Hong Kong Med J 2023 Feb;29(1):16-21 | Epub 3 Feb 2023
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE CME
Cost-minimisation analysis of intravenous versus
subcutaneous trastuzumab regimen for breast cancer management in Hong Kong
Vivian WY Lee, PharmD1; Franco WT Cheng, MClinPharm2
1 Centre for Learning Enhancement And Research, The Chinese University of Hong Kong, Hong Kong
2 Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong
Corresponding author: Prof Vivian WY Lee (vivianlee@cuhk.edu.hk)
Abstract
Introduction: In 2017, breast cancer was the most
common cancer and third leading cause of cancer
death among women in Hong Kong. Approximately
20% of patients were human epidermal growth
factor receptor-2 (HER2)-positive. This study was
conducted to investigate cost differences between
intravenous and subcutaneous trastuzumab
regimens in Hong Kong using medical resources
utilisation data from other countries.
Methods: A cost-minimisation model was developed
to compare the cost of total care, including direct
medical cost and full-time equivalent (FTE) hours.
The drug acquisition cost was obtained from the
manufacturer, whereas the costs for hospitalisation
and clinic visits were acquired from the Hong Kong
Gazette. Time (in FTE hours) was determined by
literature review. All costs were expressed in US
dollars (US$1 = HK$7.8). Costs were not discounted
because of the short time horizon. One-way
deterministic sensitivity analysis was performed to
identify the effects of changes in drug acquisition
cost, changes in FTE hours (based on confidence
intervals reported), and changes in body weight
(±20%).
Results: Literature review indicated that 0.18 FTE
hour of nursing time (7.9 hours) and 0.14 FTE hour
of pharmacist time (6.2 hours) could be saved each
week if the subcutaneous formulation was used.
Using data in 2017, after 18 cycles of treatment with
subcutaneous trastuzumab, the drug acquisition and
healthcare professional time costs were reduced by
US$9451.28 and US$566.16, respectively, yielding an
annual savings of over US$8 million.
Conclusion: The subcutaneous formulation of
trastuzumab is a potential cost-saving therapy for
HER2-positive breast cancer patients in Hong Kong.
The drug acquisition cost was the parameter with
the greatest effect on the total cost of treatment.
New knowledge added by this study
- The results of this study suggest that the subcutaneous formulation of trastuzumab would be a cost-saving therapy for HER2-positive breast cancer patients in Hong Kong.
- The drug acquisition cost was the parameter with the greatest effect on the total cost of treatment.
- The high drug acquisition cost of trastuzumab may prevent patients from receiving effective treatment.
- The subcutaneous formulation of trastuzumab is expected to remain more cost-effective, despite the potential emergence of biosimilar trastuzumab.
Introduction
In 2017, breast cancer was the most common cancer
and third leading cause of cancer death among
women in Hong Kong.1 Additionally, an estimated
20% of breast cancers in Hong Kong were human
epidermal growth factor receptor-2 (HER2)-positive.2 3
Intravenous (IV) trastuzumab, in combination
with chemotherapy, is licensed for the treatment
of HER2-positive early-stage breast cancer and
metastatic breast cancer. It must be reconstituted
into solution for loading dose infusion over a duration of 90 minutes, followed by maintenance
dose infusion over a duration of 30 minutes.4
Additionally, IV trastuzumab is dosed according to
each patient’s body weight, with a loading dose of
8 mg/kg followed by a maintenance dose of 6 mg/kg
every 3 weeks.4 This regimen consumes considerable
healthcare resources, including drug preparation
and administration time, clinic and chair time, and
physician time dedicated to patient interaction.5
A fixed-dose subcutaneous (SC) formulation
of trastuzumab was developed to allow drug
administration over approximately 5 minutes, which is much shorter than the duration of IV infusion. The
600-mg dose of SC trastuzumab every 3 weeks is non-dinferior
to the IV formulation with respect to efficacy
and tolerability.6 7 Furthermore, approximately 90%
of patients preferred SC over IV administration
of trastuzumab in the PrefHer (Preference for
subcutaneous or intravenous administration of
trastuzumab in patients with HER2-positive early
breast cancer) randomised crossover trials,8 9
which were designed to assess patient preference
and healthcare professional satisfaction with both
treatment options.
Data from other countries have demonstrated
that for SC formulation of trastuzumab, less time is
required for drug preparation and administration;
moreover, fewer consumables are used.10 11 12 13 A cost-minimisation
analysis (CMA) study in Greece
demonstrated that the total cost of therapy per
patient was 21 870 euros (€) when using the SC
formulation of trastuzumab, whereas it was €23 118
when using the IV formation of trastuzumab.
The investigators concluded that use of the SC
formulation of trastuzumab would provide cost
savings for the Greek healthcare system.10 A study
in Spain revealed similar findings: the use of the
SC formulation of trastuzumab led to a 19.4 to
28.8% cost savings in the hospital.11 Additionally, a
time-and-motion study in New Zealand compared
medical resource utilisation between the IV and SC
formulations of trastuzumab in patients with HER2-positive breast cancer. The potential cost saving
was NZ$96.94 per patient per cycle.12 Furthermore,
a time-and-motion sub-study13 from the PrefHer
trials involving eight countries (Canada, France,
Switzerland, Denmark, Italy, Russia, Spain, and
Turkey) demonstrated time savings for patient chair,
administration by healthcare professionals, and drug
preparation.
The SC formulation of trastuzumab is expected
to provide cost savings in other countries. However,
healthcare systems and modes of clinical services
differ between Hong Kong and other countries.
Therefore, this study was conducted to investigate
cost differences between IV and SC trastuzumab
regimens in Hong Kong medical settings, using
medical resources utilisation data from other
countries.
Methods
Cost methods and data sources
A CMA model was developed to compare the cost
of total care. The CMA approach was used because
the clinical efficacy and safety profiles of IV and SC
trastuzumab regimens are similar, as demonstrated
in the previous studies7 14 15; this fulfils the CMA
requirement for two treatments to demonstrate
similar efficacy. The following steps were followed
in the CMA. We compared direct medical costs
related to the IV and SC trastuzumab regimens
that produced equivalent health outcomes. The
CMA solely focuses on selection of the least costly
option. In this study, the CMA was conducted
from a hospital perspective. All direct medical
costs and full-time equivalent (FTE) hours were
included in this study. Drugs, clinic visits for drug
administration, specialist out-patient clinic visits,
and consumables were regarded as direct medical
costs. The time horizon was 18 cycles of treatment,
which mimics the duration of treatment for early-stage
HER2-positive breast cancer. Drug acquisition
cost data were obtained from the manufacturer,
whereas costs for hospitalisation and clinic visits
were acquired from the 2017 Hong Kong Gazette.16
The drug acquisition cost was based on the dose
used in previous clinical trials: IV loading dose of
8 mg/kg and maintenance dose of 6 mg/kg every
3 weeks versus SC fixed dose of 600 mg every
3 weeks. A mean body weight of 57.3 kg was used,
based on data from the 2016 Hong Kong Cancer
Registry.3
Estimated FTE hour values were obtained
from previous literature. These values were regarded
as the time (in hours) required for drug preparation
and administration, divided by 44 hours, the weekly
average working hours for such tasks. The FTE hour
values were then converted to monetary values,
calculated as the median hourly rate received by individuals in each position. In Hong Kong, nurses
and pharmacists are mainly involved in drug
preparation and administration; thus, the salaries of
these positions were used for estimation of FTE hour
values.
All costs were expressed in US dollars (US$1 = HK$7.8), using 2016 as the fiscal year. Because of
the short time horizon in the study, no costs were
discounted.
Literature review
Medical resources and FTE hour values were
determined by literature review in Embase and
MEDLINE, using the key words ‘subcutaneous’,
‘trastuzumab’, ‘time’, ‘cost’, and ‘medical resources’.
Statistical analyses
The CMA was conducted from the healthcare payer
perspective. All continuous variables were described
as means ± standard deviations and medians with
ranges.
A drug budget impact forecast analysis was
performed to determine how changes in the total
cost of treatment regimens, including direct medical
costs and FTE hours, would impact healthcare
expenditures in Hong Kong. Each individual
parameter, namely drug acquisition cost for each
formulation (±20%), patient body weight (±20%),
and time and consumables reported in the literature
(based on confidence intervals reported) were analysed independently within specified ranges,
whereas other factors were fixed at base-case values.
The analysis parameters were chosen based on the
findings in previous cost-effectiveness studies.17 A
simulation model was used to run 10 000 iterations
of the forecast model; for each iteration, model
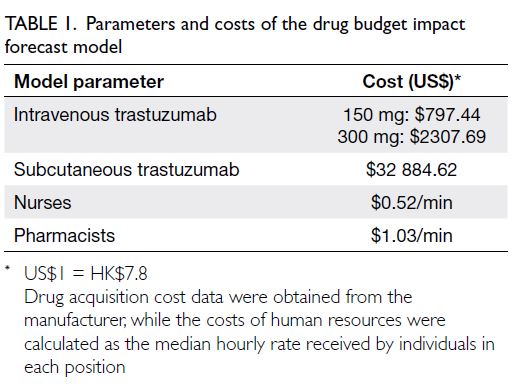
parameters were input as shown in Table 1. We
assumed that cost changes were consistent with
the beta distribution around the mean. One-way
deterministic sensitivity analysis was also performed
to evaluate the extent to which the total cost would
be affected by changes in the drug acquisition cost
for each formulation (±20%), changes in times and
consumables obtained from literature (based on
confidence intervals reported), and changes in body
weight (±20%); this approach is consistent with the
methodology used in another cost-effectiveness
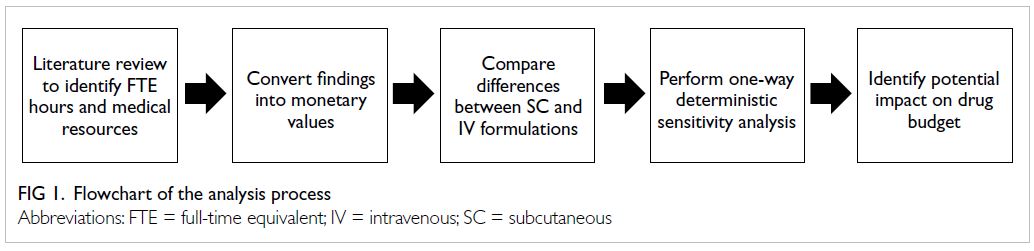
analysis focused on trastuzumab.17 Figure 1
summarises the analysis process of this study.
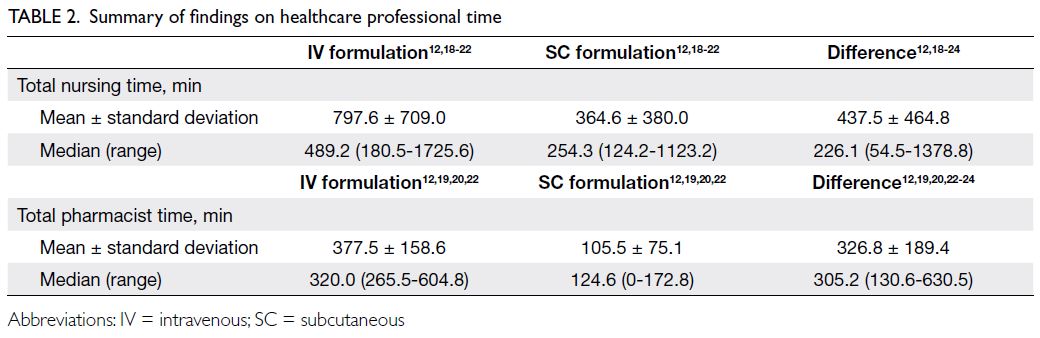
Results
In total, 11 studies were identified, eight of which were eligible for analysis.12 18 19 20 21 22 23 24 Three studies were
excluded because they did not report the time
required for administration or preparation. There
are a total of six studies with information on
pharmacist time on preparation and nursing time
on administration for IV and SC trastuzumab;
the remaining two only reported time differences
between the two formulations. Among the six studies
that reported the time for preparation, four reported
the total drug preparation time required for IV and
SC trastuzumab, whereas the remaining two only
reported time differences. If the SC formulation was
used, 0.18 FTE hour of nursing time (7.9 hours) and
0.14 FTE hour of pharmacist time (6.2 hours) could
be saved each week. Table 2 summarises the findings
from these studies.
After 18 cycles of treatment with SC
trastuzumab, the drug acquisition and healthcare
professional time costs were reduced by US$9451.28
and US$566.16, respectively, compared with IV
trastuzumab. Therefore, US$10 017.44 could be
saved for each patient who completed 18 cycles of
treatment. The cost of consumables was excluded
because only two studies reported this information, and the contributions to overall costs were minimal
(NZ$15.2712 and GBP0.6421, respectively). Table 3
summarises the direct medical costs of IV and SC
formulations.
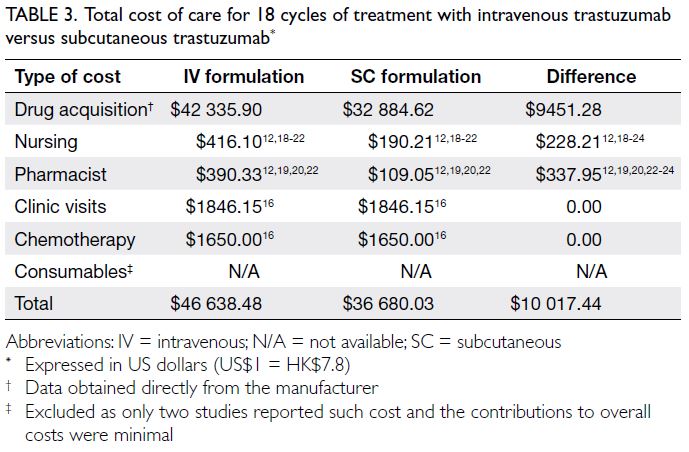
Table 3. Total cost of care for 18 cycles of treatment with intravenous trastuzumab versus subcutaneous trastuzumab
Sensitivity analysis
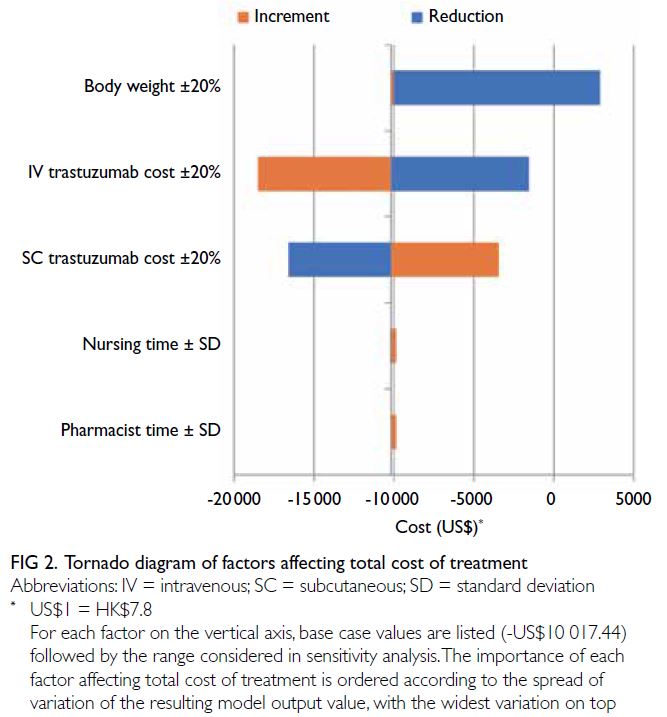
The drug budget impact forecast model was most
affected by body weight and drug acquisition cost.
Cost differences between the IV and SC formulations
were reduced by decreases in body weight and
IV trastuzumab cost, as well as an increase in SC
trastuzumab cost. The effects of changes in nursing
time and pharmacist time were smaller. Table 1
summarises the model parameters, and Figure 2
illustrates the effects of each variable on cost
differences.
Drug budget impact forecast
In 2017, 4373 women were diagnosed with invasive
breast cancer,1 and approximately 20% of them were
HER2-positive.2 3 Furthermore, trastuzumab was
the most commonly used targeted therapy (95.3%).3
Assuming that the SC formulation was used (instead
of the IV formulation) for all HER2-positive patients
receiving trastuzumab and using the 2017 data stated
here, an annual saving of over US$8.3 million could
be achieved in Hong Kong.
Discussion
The results of this study suggest that SC trastuzumab would be more cost-effective than its IV counterpart
in Hong Kong. Even if lower-cost biosimilar
trastuzumab becomes available, the SC formulation
will remain less expensive unless there is a substantial
reduction in the acquisition cost of IV trastuzumab.
As body weight decreases, the necessary
dosage and corresponding expenditures are expected
to decrease. Paradoxically, ≤20% increases in body
weight had a neutral effect in the analysis. This result
could be related to a substantial amount of drug
wastage when using weight-based IV trastuzumab,
which is consistent with previous findings.19 25
Therefore, further studies are needed to determine the optimal route of administration for patients
who are underweight or do not require full doses of
trastuzumab because of their clinical conditions.
Although the SC formulation is expected to
save time for healthcare professionals,26 27 28 the present
analysis suggests that its contribution to the total
cost of care is minimal. The cost of drug acquisition
has the greatest effect on financial burden.
The use of data from previous time-and-motion
studies in other countries may not be appropriate
for medical settings in Hong Kong. Further studies
should be conducted in Hong Kong to estimate
the actual cost savings with respect to healthcare
professional time, although theoretical time savings
may not accurately represent actual time savings
because of clinical activities conducted during
administration of trastuzumab.29 Furthermore, data
from other countries exhibited wide distributions in
terms of standard deviation and range. Nevertheless,
the influence of the SC formulation on the total cost-saving
effect may be limited, as demonstrated in the
sensitivity analysis.
Although the costs of clinic visits and
chemotherapy were assumed to be identical
throughout 18 cycles of treatment between the
two formulations, some patients can receive SC
trastuzumab in ambulatory care settings. Thus, the
mean savings may have been underestimated in our
model.
There were several limitations in this study.
First, because of the small number of studies
identified in the literature review, consumables
could not be included in the CMA. Second, societal
cost and patient preferences were not considered
because such information is unavailable in Hong
Kong. A more patient-centred approach would
provide greater insights. Third, time-and-motion
analysis and waste handling in Hong Kong were not
considered; these factors may have specific impact on
drug preparation time and administration time and
costs. Fourth, costs for adverse drug reactions were
not included because these costs were assumed to be
equal for IV and SC trastuzumab regimens. However,
this assumption may be incorrect, particularly with
regard to infusion-related reactions.
Conclusion
The results of this study suggest that the SC
formulation of trastuzumab would be a cost-saving
therapy for HER2-positive breast cancer patients
in Hong Kong. The drug acquisition cost was the
parameter with the greatest effect on the total cost
of treatment.
Author contributions
Concept or design: VWY Lee.
Acquisition of data: FWT Cheng.
Analysis or interpretation of data: Both authors.
Drafting of the manuscript: FWT Cheng.
Critical revision of the manuscript for important intellectual content: VWY Lee.
Acquisition of data: FWT Cheng.
Analysis or interpretation of data: Both authors.
Drafting of the manuscript: FWT Cheng.
Critical revision of the manuscript for important intellectual content: VWY Lee.
Both authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
Both authors disclosed no conflicts of interest.
Declaration
The datasets generated and/or analysed in this study are available from the corresponding author on reasonable request.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Not applicable because this study did not involve human participants.
References
1. Hong Kong Cancer Registry, Hospital Authority, Hong Kong SAR Government. Female breast cancer in 2017.
Available from: https://www3.ha.org.hk/cancereg/pdf/factsheet/2017/breast_2017.pdf. Accessed 11 May 2020.
2. Yau TK, Sze H, Soong IS, Hioe F, Khoo US, Lee AW. HER2 overexpression of breast cancers in Hong Kong: prevalence and concordance between immunohistochemistry and
in-situ hybridisation assays. Hong Kong Med J 2008;14:130-5.
3. Hong Kong Breast Cancer Foundation. Hong Kong Breast Cancer Registry Report No. 8. 2016. Available from: http://www.hkbcf.org/download/bcr_report8/hkbcf_report_2016_full_report.pdf. Accessed 21 May 2017.
4. Genentech. Highlights of prescribing information. 2016. Available from: http://www.gene.com/download/pdf/herceptin_prescribing.pdf. Accessed 8 Aug 2016.
5. Kruse GB, Amonkar MM, Smith G, Skonieczny DC,
Stavrakas S. Analysis of costs associated with
administration of intravenous single-drug therapies in
metastatic breast cancer in a U.S. population. J Manag Care
Pharm 2008;14:844-57. Crossref
6. Ismael G, Hegg R, Muehlbauer S, et al. Subcutaneous versus
intravenous administration of (neo)adjuvant trastuzumab
in patients with HER2-positive, clinical stage I-III breast
cancer (Hannah study): a phase 3, open-label, multicentre,
randomised trial. Lancet Oncol 2012;13:869-78. Crossref
7. Jackisch C, Kim SB, Semiglazov V, et al. Subcutaneous
versus intravenous formulation of trastuzumab for HER2-positive early breast cancer: updated results from the phase
III HannaH study. Ann Oncol 2015;26:320-5. Crossref
8. Pivot X, Gligorov J, Müller V, et al. Preference for
subcutaneous or intravenous administration of
trastuzumab in patients with HER2-positive early breast
cancer (PrefHer): an open-label randomised study. Lancet
Oncol 2013;14:962-70. Crossref
9. Pivot X, Gligorov J, Müller V, et al. Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-
positive early breast cancer: final analysis of 488 patients in
the international, randomized, two-cohort PrefHer study.
Ann Oncol 2014;25:1979-87. Crossref
10. Mylonas C, Kourlaba G, Fountzilas G, Skroumpelos A,
Maniadakis N. Cost-minimization analysis of trastuzumab
intravenous versus trastuzumab subcutaneous for the
treatment of patients with HER2+ early breast cancer
and metastatic breast cancer in Greece. Value Health
2014;17:A640-1. Crossref
11. Gutierrez F, Nazco G, Viña M, Bullejos M, Gonzalez I, Valcarcel C. Economic impact of using subcutaneous trastuzumab. Value Health 2014;17:A641. Crossref
12. North RT, Harvey VJ, Cox LC, Ryan SN. Medical resource
utilization for administration of trastuzumab in a New
Zealand oncology outpatient setting: a time and motion
study. Clinicoecon Outcomes Res 2015;7:423-30. Crossref
13. De Cock E, Pivot X, Hauser N, et al. A time and motion
study of subcutaneous versus intravenous trastuzumab in
patients with HER2-positive early breast cancer. Cancer
Med 2016;5:389-97. Crossref
14. Van den Nest M, Glechner A, Gold M, Gartlehner G. The
comparative efficacy and risk of harms of the intravenous
and subcutaneous formulations of trastuzumab in patients
with HER2-positive breast cancer: a rapid review. Syst Rev
2019;8:321. Crossref
15. Jackisch C, Stroyakovskiy D, Pivot X, et al. Subcutaneous vs
intravenous trastuzumab for patients with ERBB2-positive
early breast cancer: final analysis of the HannaH phase 3
randomized clinical trial. JAMA Oncol 2019;5:e190339. Crossref
16. Government Logistics Department, Hong Kong SAR
Government. Hospital Authority Ordinance (Chapter 113).
Revisions to list of charges. Available from: https://www.gld.gov.hk/egazette/pdf/20172124/egn201721243884.pdf. Accessed 30 May 2017.
17. Kurian AW, Thompson RN, Gaw AF, Arai S, Ortiz R, Garber AM. A cost-effectiveness analysis of adjuvant trastuzumab regimens in early HER2/neu-positive breast cancer. J Clin Oncol 2007;25:634-41. Crossref
18. Olofsson S, Norrlid H, Karlsson E, Wilking U, Ragnarson
Tennvall G. Societal cost of subcutaneous and intravenous
trastuzumab for HER2-positive breast cancer—an
observational study prospectively recording resource
utilization in a Swedish healthcare setting. Breast
2016;29:140-6. Crossref
19. Ponzetti C, Canciani M, Farina M, Era S, Walzer S. Potential
resource and cost saving analysis of subcutaneous versus
intravenous administration for rituximab in non-Hodgkin’s
lymphoma and for trastuzumab in breast cancer in 17 Italian hospitals based on a systematic survey. Clinicoecon
Outcomes Res 2016;8:227-33. Crossref
20. De Cock E, Pan YI, Tao S, Baidin P. Time savings with
trastuzumab subcutaneous (SC) injection verse
trastuzumab intravenous (IV) infusion: a time and motion
study in 3 Russian centers. Value Health 2014;17:A653. Crossref
21. Nawaz S, Samanta K, Lord S, Diment V, Mcnamara S.
Cost savings with Herceptin® (trastuzumab) SC vs
IV administration: a time & motion study. Breast
2013;22(S1):S112.
22. De Cock E, Tao S, Alexa U, Pivot X, Knoop A. Abstract P5-
15-07: Time savings with trastuzumab subcutaneous (SC)
injection vs. trastuzumab intravenous (IV) infusion: first
results from a Time-and-Motion study (T&M). Cancer Res
2014;72(24 Suppl):P5-15-07. Crossref
23. De Cock E, Tao S DM-P, Millar D CN. Time savings with
trastuzumab subcutaneous (SC) injection vs. trastuzumab
intravenous (IV) infusion: a time and motion study
in 5 Canadian centres. Proceedings of the Canadian
Association for Population Therapeutics (CAPT) Annual
Conference; 2013 Nov 17-19; Toronto, Canada.
24. Samanta K, Moore L, Jones G, Evason J, Owen G. PCN39
potential time and cost savings with herceptin (trastuzumab)
subcutaneous (SC) injection versus herceptin intravenous
(IV) infusion: results from three different English patient
settings. Value Health 2012;15:A415. Crossref
25. Nestorovska A, Naumoska Z, Grozdanova A, et al.
Subcutaneous vs intravenous administration of
trastuzumab in HER2+ breast cancer patients: a
Macedonian cost-minimization analysis. Value Health
2015;18:A463. Crossref
26. Rojas L, Muñiz S, Medina L, et al. Cost-minimization
analysis of subcutaneous versus intravenous trastuzumab
administration in Chilean patients with HER2-positive
early breast cancer. PLoS One 2020;15:e0227961. Crossref
27. O’Brien GL, O’Mahony C, Cooke K, et al. Cost
minimization analysis of intravenous or subcutaneous
trastuzumab treatment in patients with HER2-positive
breast cancer in Ireland. Clin Breast Cancer 2019;19:e440-51. Crossref
28. Lopez-Vivanco G, Salvador J, Diez R, et al. Cost
minimization analysis of treatment with intravenous or
subcutaneous trastuzumab in patients with HER2-positive
breast cancer in Spain. Clin Transl Oncol 2017;19:1454-61. Crossref
29. Papadmitriou K, Trinh XB, Altintas S, Van Dam PA,
Huizing MT, Tjalma WA. The socio-economical impact of
intravenous (IV) versus subcutaneous (SC) administration
of trastuzumab: future prospectives. Facts Views Vis
Obgyn 2015;7:176-80.