Hong Kong Med J 2022 Oct;28(5):383-91 | Epub 29 Sep 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE (HEALTHCARE IN MAINLAND CHINA)
THIRD bedside ultrasound protocol for rapid diagnosis of undifferentiated shock: a prospective observational study
P Geng, MD1 #; B Ling, MM1 #; Y Yang, MM1; Joseph Harold Walline, MD2; Y Song, MM1; M Lu, MD1; H Wang, MM1; Q Zhu, MM1; D Tan, MD1; J Xu, MD3
1 Department of Emergency Medicine, Clinical Medical College of Yangzhou University, Northern Jiangsu People’s Hospital, Yangzhou, China
2 Accident and Emergency Medicine Academic Unit, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong
3 Department of Emergency Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China
# The first two authors contributed equally to this work
Corresponding author: Dr D Tan (tandingyu1981@163.com)
Abstract
Introduction: It is clinically challenging to
differentiate the pathophysiological types of shock in
emergency situations. Here, we evaluated the ability
of a novel bedside ultrasound protocol (Tamponade/tension pneumothorax, Heart, Inferior vena cava, Respiratory system, Deep venous thrombosis/aorta dissection [THIRD]) to predict types of shock in the
emergency department.
Methods: An emergency physician performed the
THIRD protocol on all patients with shock who were
admitted to the emergency department. All patients
were closely followed to determine their final clinical
diagnoses. The kappa index, sensitivity, specificity,
positive predictive value, and negative predictive
value were calculated for the initial diagnostic
impression provided by the THIRD protocol,
compared with each patient’s final diagnosis.
Results: In total, 112 patients were enrolled in this
study. The kappa index between initial impression
and final diagnosis was 0.81 (95% confidence
interval=0.73-0.89; P<0.001). For hypovolaemic,
cardiogenic, distributive, and obstructive types
of shock, the sensitivities of the THIRD protocol
were 100%, 100%, 93%, and 100%, respectively; the
sensitivity for a ‘mixed’ shock aetiology was 86%. The
negative predictive value of the THIRD protocol for
all five types of shock was ≥96%.
Conclusion: Initial diagnostic judgements determined using the THIRD protocol showed favourable agreement with the final diagnosis
in patients who presented with undifferentiated
shock. The THIRD protocol has great potential for
use as a bedside approach that can guide the rapid
management of undifferentiated shock in emergency
settings, particularly for patients with obstructive,
hypovolaemic, or cardiogenic shock.
New knowledge added by this study
- Differentiating shock types in emergency situations is clinically challenging. We sought to assess the ability of a novel bedside ultrasound protocol (Tamponade/tension pneumothorax, Heart, Inferior vena cava, Respiratory system, Deep venous thrombosis/aorta dissection [THIRD]) in predicting shock aetiology. Shock aetiology determined using the THIRD protocol showed acceptable agreement with the final diagnosis of shock in critically ill emergency department patients.
- The THIRD protocol demonstrated very high negative predictive values when it was used to evaluate patients with hypovolaemic, cardiogenic, distributive, and obstructive shock.
- The THIRD protocol showed least sensitivity for evaluation of mixed aetiology shock.
- Our findings support incorporation of the THIRD protocol into routine emergency department assessment of patients with undifferentiated shock to help guide early treatment.
- Additional clinical assessments should be conducted to confirm a diagnosis of mixed shock made using the THIRD protocol.
Introduction
Undifferentiated shock is a common presenting
condition in the emergency department (ED)
which requires timely and effective interventions.
The rapid and accurate differentiation of possible
shock aetiologies is essential for reducing morbidity
and mortality in critically ill patients with shock.1
Patients with undifferentiated shock in the ED often
have an acute onset of severe illness, unstable vital
signs, a limited medical history, and sparse physical
examination findings.2 Point-of-care ultrasound
(POCUS) has a crucial role in the management of
undifferentiated shock because it is the only visual
imaging tool that can provide real-time information
concerning the key elements of haemodynamics.3 4
The use of POCUS in the ED has been rapidly
increasing because it is safe, reliable, non-invasive,
rapid, and repeatable at the bedside.5
The first ultrasound protocol for
undifferentiated shock was published in 20046;
since then, several additional protocols have
been developed. The results of multiple studies
have provided evidence that POCUS can help to
differentiate the cause of hypotension, identify the
most immediate life-threatening conditions, improve
diagnostic certainty, and optimise treatment.7 8
The ‘Tamponade/tension pneumothorax, Heart,
Inferior vena cava, Respiratory system, Deep venous
thrombosis/aorta dissection’ (THIRD) bedside ultrasound protocol was published in 2017; it is
the first POCUS protocol for undifferentiated
shock in emergency medicine in mainland China.9
Compared with the ‘Rapid Ultrasound for Shock
and Hypotension’ (RUSH) protocol, the THIRD
protocol has been reported to significantly increase
physician trainee self-confidence when diagnosing
undifferentiated shock.10
The THIRD protocol is now widely accepted
and regularly used by emergency physicians in
China; to our knowledge, the protocol has not been
validated in any studies thus far. This study was
conducted to examine the effectiveness and accuracy
of the THIRD protocol as an early and rapid bedside
approach for the investigation of undifferentiated
shock in emergency settings. We hypothesised that
THIRD early diagnostic predictions would not
demonstrate significant inconsistency with the final
clinical diagnosis.
Methods
Enrolment
This single-centre prospective observational study
enrolled patients with shock who presented to the
emergency intensive care unit (EICU) section of
the ED at a large, urban, tertiary teaching hospital
between October 2017 and May 2019. The ED of the
hospital has approximately 240 000 visits per year,
and >800 patients annually are admitted to the 15-bed
EICU for extended management. Shock was defined
as acute circulatory failure which led to inadequate
cellular oxygen utilisation.11 We established the
enrolment criteria for this study based on clinical
feasibility and previous literature12 13: (1) age
>18 years and <95 years; (2) systolic blood pressure
<90 mmHg or shock index (pulse/systolic blood
pressure) >1.0, confirmed after ≥3 measurements
during the first assessment; and (3) at least one of
the following symptoms or signs of hypoperfusion:
altered mental status (eg, syncope, delirium, or
unresponsiveness), respiratory distress, oliguria,
severe fatigue or discomfort, skin mottling, elevated
blood lactate, and severe chest pain or abdominal
pain. Patients with the following conditions
were excluded from the study: (1) a pre-existing
‘hypotensive’ state recorded in past medical history
or reported by the patient; (2) transfer from another
hospital with a known diagnosis of shock type; and
(3) no definite diagnosis of shock established during
hospitalisation, despite plenary discussion of their
clinical data.
Point-of-care ultrasound technique
The POCUS is routinely performed in all
hemodynamically unstable patients in our EICU.
In this study, an independent emergency physician
with specific competence in emergency ultrasound performed the THIRD protocol evaluation upon
patient arrival (Fig 1). The physician had completed a
20-hour emergency ultrasound workshop including
the THIRD protocol and had 3 years of experience
with >300 ultrasound examinations per year. The
physician was unaware of the history of present
illness or any other diagnostic test results; the
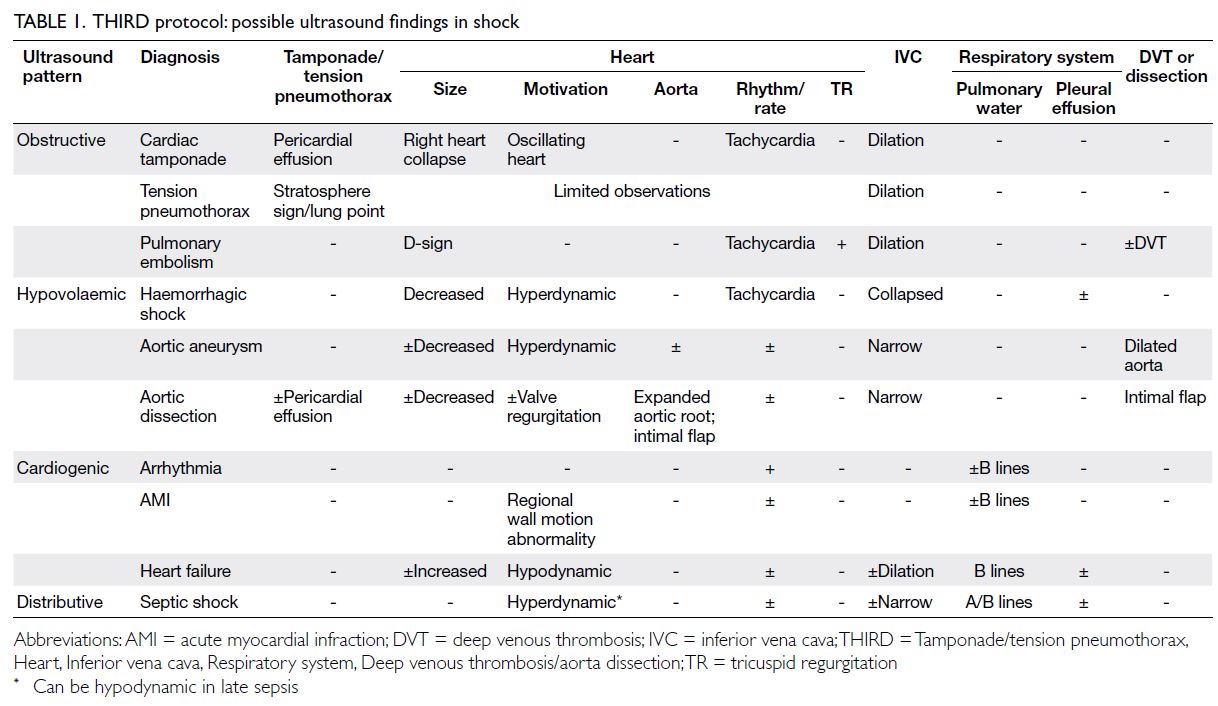
pathophysiological diagnosis of shock was made based on ultrasound findings (Table 1). Ultrasound
evaluation was performed with a Philips® Sparq
ultrasound device routinely used in the EICU.
This ultrasound system contains a high-frequency
4-12 MHz linear probe, a 2-6 MHz curvilinear probe,
and a 2-4 MHz cardiac probe.
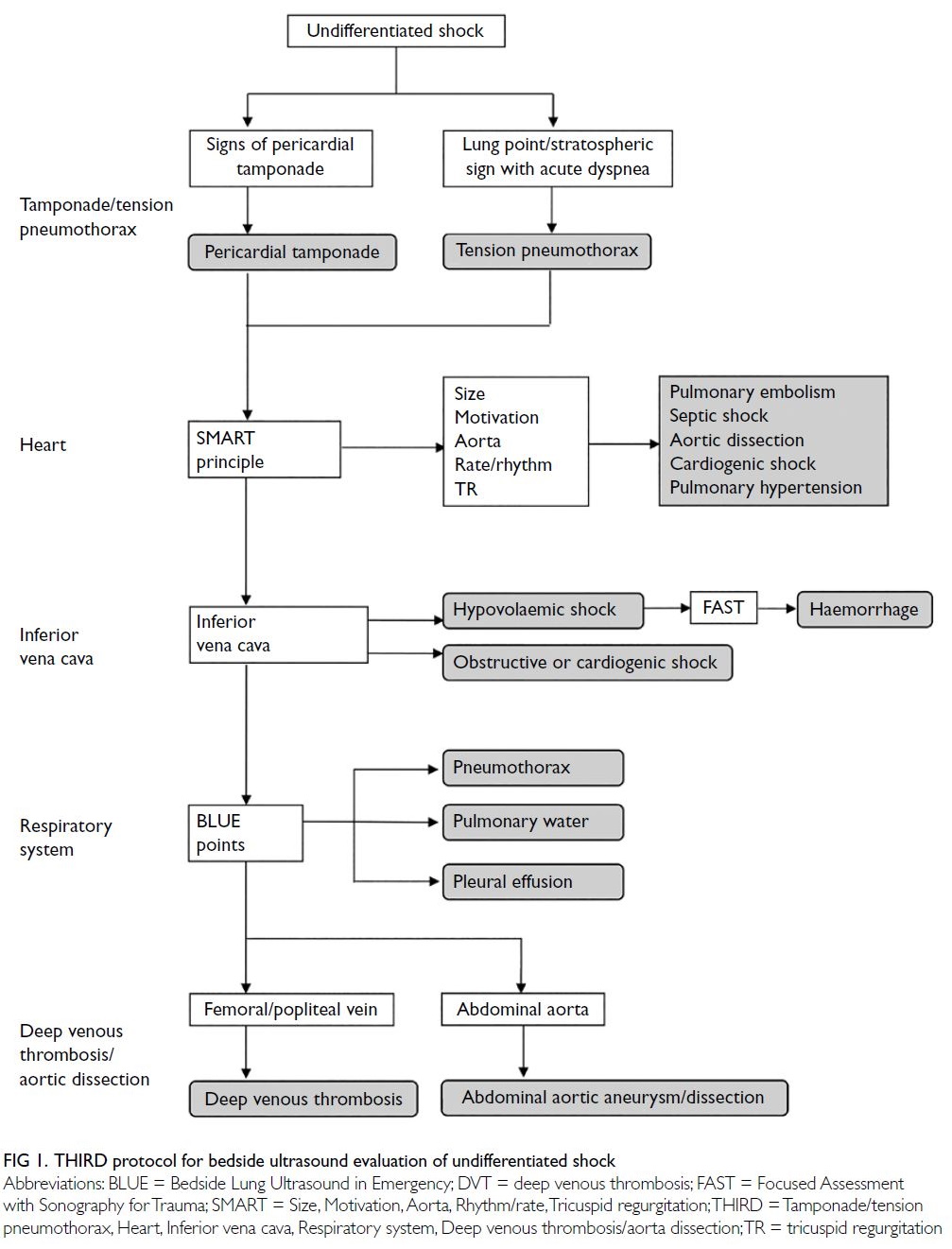
The THIRD protocol is divided into the
following five parts.
Tamponade or tension pneumothorax: First,
the subcostal cardiac view is used to determine the
presence of any pericardial effusion; then, evidence
of right atrial or right ventricular diastolic collapse
or cardiac oscillation is assessed to identify signs
of pericardial tamponade-related shock. Second,
the bilateral anterior thorax in the mid-clavicular
lines is explored to identify pleural sliding, the
‘seashore’ sign, A lines, and B lines. If the above
signs are not found and a ‘stratosphere’ sign or lung
point is identified, tension pneumothorax–related
obstructive shock is suspected.
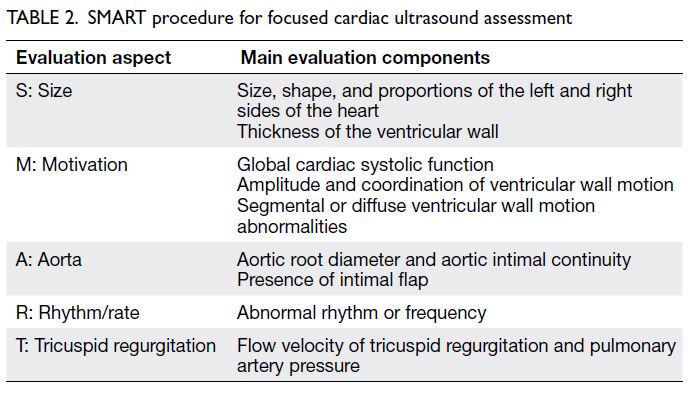
Heart: The SMART (Size, Motivation, Aorta,
Rhythm/rate, Tricuspid regurgitation) procedure
is used to assess the heart size, shape, and wall
motion; aortic diameter; presence of an aortic
intimal flap; cardiac rhythm and rate; and presence
of tricuspid regurgitation on the parasternal long-axis,
parasternal short-axis, and apical four-chamber
views. These assessments help to clarify the cause
and type of shock with respect to cardiac function
(Table 2).
Inferior vena cava: The subcostal longitudinal
acoustic window is used to localise the inferior vena
cava. The diameter and respiratory variation rate
of the inferior vena cava are measured to estimate
central venous pressure, assess right heart function
and overall blood volume, and evaluate indirect
evidence of shock caused by hypovolaemia, right
heart failure, pulmonary embolism, or pulmonary
hypertension.
Respiratory system: Lung ultrasound
assessment is performed using a symmetrical three-point
technique to identify common lung ultrasound
signs (eg, pleural fluid, pleural sliding, A lines, B
lines, shred sign, and lung rockets). These signs are
indicators of shock caused by lung consolidation,
massive pleural effusion/haemorrhage, or other
aetiologies such as pulmonary oedema.
Deep venous thrombosis or aortic dissection:
The acoustic windows of the bilateral symmetrical
inguinal areas and popliteal fossae are used to detect and assess the compressibility of the femoral veins
and popliteal veins; these assessments facilitate the
identification of deep vein thromboses. Because
pulmonary emboli are commonly associated with
deep venous thrombosis from the lower extremities,
this ultrasound technique is an indirect test for shock
caused by pulmonary emboli. Scans of the abdominal
aorta in horizontal sections of the peritoneal trunk,
superior mesenteric artery, and renal artery are then
conducted to determine whether aortic dissection or
aneurysm is present.
Clinical evaluation and final diagnosis
Upon admission to the EICU, the following
information was recorded for all enrolled patients:
demographic data, co-morbidities, APACHE II (acute
physiology and chronic health evaluation II) score,
need for mechanical ventilation, and physiological
data (eg, mean arterial pressure, shock index, lactate
level, and central venous oxygen saturation). All
patients were closely followed to confirm their final
diagnosis of shock. A panel of three board-certified
physicians (D Tan, emergency physician; J Ye,
radiologist; and J Zhang, cardiologist) established
the diagnosis of shock type based on all relevant
clinical data including history of present illness,
signs, auxiliary examination results. Disagreements
concerning diagnosis were resolved using a majority
vote approach. Patients were excluded if their
diagnosis could not be agreed upon by at least two
physicians.
Statistical analysis
Statistical analysis was performed using SPSS
(Windows version 21; IBM Corp., Armonk [NY], United States). Sample size calculations were
performed prior to enrolment; to detect a protocol
accuracy of >90% using the kappa method and
considering an anticipated 10% rate of exclusion
or dropout,14 at least 100 patients were required.
Thus, we planned a sample size of >110 patients
in this study. We calculated the kappa index
between the diagnosis of shock type according
to the THIRD protocol and the final diagnosis of
shock. Additionally, we separately assessed the
kappa agreement and reliability indices (sensitivity,
specificity, positive predictive value [PPV] and
negative predictive value [NPV])of the THIRD
protocol for each type of shock. For this analysis, we
excluded patients without a definite final diagnosis
of shock type.
Results
Patient characteristics and final clinical diagnoses
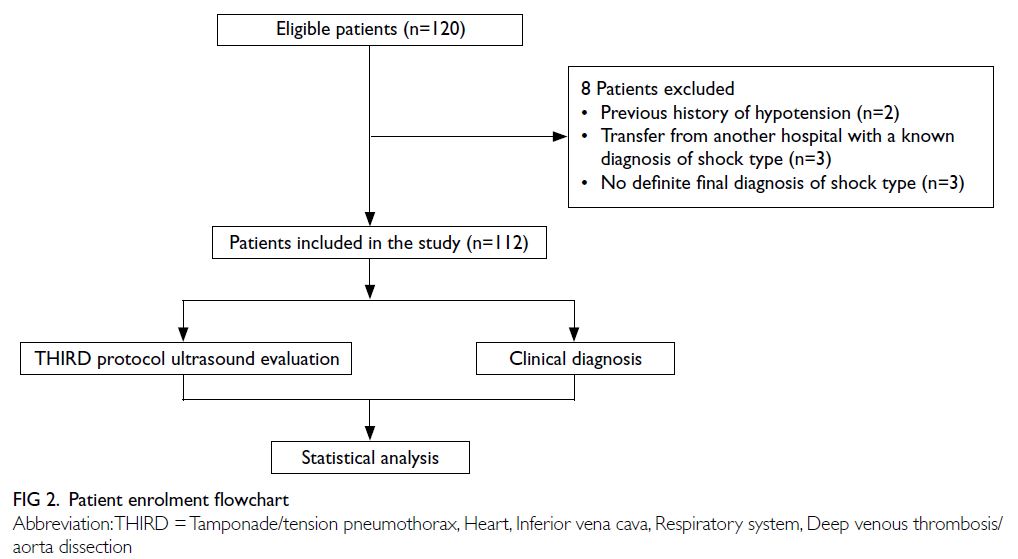
In total, 120 patients were enrolled; eight patients
were excluded prior to the analysis (two patients had
a history of hypotension in their previous medical
records, three patients were transferred from another
hospital with a known diagnosis of shock type, and
three patients did not have a definite final diagnosis
of shock type) [Fig 2]. In the final sample size of
112 patients, 54% were men, with a mean age of
66.5 ± 13.5 years and a mean arterial pressure of
51.2 ± 10.9 mmHg at presentation to the EICU.
The mean duration of a complete THIRD protocol
evaluation was 9.1 ± 1.5 minutes. The baseline
characteristics of enrolled patients are shown in
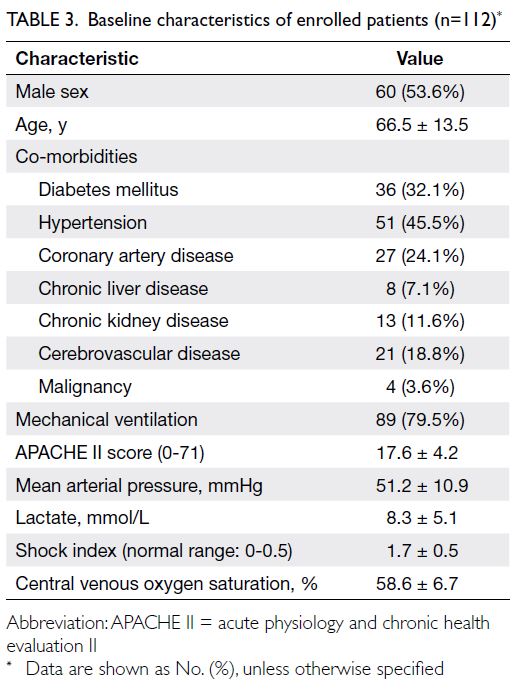
Table 3. The final charted clinical diagnoses of the
112 patients are reported in Table 4.
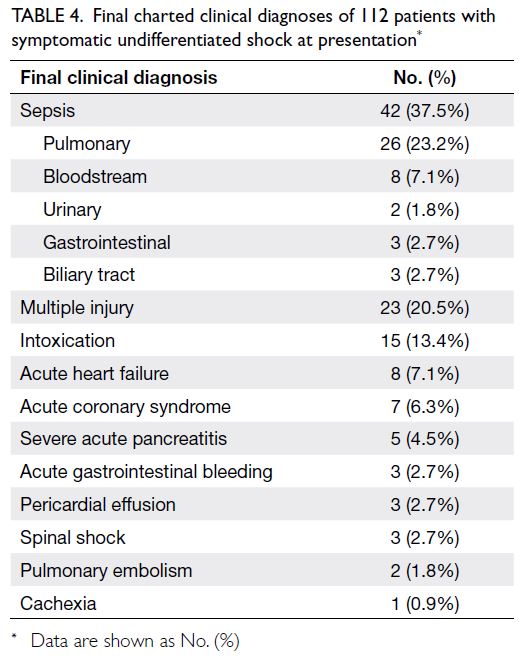
Table 4. Final charted clinical diagnoses of 112 patients with symptomatic undifferentiated shock at presentation
The final clinical diagnoses of the 112 patients,
based on chart assessment by the three auditors and
the THIRD protocol evaluation results, are shown
in Table 5. The most common type of shock in our
study was distributive shock (36 patients, 32.1%). The kappa index for general agreement between final
clinical diagnosis and the type of shock identified
using the THIRD protocol was 0.81 (95% confidence
interval=0.73-0.89; P<0.001) for all patients. Table 6
shows the kappa index, sensitivity, specificity, PPV,
and NPV of the THIRD protocol for determining
each type of shock among patients with definite final
diagnoses.
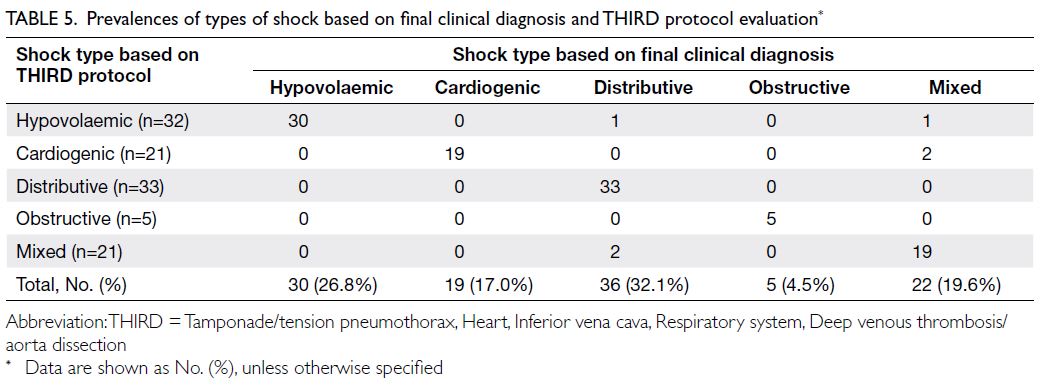
Table 5. Prevalences of types of shock based on final clinical diagnosis and THIRD protocol evaluation
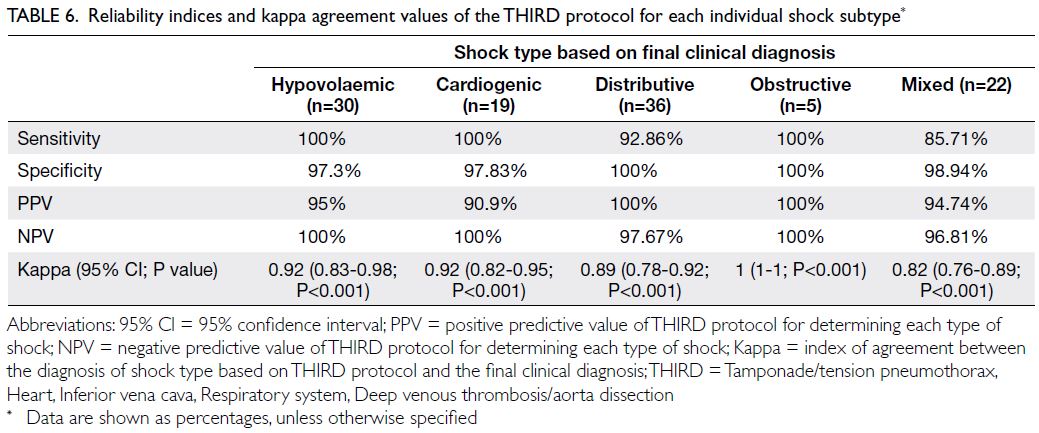
Table 6. Reliability indices and kappa agreement values of the THIRD protocol for each individual shock subtype
Hypovolaemic shock
Using the THIRD protocol, 32 patients had a
diagnosis of hypovolaemic shock. The causes of
hypovolaemic shock were traumatic bleeding
(n=23), sepsis (n=2), acute gastrointestinal bleeding
(n=3), cachexia (n=1), and pancreatitis (n=1). The
remaining two patients included in the 32 patients
were misdiagnosed with hypovolaemic shock based
on their ultrasound findings, but the final clinical
diagnoses were mixed shock (n=1) and distributive
shock secondary to sepsis (n=1) [97.3% specificity
and 95% PPV].
Cardiogenic shock
Using the THIRD protocol, 21 patients had a
diagnosis of cardiogenic shock. Of them, 19 patients
had a final clinical diagnosis of cardiogenic shock
due to decompensated heart failure (n=8), acute
myocardial infarction (n=7), and intoxication
(n=4). The final clinical diagnosis for the remaining
two patients was mixed aetiology shock (97.83%
specificity and 90.9% PPV). The agreement between
ultrasound findings and the final diagnosis was 92%
(P<0.001) for cardiogenic shock.
Distributive shock
Using the THIRD protocol, 33 patients were
diagnosed with distributive shock. Of them,
30 patients had a final clinical diagnosis of sepsis
(concurrent pneumonia [n=26], concurrent
cholangitis [n=2], concurrent urinary tract
infections [n=2]) and three patients had a final
clinical diagnosis of neurogenic aetiologies. Three
other patients had a final clinical diagnosis of
distributive shock, who were initially misdiagnosed
using the THIRD protocol with hypovolaemic (n=1)
and mixed aetiology shock (n=2). The agreement
between ultrasound findings and the final diagnosis
was 89% (P<0.001) for distributive shock.
Obstructive shock
Using the THIRD protocol, five patients were
diagnosed with obstructive shock, and all five of
them had a final clinical diagnosis of obstructive
shock (cardiac tamponade [n=3], large, acute
pulmonary embolism [n=2]). The agreement
between ultrasound findings and the final diagnosis
was 100% (P<0.001) for obstructive shock.
Mixed aetiology shock
Using the THIRD protocol, 21 patients were
diagnosed with mixed aetiology shock. Of them,
19 had a final clinical diagnosis of mixed aetiology
shock (sensitivity of 90.4%). The remaining two
patients were misdiagnosed with mixed aetiology
shock; the final clinical diagnosis was distributive
shock (n=2). Three other patients had a final clinical
diagnosis of mixed aetiology shock, who were
initially misdiagnosed using the THIRD protocol
with hypovolaemic (n=1) or cardiogenic shock (n=2).
The THIRD protocol had the lowest agreement (82%,
P<0.001) with the final diagnosis for mixed aetiology
shock.
Discussion
In this prospective study, the primary diagnosis after
implementation of the THIRD protocol in patients
with undifferentiated shock was highly concordant
with the final clinical diagnosis. The protocol
was highly effective in guiding the rapid bedside
management of undifferentiated shock in emergency
settings, particularly for patients with obstructive, hypovolaemic, or cardiogenic shock.
Point-of-care ultrasound is the only tool
available at the bedside that can rapidly reveal
acute pathophysiology and establish key diagnoses
to guide targeted interventions.15 As in other
disciplines, POCUS has been commonly used in
emergency medicine; it is an essential skill for
emergency physicians. In mainland China, bedside
ultrasound has been used in EDs since 2006, mainly
for guidance during vascular puncture procedures
and for the assessment of free intraperitoneal fluid.16
After a decade of rapid development, nearly half of
EDs in China have dedicated bedside ultrasound
equipment.17 The applications of POCUS have
gradually expanded to undifferentiated hypotension,
shortness of breath, chest pain, sepsis, and cardiac
arrest, as well as other clinical manifestations.
To our knowledge, the THIRD protocol is
the first ultrasound protocol for assessment of
undifferentiated shock in mainland China, and this
is the first study to validate the effectiveness and
accuracy of the protocol. This study demonstrated
favourable general agreement between the final
clinical diagnosis of shock and the results of this early bedside ultrasound assessment (kappa=0.81). Similar
to the RUSH protocol,13 the highest agreements
were observed in patients with hypovolaemic,
cardiogenic, and obstructive shock (kappa values
of 0.92, 0.92, and 1.00, respectively). The NPVs for
these shock types were all 100%, suggesting that
the THIRD protocol can reliably exclude these
types of shock. Clinically significant hypovolaemia,
cardiac dysfunction, cardiac tamponade, pulmonary
embolism, and tension pneumothorax are readily
identifiable on ultrasound; the corresponding signs
facilitate rapid diagnosis and ensure minimal delays
in life-saving interventions for these conditions.4 18
The sensitivity, NPV, and kappa values of
the THIRD protocol were lower for distributive or
mixed shock than for the other three types of shock.
Sepsis was the main cause of distributed shock in
our study; high cardiac output with reduced vascular
resistance is the main pathophysiological feature of
this type of shock.19 A plausible explanation for this
pathophysiological feature is that a hyperdynamic
heart is not specific to distributive shock, and
a decrease in vascular resistance lacks specific
ultrasound signs. Our protocol had the least
sensitivity and agreement for mixed aetiology shock.
Considering this increased uncertainty, caution is
advised when making a diagnosis of a ‘mixed’ type of
shock.
Since its initial use in 2001 by Rose et al,20
POCUS has been increasingly used in the
management of patients with undifferentiated shock
in the ED. Furthermore, >15 ultrasound protocols for
hypotension have been developed since 2001.7 These
protocols consist of items such as echocardiography,
transthoracic scanning, evaluation of the inferior
vena cava and aorta, assessment of free fluid in
the abdominal cavity, and detection of deep vein
thrombosis. The overall goal of these protocols is to
provide a comprehensive and practical approach for
the classification of clinical syndromes that involve
circulatory failure—syndromes which lack specificity
and may have substantially different possible
treatments—into four specific and manageable types
of shock.
The RUSH protocol is one of the most frequently
used POCUS protocols for undifferentiated shock.21
Multiple studies have shown that the kappa index of
the RUSH protocol–based ultrasound diagnosis and
the final clinical diagnosis is approximately 0.7.13 14 22
The RUSH protocol is used to find ultrasound
abnormalities in three major aspects of a patient’s
physiology, including ‘pump, tank, and pipe’.23 Unlike
the RUSH protocol or other existing POCUS shock
protocols (eg, ACES,24 UHP,20 or FATE25), each
letter of the THIRD protocol represents a specific
ultrasound assessment. The THIRD protocol is
easy for clinicians to remember and can be used
as a practical checklist for ultrasound examination during the management of patients with shock. This
might explain why trainees had greater confidence
and performance when using the THIRD protocol
than when using the RUSH protocol in a training
curriculum.10
There were some limitations in this study. First, we did not exclude certain patients, such as patients
with traumatic injuries or gastrointestinal bleeding.
However, trauma and gastrointestinal bleeding are
common among patients who present to our ED,
and the inclusion of these patients ensures that
our shock assessment is consistent with real-world
emergency settings. Second, we did not compare
the THIRD protocol with other protocols, such as
the RUSH protocol; thus, we cannot conclusively
state whether the THIRD and RUSH protocols are
equally effective. Third, we did not assess the impact
of the THIRD protocol on subsequent treatment. In
a previous study, 24.6% of patients had a statistically
significant change in their management after a
POCUS protocol examination.12
In conclusion, this study demonstrated that
the initial diagnostic judgements obtained using
the THIRD protocol in the ED are consistent
with the final diagnosis in patients who present
with undifferentiated shock. The findings in this
study encourage the incorporation of the THIRD
protocol into routine ED assessment of patients
with undifferentiated shock to help guide early
interventions. The impact of the THIRD protocol
on the outcomes of patients with shock, as well as
comparisons of the effectiveness and accuracy of
the THIRD protocol with other POCUS protocols,
should be the focus of future studies.
Author contributions
Concept or design: P Geng, B Ling, J Xu, D Tan.
Acquisition of data: B Ling, Y Song, H Wang, Q Zhu, Y Yang, M Lu.
Analysis or interpretation of data: Y Song, H Wang, Q Zhu, Y Yang, M Lu.
Drafting of the manuscript: B Ling, JH Walline, D Tan, P Geng.
Critical revision of the manuscript for important intellectual content: D Tan, JH Walline, J Xu, P Geng.
Acquisition of data: B Ling, Y Song, H Wang, Q Zhu, Y Yang, M Lu.
Analysis or interpretation of data: Y Song, H Wang, Q Zhu, Y Yang, M Lu.
Drafting of the manuscript: B Ling, JH Walline, D Tan, P Geng.
Critical revision of the manuscript for important intellectual content: D Tan, JH Walline, J Xu, P Geng.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgement
We are grateful to J Ye and J Zhang for their contributions to establish the diagnosis of shock type in this study.
Funding/support
This work was supported by the Rui E Special Fund for Emergency Medicine Research (R2017003), the Yangzhou Science and Technology Development Plan (YZ2018090),
the Yangzhou Phase III ‘Talent Cultivation Program’ Support
Project (2018034), the Hospital-Level Support Project of
Northern Jiangsu People’s Hospital (yzucms2018943), and the
Hospital-Level Support Project of Northern Jiangsu People’s
Hospital (fcjs201708, fcjs201842).
Ethics approval
This study protocol was approved by the Institutional Ethics
Committee of Northern Jiangsu People’s Hospital (Ref:
fcjs2017008). Written informed consent was obtained from
all patients (or next of kin, if the patient was unable to provide
informed consent). The study was registered in the Chinese
Clinical Trial Registry (Ref: ChiCTR2000031072).
References
1. Hiemstra B, Eck RJ, Keus F, van der Horst IC. Clinical
examination for diagnosing circulatory shock. Curr Opin
Crit Care 2017;23:293-301. Crossref
2. Goodwin M, Ito K, Gupta AH, Rivers EP. Protocolized
care for early shock resuscitation. Curr Opin Crit Care
2016;22:416-23. Crossref
3. Sasmaz MI, Gungor F, Guven R, Akyol KC, Kozaci N,
Kesapli M. Effect of focused bedside ultrasonography in
hypotensive patients on the clinical decision of emergency
physicians. Emerg Med Int 2017;2017:6248687. Crossref
4. Koster G, van der Horst IC. Critical care ultrasonography
in circulatory shock. Curr Opin Crit Care 2017;23:326-33. Crossref
5. Whitson MR, Mayo PH. Ultrasonography in the emergency
department. Crit Care 2016;20:227. Crossref
6. PRISM Investigators; Rowan KM, Angus DC, Bailey M, et al.
Early, goal-directed therapy for septic shock—a patient-level
meta-analysis. N Engl J Med 2017;376:2223-34. Crossref
7. Mok KL. Make it SIMPLE: enhanced shock management by focused cardiac ultrasound. J Intensive Care 2016;4:51. Crossref
8. Alpert EA. The ABCDs of ResUS—Resuscitation
ultrasound. Cureus 2019;11:e4616. Crossref
9. Unexplained shock emergency ultrasound clinical practice
expert consensus group. Expert consensus on emergency
ultrasound practice for unexplained shock [in Chinese].
Chin J Emerg Med 2017;26:498-506.
10. Shi D, Liu J, Xu J, Zhu H, Yu X. Evaluation of a new goal-directed
training curriculum for point-of-care ultrasound
in the emergency department: impact on physician self-confidence
and ultrasound skills. Eur J Trauma Emerg Surg
2021;47:435-44. Crossref
11. Cecconi M, De Backer D, Antonelli M, et al. Consensus
on circulatory shock and hemodynamic monitoring. Task
force of the European Society of Intensive Care Medicine.
Intensive Care Med 2014;40:1795-815. Crossref
12. Shokoohi H, Boniface KS, Pourmand A, et al. Bedside
ultrasound reduces diagnostic uncertainty and guides
resuscitation in patients with undifferentiated hypotension.
Crit Care Med 2015;43:2562-9. Crossref
13. Ghane MR, Gharib MH, Ebrahimi A, et al. Accuracy of
rapid ultrasound in shock (RUSH) exam for diagnosis of
shock in critically ill patients. Trauma Mon 2015;20:e20095.
14. Volpicelli G, Lamorte A, Tullio M, et al. Point-of-care
multiorgan ultrasonography for the evaluation
of undifferentiated hypotension in the emergency
department. Intensive Care Med 2013;39:1290-8. Crossref
15. Sun JT. New advances in emergency ultrasound protocols for shock. J Med Ultrasound 2017;25:191-4. Crossref
16. Vincent JL, De Backer D. Circulatory shock. N Engl J Med
2013;369:1726-34. Crossref
17. Shi D, Walline JH, Yu X, Xu J, Song PP, Zhu H. Evaluating
and assessing the prevalence of bedside ultrasound
in emergency departments in China. J Thorac Dis
2018;10:2685-90. Crossref
18. Bernier-Jean A, Albert M, Shiloh AL, Eisen LA,
Williamson D, Beaulieu Y. The diagnostic and therapeutic
impact of point-of-care ultrasonography in the intensive
care unit. J Intensive Care Med 2017;32:197-203. Crossref
19. Russell JA. Vasopressor therapy in critically ill patients
with shock. Intensive Care Med 2019;45:1503-17. Crossref
20. Rose JS, Bair AE, Mandavia D, Kinser DJ. The UHP
ultrasound protocol: a novel ultrasound approach to the
empiric evaluation of the undifferentiated hypotensive
patient. Am J Emerg Med 2001;19:299-302. Crossref
21. Keikha M, Salehi-Marzijarani M, Soldoozi Nejat R, Sheikh
Motahar Vahedi H, Mirrezaie SM. Diagnostic accuracy
of rapid ultrasound in shock (RUSH) Exam; a systematic
review and meta-analysis. Bull Emerg Trauma 2018;6:271-8.
22. Ghane MR, Gharib M, Ebrahimi A, et al. Accuracy of early
rapid ultrasound in shock (RUSH) examination performed
by emergency physician for diagnosis of shock etiology in
critically ill patients. J Emerg Trauma Shock 2015;8:5-10. Crossref
23. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH
exam: Rapid Ultrasound in SHock in the evaluation of the
critically ill. Emerg Med Clin North Am 2010;28:29-56,
vii. Crossref
24. Atkinson PR, McAuley DJ, Kendall RJ, et al. Abdominal
and Cardiac Evaluation with Sonography in Shock (ACES):
an approach by emergency physicians for the use of
ultrasound in patients with undifferentiated hypotension.
Emerg Med J 2009;26:87-91. Crossref
25. Sordillo LA, Pu Y, Pratavieira S, Budansky Y, Alfano RR.
Deep optical imaging of tissue using the second and
third near-infrared spectral windows. J Biomed Opt
2014;19:056004. Crossref