1
year hinders the prediction of long-term refractive
outcomes in individual patients. When selecting
target refraction in infants, low to moderate
hyperopia (<+7.00 D) should be considered to
balance the avoidance of high myopia in adulthood
with the risk of worse long-term visual acuity related
to high postoperative hyperopia
Author contributions
Concept or design: All authors.
Acquisition of data: JJT Chan.
Analysis or interpretation of data: JJT Chan.
Drafting of the manuscript: JJT Chan.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: JJT Chan.
Analysis or interpretation of data: JJT Chan.
Drafting of the manuscript: JJT Chan.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
As an editor of the journal, JC Yam was not involved in the peer review process. Other authors have disclosed no conflicts of interest.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Ethics approval was granted by the Research Ethics Committee (Kowloon Central/Kowloon East), Hospital Authority (Ref No.: KC/KE-19-0059/ER-4). The requirement for patient consent was waived by the ethics board due to the retrospective nature of the study. The
study is conducted in accordance with the ethical principles of
the Declaration of Helsinki.
References
1. Kumar P, Lambert SR. Evaluating the evidence for and against the use of IOLs in infants and young children.
Expert Rev Med Devices 2016;13:381-9. Crossref
2. Infant Aphakia Treatment Study Group; Lambert SR,
Lynn MJ, et al. Comparison of contact lens and intraocular
lens correction of monocular aphakia during infancy: a
randomized clinical trial of HOTV optotype acuity at
age 4.5 years and clinical findings at age 5 years. JAMA
Ophthalmol 2014;132:676-82. Crossref
3. Lambert SR, Aakalu VK, Hutchinson AK, et al. Intraocular
lens implantation during early childhood: a report by the
American Academy of Ophthalmology. Ophthalmology
2019;126:1454-61. Crossref
4. Valera Cornejo DA, Flores Boza A. Relationship between
preoperative axial length and myopic shift over 3 years after
congenital cataract surgery with primary intraocular lens
implantation at the National Institute of Ophthalmology of
Peru, 2007-2011. Clin Ophthalmol 2018;12:395-9. Crossref
5. McClatchey SK, Parks MM. Theoretic refractive changes after lens implantation in childhood. Ophthalmology
1997;104:1744-51. Crossref
6. Enyedi LB, Peterseim MW, Freedman SF, Buckley EG. Refractive changes after pediatric intraocular lens
implantation. Am J Ophthalmol 1998;126:772-81. Crossref
7. Astle WF, Ingram AD, Isaza GM, Echeverri P. Paediatric pseudophakia: analysis of intraocular lens power and myopic shift. Clin Experiment Ophthalmol 2007;35:244-51. Crossref
8. Yam JC, Wu PK, Ko ST, Wong US, Chan CW. Refractive changes after pediatric intraocular lens implantation in
Hong Kong children. J Pediatr Ophthalmol Strabismus
2012;49:308-13.
9. Weakley DR Jr, Lynn MJ, Dubois L, et al. Myopic shift 5 years after intraocular lens implantation in the Infant Aphakia Treatment Study. Ophthalmology 2017;124:822-7. Crossref
10. Magli A, Forte R, Carelli R, Rombetto L, Magli G. Long-term outcomes of primary intraocular lens implantation for unilateral congenital cataract. Semin Ophthalmol
2015;31:1-6. Crossref
11. Lowery RS, Nick TG, Shelton JB, Warner D, Green T. Long-term
visual acuity and initial postoperative refractive error
in pediatric pseudophakia. Can J Ophthalmol 2011;46:143-7. Crossref
12. Vasavada A, Chauhan H. Intraocular lens implantation in infants with congenital cataracts. J Cataract Refract Surg 1994;20:592-8. Crossref
13. Plager DA, Yang S, Neely D, Sprunger D, Sondhi N. Complications in the first year following cataract surgery with and without IOL in infants and older children. J
AAPOS 2002;6:9-14. Crossref
14. Gordon RA, Donzis PB. Refractive development of the human eye. Arch Ophthalmol 1985;103:785-9. Crossref
15. Indaram M, VanderVeen DK. Postoperative refractive
errors following pediatric cataract extraction with
intraocular lens implantation. Semin Ophthalmol
2018;33:51-8. Crossref
16. Yam JC, Tang SM, Kam KW, et al. High prevalence of
myopia in children and their parents in Hong Kong
Chinese population: the Hong Kong Children Eye Study.
Acta Ophthalmol 2020;98:e639-48. Crossref
17. Negalur M, Sachdeva V, Neriyanuri S, Ali M, Kekunnaya R.
Long-term outcomes following primary intraocular lens
implantation in infants younger than 6 months. Indian J
Ophthalmol 2018;66:1088-93. Crossref
18. Fan DS, Rao SK, Yu CB, Wong CY, Lam DS. Changes in
refraction and ocular dimensions after cataract surgery
and primary intraocular lens implantation in infants. J
Cataract Refract Surg 2006;32:1104-8. Crossref
19. Lu Y, Ji YH, Luo Y, Jiang YX, Wang M, Chen X. Visual
results and complications of primary intraocular lens
implantation in infants aged 6 to 12 months. Graefes Arch
Clin Exp Ophthalmol 2010;248:681-6. Crossref
20. O’Keefe M, Fenton S, Lanigan B. Visual outcomes and
complications of posterior chamber intraocular lens
implantation in the first year of life. J Cataract Refract Surg
2001;27:2006-11. Crossref
21. Hoevenaars NE, Polling JR, Wolfs RC. Prediction error and myopic shift after intraocular lens implantation in paediatric cataract patients. Br J Ophthalmol 2011;95:1082-5. Crossref
22. McClatchey SK, Dahan E, Maselli E, et al. A comparison of the rate of refractive growth in pediatric aphakic and pseudophakic eyes. Ophthalmology 2000;107:118-22. Crossref
23. Lambert SR, Archer SM, Wilson ME, Trivedi RH, del Monte
MA, Lynn M. Long-term outcomes of undercorrection
versus full correction after unilateral intraocular lens
implantation in children. Am J Ophthalmol 2012;153:602-8.e1. Crossref
24. Barry JS, Ewings P, Gibbon C, Quinn AG. Refractive outcomes after cataract surgery with primary lens
implantation in infants. Br J Ophthalmol 2006;90:1386-9. Crossref
25. Plager DA, Kipfer H, Sprunger DT, Sondhi N, Neely DE. Refractive change in pediatric pseudophakia: 6-year
follow-up. J Cataract Refract Surg 2002;28:810-5. Crossref
26. Gouws P, Hussin HM, Markham RH. Long term results of primary posterior chamber intraocular lens implantation for congenital cataract in the first year of life. Br J
Ophthalmol 2006;90:975-8. Crossref
27. Morgan IG, French AN, Ashby RS, et al. The epidemics
of myopia: aetiology and prevention. Prog Retin Eye Res
2018;62:134-49. Crossref
28. Pascual M, Huang J, Maguire MG, et al. Risk factors for amblyopia in the vision in preschoolers study.
Ophthalmology 2014;121:622-9.e1. Crossref
29. Drews-Botsch CD, Hartmann EE, Celano M, Infant
Aphakia Treatment Study Group. Predictors of adherence
to occlusion therapy 3 months after cataract extraction in
the Infant Aphakia Treatment Study. J AAPOS 2012;16:150-5. Crossref
30. O’Hara MA. Pediatric intraocular lens power calculations. Curr Opin Ophthalmol 2012;23:388-93. Crossref

