© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
CASE REPORT
Persistent fever in a child with eosinophilia and
systemic symptoms: a case report
CS Wai, MB, ChB, MRCPCH; WF Hui, MB, ChB, MRCPCH; Karen KY Leung, MB, BS, MRCPCH; KL Hon, MB, BS, MD
Department of Paediatrics and Adolescent Medicine, Hong Kong Children’s Hospital, Hong Kong
Corresponding author: Dr KL Hon (ehon@hotmail.com)
Case
In October 2019, an 8-year-old girl with Diamond–Blackfan anaemia on long-term maintenance steroid
therapy was admitted to a paediatric unit with
meningitis. The meningitis was caused by extended
spectrum beta-lactamase-producing Klebsiella pneumonia that was sensitive to meropenem but
resistant to cephalosporin and penicillin group
of antibiotics. She was treated with intravenous
meropenem and her fever subsided.
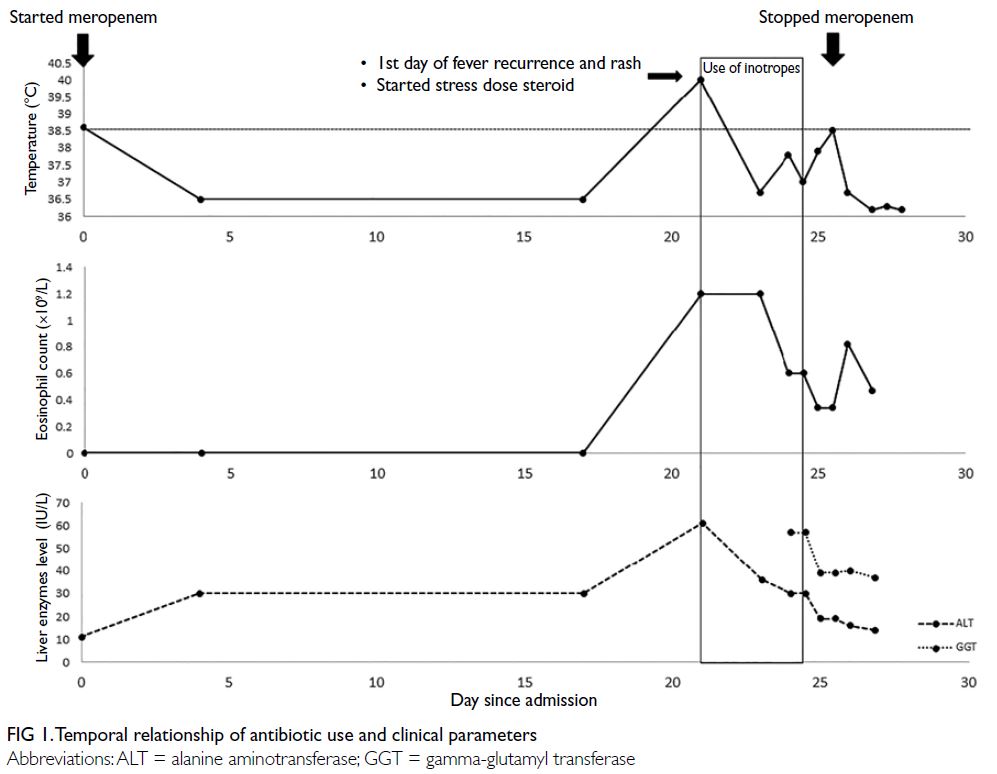
On day 21 of meropenem treatment the
patient’s fever recurred with temperature of 39.7°C
and associated abdominal pain, diarrhoea, and rash
(Fig 1). Physical examination revealed a generalised
blanchable erythematous maculopapular rash over her face, torso, and limbs with no mucosal
involvement or lymphadenopathy (Fig 2). She was
tachycardic at 150 beats per minute but her blood
pressure was normal. She was given fluid boluses
and managed in the paediatric intensive care unit
with a noradrenaline infusion (0.05 μg/kg/min),
prednisolone (increased to 20 mg daily), and the
addition of vancomycin.
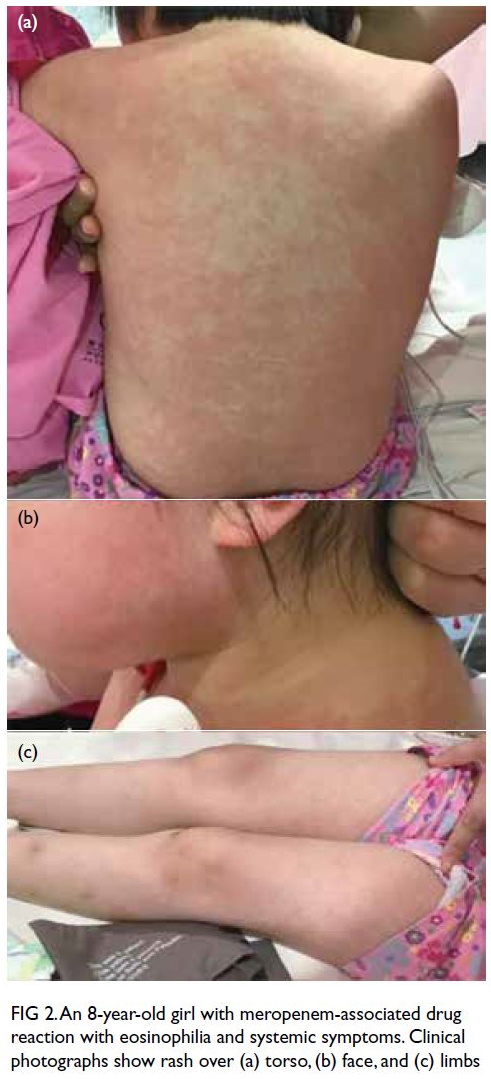
Figure 2. An 8-year-old girl with meropenem-associated drug reaction with eosinophilia and systemic symptoms. Clinical photographs show rash over (a) torso, (b) face, and (c) limbs
Autoimmune disease and infection workup
including serology for Epstein–Barr virus,
cytomegalovirus, and human herpesvirus 6 and stool
culture for ova, cysts, and parasites examination
were all negative. The patient’s C-reactive protein
level was 166.43 mg/L (normal range <10 mg/L)
and procalcitonin was 3.63 ng/mL (normal range <0.5 ng/mL). There was evolving eosinophilia since
fever recurrence. The absolute eosinophil count
was elevated to 1.2×109/L (18.9% of total white cell
count) on the first day of fever recurrence with a
peak of 1.4×109/L (35.4% of total white cell count).
Gamma-glutamyl transferase level was elevated
(57 IU/L). Skin biopsy showed non-specific
perivascular lymphocyte infiltration with no
eosinophils. There was no erythema multiforme-like
pattern, spongiosis, interface change or vasculitis
and staining showed no fungus.
Meropenem-associated drug reaction with
eosinophilia and systemic symptoms (DRESS)
was suspected in view of her fever, rash, persistent
eosinophilia, and deranged liver function alongside prolonged meropenem use and a negative infection
workup. Meropenem was therefore stopped, and her
fever subsided after 6 hours. She was also weaned off
inotrope support. She remained hemodynamically
stable and the rash did not worsen and gradually
subsided in 48 hours. She was discharged from the
paediatric intensive care unit 2 days later. There
was no residual rash and eosinophil count had
normalised 2 weeks after onset of DRESS symptoms.
Discussion
Children with fever and rashes pose challenges in diagnosis and management. Differential diagnoses
must consider “drug versus bug” scenarios.1 Drug
reaction with eosinophilia and systemic symptoms
is a rare reaction to certain medications and involves
a widespread skin rash, fever, swollen lymph nodes,
and characteristic blood abnormalities including
eosinophilia, thrombocytopenia and atypical
lymphocytosis.2 Drug reaction with eosinophilia and
systemic symptoms is classified as a form of severe
cutaneous adverse reaction that usually develops
within 6 weeks of initiation of the suspected drug.3 It
may be associated with hepatitis, carditis, interstitial
nephritis or interstitial pneumonitis and is potentially
life-threatening with a reported mortality rate up to
10%.2 The estimated incidence of DRESS is 1 in 1000
to 1 in 10000 drug exposures and is more commonly
observed in adults than children.4
The RegiSCAR (European Registry of Severe
Cutaneous Adverse Reactions) developed a scoring
system in 2007 to classify patients as having no,
possible, probable, or definite DRESS based on
clinical features and laboratory findings.5 Our
patient had a score of 3 and was classified as possible
DRESS, with extreme eosinophilia, rash with
>50% body surface area involvement and negative
microbiological investigations. One point was
deducted from the score for “rash resolution more
than 15 days”. Her aminotransferase and bilirubin
level remained normal throughout although gamma-glutamyl
transferase was elevated. Skin biopsy
revealed perivascular lymphocytosis, a commonly
observed feature among patients with DRESS.5
Skin biopsy is indicated as one of the parameters in
RegiSCAR if facilities are available.
Carbapenems, as members of the beta-lactam
antibiotics, are reported to pose a low risk of allergic
reaction or DRESS. The incidence of rash, pruritis
and urticaria after use of carbapenem has been
reported to be only 0.3 to 3.7%, with rash reported in
only 1.4% of patients treated with meropenem.6 Early
suspicion of DRESS is crucial as discontinuation
of the culprit drug can prevent development of
potentially life-threatening complications. This case
illustrates that all sepsis symptomatology subsided
in a febrile patient with rash and eosinophilia, not by
adding more drugs but by considering the possibility of severe cutaneous adverse reactions and removing
the culprit drug.
Author contributions
All authors contributed to the concept or design of the study,
acquisition of the data, analysis or interpretation of the
data, drafting of the manuscript, and critical revision of the
manuscript for important intellectual content. All authors
had full access to the data, contributed to the study, approved
the final version for publication, and take responsibility for its
accuracy and integrity.
Conflicts of interest
As an editor of the journal, KL Hon was not involved in the peer review process. Other authors have no conflicts of
interest to disclose.
Funding/support
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The patient was treated in accordance with the Declaration of Helsinki and a parent provided informed consent for all
treatments and procedures. Consent was obtained from the
patient and her parents for publication.
References
1. Hon KL, Choi CL. Steven Johnson syndrome: drug or bug? Indian J Pediatr 2016;83:1508-9. Crossref
2. Walsh SA, Creamer D. Drug reaction with eosinophilia and
systemic symptoms (DRESS): a clinical update and review
of current thinking. Clin Exp Dermatol 2011;36:6-11. Crossref
3. Adler NR, Aung AK, Ergen EN, Trubiano J, Goh MS,
Phillips EJ. Recent advances in the understanding of severe
cutaneous adverse reactions. Br J Dermatol 2017;177:1234-47. Crossref
4. Shiohara T, Kano Y. Drug reaction with eosinophilia and
systemic symptoms (DRESS): incidence, pathogenesis and
management Expert Opin Drug Saf 2017 2020;16:139-47.
5. Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug
reaction with eosinophilia and systemic symptoms
(DRESS): an original multisystem adverse drug reaction.
Results from the prospective RegiSCAR study. Br J
Dermatol 2013;169:1071-80. Crossref
6. Linden P. Safety profile of meropenem: an updated review
of over 6,000 patients treated with meropenem. Drug Saf
2007;30:657-68. Crossref