© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Neutropenia and anaemia secondary to copper
deficiency in a child receiving long-term jejunal feeding: a case report
WY Leung, MRCPCH1; CC So, FRCPath2,3,4; Godfrey CF Chan, FRCPCH1,5; SY Ha, FRCPCH1,5; Alan KS Chiang, FRCP1,5; Daniel KL Cheuk, FHKAM (Paediatrics)1,5
1 Department of Paediatrics and Adolescent Medicine, Hong Kong Children’s Hospital, Hong Kong
2 Department of Pathology, Hong Kong Children’s Hospital, Hong Kong
3 Department of Pathology, Queen Elizabeth Hospital, Hong Kong
4 Department of Pathology, The University of Hong Kong, Hong Kong
5 Department of Paediatrics and Adolescent Medicine, The University of Hong Kong, Hong Kong
Corresponding author: Dr WY Leung (lwy729@ha.org.hk)
Case report
In January 2017, a girl was born full term to non-consanguineous
Chinese parents. Oesophageal
atresia with a distal tracheo-oesophageal fistula was
diagnosed soon after birth. Emergency surgical repair
was attempted on day 1 of life but was complicated
by complete transection of the left main bronchus
that was subsequently repaired. The tracheo-oesophageal
fistula was divided and a gastrostomy
created. End-to-end anastomosis of the proximal and
distal oesophagus was performed at age 6 months.
Gastrostomy feeding was switched to jejunal feeding
at 7 months because of gastroesophageal reflux
and recurrent aspiration pneumonia. Attempted
fundoplication at 17 months failed due to a small
tubular stomach. She was prescribed oral ranitidine
30 mg three times daily.
At birth, the child’s haematology results
were normal but by age 2 years she had developed
persistent severe neutropenia (lowest neutrophil
count 0.13 × 109/L) and microcytic anaemia, with
a lowest haemoglobin of 6.6 g/dL and lowest mean
corpuscular volume of 65.5 fL. Platelet count was
normal and she had no major infection or signs of
anaemia. She was on continuous jejunal feeding
with high-energy infant formula (Infatrini; Nutricia,
Zoetermeer, The Netherlands) 640 mL daily as well
as iron(III)-hydroxide polymaltose complex (IPC)
25 mg daily and multivitamin drops (Poly-Vi-Sol;
Mead-Johnson Nutrition, Chicago [IL], United
States) 1 mL daily. The high-energy infant formula
provided 416 μg of copper daily, equivalent to 1.2
times the recommended dietary allowance.
Growth was satisfactory with her body weight
at the 25th centile and height at the 10th centile. No
new physical sign was detected. Peripheral blood
smear showed occasional macro-ovalocytes. A
dimorphic red cell picture was not seen and there
were no hypersegmented neutrophils. Serum iron of
4.6 μmol/L (normal, 4-25 μmol/L), total iron-binding capacity of 103 μmol/L (normal, 41-77 μmol/L), and
transferrin saturation of 4% (normal, 7%-44%) were
suggestive of iron deficiency. Haemoglobin pattern
analysis was negative for thalassaemia. Serum active
vitamin B12 level was low at 23.6 pmol/L (normal,
>46.2 pmol/L) and serum and red blood cell folate,
serum bilirubin and lactate dehydrogenase levels
were normal. Direct antiglobulin test, antinuclear
antibody, C3, C4, anti-intrinsic factor antibody,
antiparietal cell antibody and antineutrophil
antibody were all negative.
Intramuscular vitamin B12 was given and
ranitidine was stopped. The dose of IPC was
increased to 15 mg twice daily and administered via
the gastric tube.
Despite normalisation of serum iron and
vitamin B12 level, anaemia and neutropenia persisted.
Haemoglobin further dropped to 6.6 g/dL and red
blood cell transfusion was required. She responded
to a dose of granulocyte colony-stimulating factor
with the neutrophil count rising from 0.25 × 109/L to
1.74 × 109/L. Nonetheless, her neutrophil count
dropped to 0.33 × 109/L 5 days later. Bone marrow
aspiration and trephine biopsy was performed
to evaluate the cause of refractory cytopenias
and revealed reduced granulopoiesis, reactive
histiocytosis and iron block. Vacuolated myeloid
and erythroid precursors were observed (Fig 1).
Megakaryopoiesis was adequate and no sideroblasts
were evident on iron staining. These findings
suggested copper deficiency or zinc toxicity. Serum
copper was subsequently found to be <2.0 μmol/L
(normal, 13-24 μmol/L), consistent with severe
copper deficiency, whilst serum zinc level was
normal.
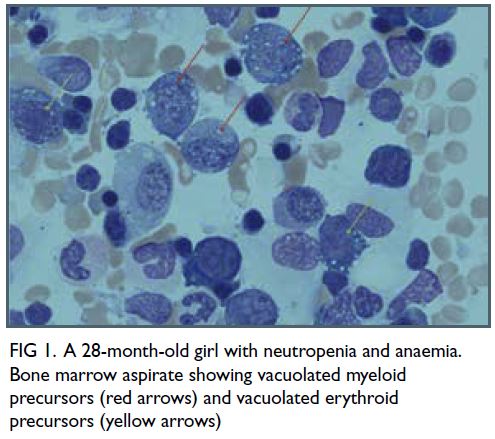
Figure 1. A 28-month-old girl with neutropenia and anaemia. Bone marrow aspirate showing vacuolated myeloid precursors (red arrows) and vacuolated erythroid precursors (yellow arrows)
Copper deficiency was treated with mineral
mixture powder (Seravit; Nutricia) via the gastric
tube from age 28 months as direct copper supplement
was not available. The mineral mixture powder
was administered at a dose of 2.5 g daily, providing 115 μg copper each day. On day 9 after
commencement of copper supplementation, the
absolute neutrophil count increased from the lowest
level of 0.29 × 109/L to 1.09 × 109/L and haemoglobin
level increased from the lowest level of 9.6 g/dL to
10.6 g/dL. The mineral mixture powder was further
increased to 2.5 g twice daily. A small amount of milk
was introduced to the stomach to allow absorption
of the micronutrients. On day 16, the haemoglobin
and neutrophil count normalised (haemoglobin, 12.2 g/dL; absolute neutrophil count, 2.03 × 109/L)
and by 7 weeks, serum copper had normalised. The
mineral mixture powder was stopped after 8 weeks,
at age 30 months. At age 35 months, haemoglobin,
neutrophil count, copper, and iron levels remained
normal (Fig 2).
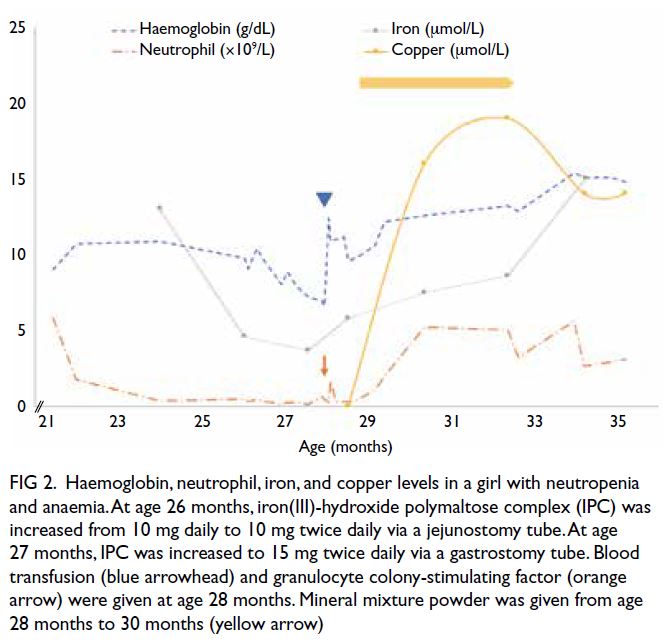
Figure 2. Haemoglobin, neutrophil, iron, and copper levels in a girl with neutropenia and anaemia. At age 26 months, iron(III)-hydroxide polymaltose complex (IPC) was increased from 10 mg daily to 10 mg twice daily via a jejunostomy tube. At age 27 months, IPC was increased to 15 mg twice daily via a gastrostomy tube. Blood transfusion (blue arrowhead) and granulocyte colony-stimulating factor (orange arrow) were given at age 28 months. Mineral mixture powder was given from age 28 months to 30 months (yellow arrow)
Discussion
The index patient presented with haematological
abnormalities due to acquired copper deficiency
following long-term jejunal feeding, which is not well
reported in the literature.1 Jacobson et al2 reported
three paediatric patients with exclusive jejunal
feeding who developed cytopenias, one of whom
had concurrent combined iron and vitamin B12
deficiency similar to our patient. Premature infants
and children with intestinal failure on parenteral
nutrition with inadequate copper supplementation
are also at increased risk of acquired copper
deficiency.3
Although the amount of iron, copper and
vitamin B12 provided was above the recommended
dietary requirement, deficiencies occurred because
of problems in absorption. Most copper absorption
occurs in the stomach and proximal duodenum.
The acidic environment in the stomach facilitates
solubilisation by dissociating it from copper-containing
dietary macromolecules. Jejunal
administration of IPC, multivitamin drops and
infant formula bypassed the stomach and ranitidine
reduced the acidity of the jejunal environment.
Copper-dependent enzymes are essential for
normal function of the haematopoietic, skeletal,
and central nervous systems. Although absent in the
index patient, clinical signs of copper deficiency such
as fragile, abnormally formed hair, depigmentation
of the skin, oedema, myeloneuropathy, ataxia
and cognitive deficits should be actively sought.
Anaemia and neutropenia are the predominant
haematological manifestations. Thrombocytopenia
rarely occurs. Serum ferritin and erythropoietin
are usually elevated. Serum ceruloplasmin is
low. Anaemia is caused by the reduced activity
of ceruloplasmin ferroxidase, copper/zinc
superoxidase and cytochrome-c oxidase.4 Upon
copper supplementation, neutropenia typically
improves within a few weeks and anaemia improves
within a few months. Cytoplasmic vacuolation in
erythroid and myeloid precursors is the prominent
feature in the bone marrow. Dysplastic features, such
as megaloblastic changes and ring sideroblasts, may
be observed.4 Vacuolated erythroblasts and myeloid
precursors are classically observed in Pearson
syndrome,5 acute alcoholism, chloramphenicol and
linezolid toxicity, acute erythroid leukaemia and
acute metabolic disturbance. These causes were
unlikely in the index patient in the absence of an
associated history or pathological features. Primary myelodysplasia is an important differential diagnosis
but blood count recovery on copper replacement
would not be expected.
This case highlights the importance of
nutritional monitoring in patients receiving
exclusive jejunal feeding. We recommend checking
full blood count, liver and renal function tests,
electrolytes, iron profile, vitamin B12, copper and
zinc level every 3 months. Unexplained anaemia
or neutropenia should prompt investigations
for possible micronutrient deficiency to avoid
unnecessary invasive investigations.
Author contributions
Concept or design: All authors.
Acquisition of data: WY Leung, CC So.
Analysis or interpretation of data: WY Leung.
Drafting of the manuscript: WY Leung, CC So.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: WY Leung, CC So.
Analysis or interpretation of data: WY Leung.
Drafting of the manuscript: WY Leung, CC So.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Funding/support
This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki. The parent of the patient provided written consent
for publication.
References
1. Barraclough H, Cooke K. Are patients fed directly into the jejunum at risk of copper deficiency? Arch Dis Child
2019;104:817-9. Crossref
2. Jacobson AE, Kahwash SB, Chawla A. Refractory
cytopenias secondary to copper deficiency in children
receiving exclusive jejunal nutrition. Pediatr Blood Cancer
2017;64:e26617. Crossref
3. Leite HP, Koch Nogueira PC, Uchoa KM, Carvalho de
Camargo MF. Copper deficiency in children with intestinal
failure: risk factors and influence on hematological
cytopenias. JPEN J Parenter Enteral Nutr 2021;45:57-64. Crossref
4. Chen CC, Takeshima F, Miyazaki T, et al. Clinicopathological
analysis of hematological disorders in tube-fed patients
with copper deficiency. Intern Med 2007;46:839-44. Crossref
5. Knerr I, Metzler M, Niemeyer CM, et al. Hematologic
features and clinical course of an infant with Pearson
syndrome caused by a novel deletion of mitochondrial
DNA. J Pediatr Hematol Oncol 2003;25:948-51. Crossref

