Hong Kong Med J 2022 Aug;28(4):300–5 | Epub 17 Mar 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Rapid antigen test during a COVID-19 outbreak
in a private hospital in Hong Kong
Jonpaul ST Zee, FRCPath, FHKAM (Medicine)1,2; Chris TL Chan, BSc (Hons) (UK), PhD (HK)1; Alex CP Leung, MMedsc (HKU)1; Bella PW Yu, MNurs2; Jhan Raymond L Hung, MNurs2; Queenie WL Chan, BScN, FHKAN (Medicine-Infection Control)2; Edmond SK Ma, MD (HK), FRCPath1; KH Lee, MMed Sc (HKU), FHKAM (Community Medicine)3; CC Lau, MB, BS, FHKAM (Emergency Medicine)3; Raymond WH Yung, MB, BS, FHKCPath1,2,3
1 Department of Pathology, Hong Kong Sanatorium & Hospital, Hong Kong
2 Infection Control Team, Hong Kong Sanatorium & Hospital, Hong Kong
3 Hospital Administration, Hong Kong Sanatorium & Hospital, Hong Kong
Corresponding author: Dr Jonpaul ST Zee (jonpaul.st.zee@hksh.com)
Abstract
Introduction: In response to two nosocomial
clusters of coronavirus disease 2019 (COVID-19)
in our hospital, we adopted a series of strict
infection control measures, including regular rapid
antigen test (RAT) screening for high-risk patients,
visitors, and healthcare workers. We evaluated the
diagnostic performance of a locally developed RAT,
the INDICAID COVID-19 Rapid Antigen Test
(Phase Scientific, Hong Kong), using respiratory
samples from both symptomatic and asymptomatic
individuals.
Methods: Real-time reverse-transcription
polymerase chain reaction (rRT-PCR)–confirmed
deep throat saliva (DTS) and pooled nasopharyngeal
swab and throat swab (NPS/TS) samples collected
from 1 November to 30 November 2020 were tested
by INDICAID. Screening RATs were performed on
asymptomatic healthcare workers during a 16-week
period (1 December 2020 to 22 March 2021).
Results: In total, 20 rRT-PCR-confirmed samples
(16 DTS, four pooled NPS/TS) were available for
RAT. Using the original sample, RAT results were
positive in 17/20 samples, indicating 85% sensitivity
(95% confidence interval [CI]=62.11%-96.79%).
Negative RAT results were associated with higher
cycle threshold (Ct) values. For samples with Ct
values <25, the sensitivity was 100%. Of the 49801 RATs collected from healthcare workers, 33
false positives and one rRT-PCR-confirmed case
were detected. The overall specificity was 99.93%
(95% CI=99.91%-99.95%). The positive and negative
predictive values were 2.94% (95% CI=2.11%-4.09%)
and 100%, respectively.
Conclusions: The INDICAID COVID-19 RAT demonstrated good sensitivity for specimens with
high viral loads and satisfactory specificity for low-risk,
asymptomatic healthcare workers.
New knowledge added by this study
- Rapid antigen tests (RATs) are simple and rapid; they have high sensitivity for specimens with high viral loads. When RATs were applied as point-of-care tests, using specimens intended analysis by for real-time reverse-transcription polymerase chain reaction (rRT-PCR), infected patients could be identified before molecular results were available.
- The use of RATs to regularly screen asymptomatic high-risk patients, visitors, and healthcare workers during a coronavirus disease 2019 outbreak led to successful control of the nosocomial outbreak and prevented further entry of community-acquired infections into the hospital.
- The use of screening RATs and the establishment of a registration system for patient visitors led to minimal laboratory service disruption; visitation policies were maintained without reducing infection control measures.
- RATs are appropriate for the screening of individuals with recent exposure or early symptoms because of their high sensitivities for specimens with high viral loads.
- RATs can be used in conjunction with rRT-PCR in outbreak situations to allow the rapid triage and isolation of infected individuals before confirmatory rRT-PCR results are available.
- Regular RAT screening for asymptomatic high-risk patients, visitors, and healthcare workers is useful for preventing nosocomial outbreaks while causing minimal disturbances to laboratory services and visitation policies.
Introduction
Rapid diagnosis of coronavirus disease 2019
(COVID-19) is crucial, particularly during an
outbreak situation when the segregation and
immediate isolation of infected individuals are
critical. This is because up to half of severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2)
infections are asymptomatic; moreover, infection
transmission can be greater during the pre-symptomatic
phase than during the symptomatic
phase, leading to silent transmission.1 2 The ideal
diagnostic test should be easy to perform and
interpret; it should also have a rapid turnaround
time. Despite higher costs and greater technical
demands, the detection of unique viral sequences
(eg, E, RdRP, N, and S genes) by nucleic acid
amplification tests such as real-time reverse-transcription
polymerase chain reaction (rRT-PCR)
remains the ‘gold standard’ for diagnosis because of
superior sensitivity and specificity.3 Although most
contemporary automated PCR platforms are capable
of integrated sample preparation, amplification, and
software-assisted result interpretation, most such
tests require approximately 1 hour to perform; this
duration excludes specimen transportation time
from the bedside or the field to the laboratory,
as well as time for preparation by laboratory
personnel. In contrast, rapid antigen tests (RATs;
ie, immunochromatographic membrane assays), commonly known as lateral flow assays, are
gaining popularity. Rapid antigen tests are rapid,
easily deployable in the field without the need for
specialised equipment, and relatively inexpensive;
they require only minimal training for performance
and subsequent interpretation of the results. Despite
their lower sensitivities, several antigen-based
diagnostic tests have received in vitro diagnostics
emergency use authorisations from the United States
Food and Drug Administration4 and are considered
valuable for reducing transmission through the early
detection of highly infectious cases and facilitation
of contact tracing.5
Since the first local case of COVID-19 were
confirmed on 4 February 2020, Hong Kong has
experienced four waves of COVID-19 surges with
over 11 000 cases reported. The fourth wave, which
began in late October/early November, primarily
comprised multiple clusters of locally acquired
infections that involved food premises, construction
sites, nursing homes, and dancing/singing venues.6
In November 2020, two clusters of nosocomial
transmission of COVID-19 were found in a private
ward and the renal dialysis unit of Hong Kong
Sanatorium & Hospital. In both clusters, the source
of nosocomial infection could be traced back to
visitors and relatives of patients who belonged to the
largest local COVID-19 cluster–the dancing/singing
cluster. As a precautionary measure against future
transmission, the hospital subsequently adopted a
strict registration policy for patient visitors. Each
patient could register a maximum of three visitors;
each patient visitor was required to undergo RATs
at 3-day intervals. Single RATs were required for
other hospital visitors, including technicians and
contractors who remained in clinical areas for
>1 hour. In addition to the mandatory pre-admission
PCR screening for all in-patients, PCR was repeated
at 7-day intervals for long-term in-patients. For
haemodialysis and oncology patients who required
frequent visits, RATs were required at 3-day intervals
or before each haemodialysis session, in addition to a
weekly PCR test. Single RATs were also required for
out-patient visits that involved mask-off procedures,
such as dental procedures, rhinoscopy, lung function
tests, or gastroscopy. In this study, we evaluated
the diagnostic performance of the INDICAID
COVID-19 Rapid Antigen Test (Phase Scientific,
Hong Kong) using respiratory samples submitted by
patients and staff members.
Methods
Clinical specimens
The rRT-PCR-confirmed SARS-CoV-2-positive
respiratory specimens, including posterior
pharyngeal saliva (ie, deep throat saliva; DTS) and
pooled nasopharyngeal swab and throat swab (NPS/TS), submitted to our laboratory during 1 to
30 November 2020 were subjected to additional
RATs. Deep throat saliva specimens were self-collected,
in accordance with instructions from
local health authorities.7 8 A video with detailed
instructions was shown to all patients before the
collection of their DTSs in a well-ventilated area
with a hand-washing facility. Each DTS was spit into
an empty sterile container, which was then double-bagged
and submitted to the designated collection
point in our hospital. The NPS/TS specimens were
collected by healthcare workers in full personal
protection equipment using a Dryswab™ PurFlock®
(Medical Wire, United Kingdom) for nasal swabbing
and a flocked swab (Taizhou Sun Trine Biotechnology
Co, Ltd, Taizhou City, China) for throat swabbing.
Both swabs were submerged in the same viral
transport medium (Biologix, Shandong, China),
then double bagged and immediately transferred
to the laboratory. Nasal swabs collected for the
screening of asymptomatic hospital staff members
from 1 December 2020 to 22 March 2021 were
included for analysis. Nasal swabs were collected
by healthcare workers using swabs provided by the
RAT manufacturer. Each swab was inserted 2.5 cm
into each nostril, twisted for 5 seconds, and then
swirled in buffer solution at least 20 times.
Severe acute respiratory syndrome
coronavirus 2 detection by nucleic acid
amplification test
Deep throat saliva specimens (approximately 500 µL) from patients were mixed in a 1:1 (v/v) ratio with
Sputasol (Oxoid, England), vortexed for 1 minute
to reduce viscosity, and spun for 1 minute. An
approximately 300-µL aliquot of the mixture was
transferred to the Xpert® Xpress SARS-CoV-2
cartridge. Nucleic acid amplification tests of DTS
and pooled NPS/TS were performed in accordance
with the manufacturer’s protocol.
Rapid antigen test
The INDICAID COVID-19 Rapid Antigen Test is an
immunochromatographic membrane assay intended
for the qualitative detection of SARS-CoV-2
nucleocapsid antigens in nasal swab and NPS
samples. The SARS-CoV-2-specific monoclonal
antibodies and a control antibody are immobilised
at the test line (T) region and control line (C)
region of a nitrocellulose membrane in a plastic
cassette. Monoclonal anti-SARS-CoV-2 antibodies
conjugated with red colloidal gold particles are used
to detect the SARS-CoV-2 antigen. In accordance
with the test protocol, the collected nasal swab or
NPS was swirled 20 times in the buffer solution;
three drops of the buffer solution were then applied
to the sample well. When the SARS-CoV-2 antigen was present, it bound to the antibody-gold conjugate
to form an immunocomplex. The immunocomplex
then travelled across the strip via capillary action
and bound to the SARS-CoV-2 antibodies at the test
line (T), forming a visible red line. The test result
was intended to be read between 20 and 25 minutes
after sample application to the well. The result was
considered invalid if the control line was invisible
(Fig). The result was considered false positive
if a subsequent PCR result was negative, or the
positive band was not reproducible upon repeated
assessment with a new INDICAID kit.
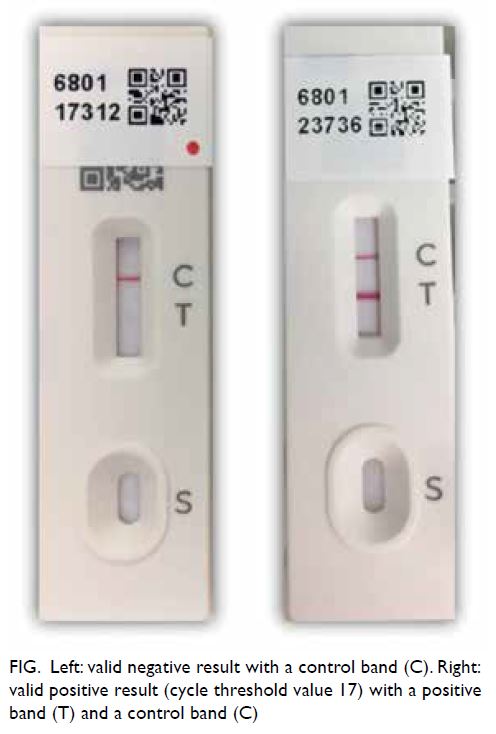
Figure. Left: valid negative result with a control band (C). Right: valid positive result (cycle threshold value 17) with a positive band (T) and a control band (C)
For RATs using DTS specimens, a 50-µL
aliquot of Sputasol-treated DTS was mixed with
100 µL of INDICAID buffer. An approximately
100-µL aliquot of the mixture was then transferred
to the sample well of the INDICAID kit.
For RATs using pooled NPS/TS specimens, a
50-µL aliquot of viral transport medium was added
to the INDICAID buffer solution; a 100-µL aliquot of
the mixture was then transferred to the sample well
of the INDICAID kit.
Data analysis
To evaluate RAT sensitivity, we calculated the
proportion of rRT-PCR-confirmed SARS-CoV-2-positive respiratory specimens that were correctly identified as positive by the RAT. Nasal swabs from
asymptomatic hospital staff were used for evaluation
of the RAT false positive rate, specificity, positive
predictive value, and negative predictive value.
Statistical tests were performed using MedCalc®
(https://www.medcalc.org/).
Results
In total, 20 PCR positive samples (16 DTS, four
pooled NPS/TS) were available for further testing
by RAT (Table 1). These specimens belonged to
18 symptomatic or asymptomatic patients who
attended the hospital’s out-patient department
and two hospital staff members who had positive
screening results during contact tracing of a
nosocomial cluster of COVID-19. Using the original
sample, RATs yielded positive results in 17 samples,
demonstrating 85% sensitivity (95% confidence
interval [CI]=62.11%-96.79%). Negative RAT results
were associated with higher cycle threshold (Ct)
values. For samples with Ct values <25 (Xpert Xpress
SARS-CoV-2), the sensitivity was 100%.
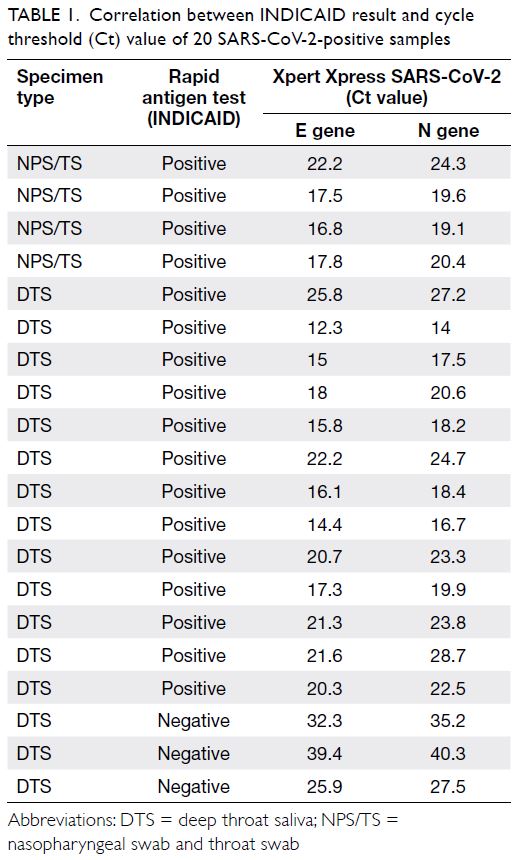
Table 1. Correlation between INDICAID result and cycle threshold (Ct) value of 20 SARS-CoV-2-positive samples
In total, 49 801 RAT screenings were performed
on asymptomatic healthcare workers during 16 weeks from 1 December 2020 to 22 March 2021 (Table 2).
In all, 33 false positives and one PCR-confirmed
case were detected during this period. In the first
week of hospital-wide staff screening, all specimens
with positive RAT results exhibited negative PCR
results. Importantly, these false positives were not
reproducible by a repeat RAT, and many of them were
caused by delays in reading the results (>25 min).
Therefore, staff members were subsequently advised
to strictly adhere to the manufacturer’s instructions;
PCR was not performed unless a repeat RAT also
yielded positive results. We also ensured that the
healthcare workers with positive screening results
were asymptomatic and did not have any recent
exposure to confirmed cases; otherwise, rRT-PCR
was performed. The reported false positive rate
greatly decreased in subsequent weeks. The false
positive rate of INDICAID was approximately
1/1509 tests in our cohort. The overall specificity
was 99.93% (95% CI=99.91%-99.95%). The positive
predictive value was 2.94% (95% CI=2.11%-4.09%),
while the negative predictive value was 100%.
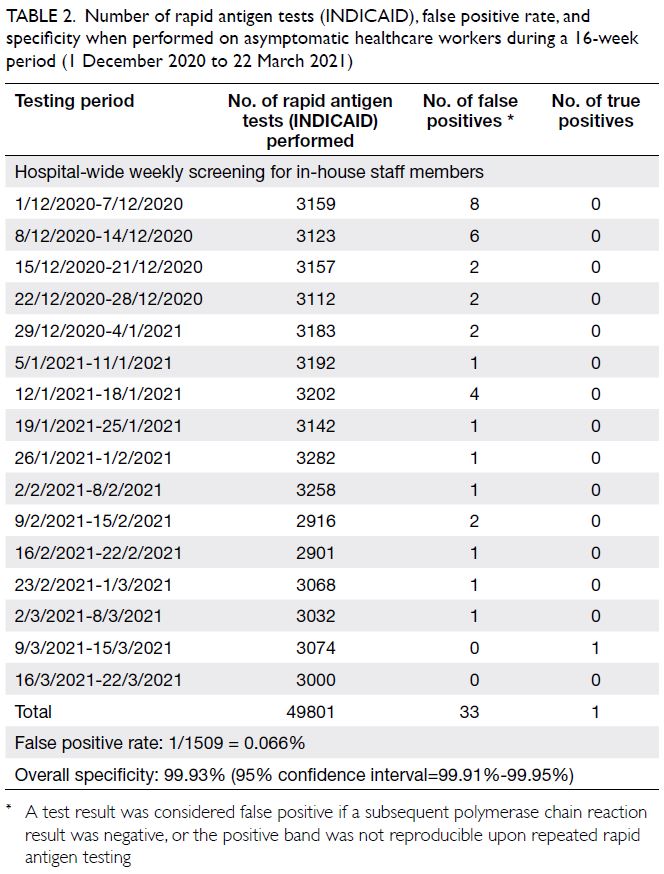
Table 2. Number of rapid antigen tests (INDICAID), false positive rate, and specificity when performed on asymptomatic healthcare workers during a 16-week period (1 December 2020 to 22 March 2021)
A staff member from the Engineering and
Maintenance Department exhibited positive RAT
results during his pre-symptomatic period in March
2021. He subsequently exhibited positive rRT-PCR
results (Ct values of approximately 20) and developed
mild upper respiratory tract symptoms. This staff
member had no known exposure to a confirmed
COVID-19 case but had received physiotherapy
in the hospital during the incubation period. He
did not have any direct patient contact. His close
contacts, including co-workers who shared the
same workspace and his attending physiotherapist,
were offered immediate screening. All of his close
contacts were quarantined, but no secondary cases
were identified.
Discussion
The RAT used in this study was a SARS-CoV-2
antigen lateral flow assay with a reported detection
limit of 140 TCID50/swab; it has positive and
negative percent agreements of 96% (95% CI=86.3%-99.5%) and 100% (95% CI=92.9%-100%), respectively,
when performed on contrived samples near the
test’s limit of detection (2xLoD) and simulated
negative matrix. Although the manufacturer does
not specifically recommend the use of DTS and
pooled NPS/TS specimens, our evaluation showed a
satisfactory sensitivity for these samples, particularly
for samples with high viral loads (100% sensitivity
for Ct values <25). The INDICAID test specificity
was high; however, the positive predictive value
was only 2.94% (95% CI=2.11%-4.09%). This finding
was presumably caused by low disease prevalence
in our cohort because all RATs were performed on
asymptomatic healthcare workers without exposure
history.
In a Cochrane review of five studies regarding
SARS-CoV-2 RATs, their sensitivities considerably
varied (mean, 56.2%; 95% CI=29.5%-79.8%), while
their specificities were consistently high (mean,
99.5%; 95% CI=98.1%-99.9%).9 The World Health
Organisation recommends the use of SARS-CoV-2
RATs for screening to support outbreak investigations
and contact tracing for rapid isolation of positive
cases; they should also be used in communities
with widespread transmission where the nucleic
acid amplification test capacity is limited, although
such tests should meet the minimum performance
requirements of ≥80% sensitivity and ≥97%
specificity. Moreover, a negative RAT result should be
considered presumptive and insufficient for removal
of a contact from quarantine requirements.10 The
European Centre for Disease Prevention and Control
has higher performance requirements of ≥90%
sensitivity and ≥97% specificity for SARS-CoV-2
RATs. The positive predictive value of any clinical
test could be influenced by the pretest probability.
Therefore, both the World Health Organisation and
the European Centre for Disease Prevention and
Control do not recommend the use of SARS-CoV-2
RATs on asymptomatic individuals without contact
history and in low prevalence communities (eg,
<10%).5 10 The United States Centers for Disease
Control and Prevention has provided an antigen
test algorithm that focuses on pretest probability: a
negative RAT result should be confirmed by a nucleic
acid amplification test in situations where the pretest
probability is high, while a negative antigen test
could indicate the absence of SARS-CoV-2 infection
in an asymptomatic individual who had no known
exposure to a COVID-19 case within the previous
14 days.11
Rapid antigen test sensitivity is higher during
the early course of infection (5-7 days after symptom
onset) when both viral load and infectivity are at their
peaks.9 10 12 13 14 A negative RAT result is insufficient
to rule out infection, although it is associated with
lower infectivity. In a field evaluation of the Panbio™
COVID-19 Ag Rapid Test Device for symptomatic
patients (n=412) attending primary healthcare
centres, SARS-CoV-2 could not be cultured from
specimens that yielded rRT-PCR+/RAT– results
(n=11); the authors of the study concluded that
patients with RT-PCR-proven COVID-19 and
negative RAT results were unlikely to be infectious.15
Because of their timeliness and simplicity, RATs
provide added value for contact tracing and patient
triage. Considering the limitations of RATs, we
used them as screening tools for people who were
at highest risk of SARS-CoV-2 transmission, such
as immunocompromised oncology and renal failure
patients who attended out-patient chemotherapy
and haemodialysis treatment centres, as well as out-patients
who underwent mask-off procedures. Our frequent screening approach constituted an attempt
to compensate for the moderate sensitivity of the
RAT. The scale of screening in our hospital was very
large and could only be achieved by a point-of-care
test that permitted decentralised testing (ie, at the
site of clinical encounter); this allowed minimal
impact to our daily laboratory operation.
Among the 49 801 RATs performed for weekly
staff screening during the 16-week study period, only
one PCR-confirmed case was detected. Although
the cost-effectiveness has not been determined, the
early case detection could have prevented a major
nosocomial outbreak and service disruption affecting
the Engineering and Maintenance Department and
the Physiotherapy Department.
To control the fourth wave of COVID-19 in
Hong Kong, authorities repeatedly enforced
lockdowns within communities containing multiple
cases of COVID-19; this facilitated mandatory
testing of all residents in those communities. When
respiratory samples were collected for complementary
RAT and PCR assessments, positive results could be
obtained before molecular results were available. Rapid antigen tests allowed rapid specimen triage
and the preliminary isolation of individuals with
presumptive positive results. This type of dual-track
testing was also used during screening of a local
community outbreak (personal communication). In
addition to the screening function, RATs have been
utilised by some laboratories for secondary rapid
confirmation of positive rRT-PCR results.
Our study had several limitations. First, we
could not evaluate the diagnostic sensitivity of the
INDICAID test using the recommended types of
specimens (ie, nasal swab and NPS) because most of
our patient samples were DTS and pooled NPS/TS.
Second, asymptomatic infections with viral loads
below the INDICAID detection limit could have
been missed because no parallel rRT-PCR analyses
were conducted. Third, the effects of mutant
SARS-CoV-2 strains on the INDICAID detection
limit were not evaluated.
In conclusion, RATs are rapid and simple
point-of-care tools that can shorten the COVID-19
testing turnaround time; they can be used in many
different strategies. Our study showed that the
INDICAID COVID-19 RAT has good sensitivity
for specimens with high viral loads and satisfactory
specificity for low-risk, asymptomatic healthcare
workers.
Author contributions
Concept or design: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: JST Zee.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: JST Zee.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
As an epidemiology adviser of the journal, ESK Ma was not involved in the peer review process. Other authors have
disclosed no conflicts of interest.
Acknowledgement
The authors acknowledge the excellent work and contributions
by staff members at the Clinical Pathology Laboratory,
Infection Control Team, and Audit Office of Quality and
Safety Division of Hong Kong Sanatorium & Hospital.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study obtained ethics approval (RC-2021-08) from the Research Ethics Committee of the Hong Kong Sanatorium &
Hospital Medical Group.
References
1. Ren R, Zhang Y, Li Q, et al. Asymptomatic SARS-CoV-2 infections among persons entering China from April 16 to
October 12, 2020. JAMA 2021;325:489-92. Crossref
2. Li F, Li YY, Liu MJ, et al. Household transmission of
SARS-CoV-2 and risk factors for susceptibility and
infectivity in Wuhan: a retrospective observational study.
Lancet Infect Dis 2021;21:617-28. Crossref
3. World Health Organization. Diagnostic testing for
SARS-CoV-2 Interim guidance, 11 September 2020.
Available from: https://apps.who.int/iris/handle/10665/334254. Accessed 11 Sep 2020.
4. US Food and Drug Administration. In vitro diagnostics EUAs. Available from: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas. Accessed 6 May 2021.
5. European Centre for Disease Prevention and Control. Options for the use of rapid antigen tests for COVID-19 in the EU/EEA and the UK. 19 November 2020. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Options-use-of-rapid-antigen-tests-for-COVID-19.pdf. Accessed 6 May 2021.
6. Liu Y, Gu Z, Liu J. Uncovering transmission patterns
of COVID-19 outbreaks: a region-wide comprehensive
retrospective study in Hong Kong. EClinicalMedicine
2021;36:100929. Crossref
7. Centre for Health Protection, Department of Health, Hong
Kong SAR Government. Information sheet on deep throat
saliva (DTS) collection. Available from: https://www.chp.gov.hk/files/pdf/information_sheet_on_dts_en.pdf. Accessed 6 May 2021.
8. Hospital Authority, Hong Kong SAR Government.
Patient information sheet on deep throat saliva collection.
Available from: https://www.ha.org.hk/haho/ho/cc/Information_sheet_en_txt.pdf. Accessed 6 May 2021.
9. Dinnes J, Deeks JJ, Adriano A, et al. Rapid, point-of-care antigen
and molecular-based tests for diagnosis of SARS-CoV-2
infection. Cochrane Database Syst Rev 2020;(8):CD013705. Crossref
10. World Health Organization. SARS-CoV-2 antigen-detecting
rapid diagnostic tests: an implementation guide.
Available from: https://www.who.int/publications/i/item/9789240017740. Accessed 6 May 2021.
11. US Centers for Disease Control and Prevention. Using
antigen tests. Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html. Accessed 16 Dec 2020.
12. Li D, Li J. Immunologic testing for SARS-CoV-2 infection from the antigen perspective. J Clin Microbiol
2021;59:e02160-20. Crossref
13. Prince-Guerra JL, Almendares O, Nolen LD, et al.
Evaluation of Abbott BinaxNOW rapid antigen test for
SARS-CoV-2 infection at two community-based testing
sites—Pima County, Arizona, November 3-17, 2020.
MMWR Morb Mortal Wkly Rep 2021;70:100-5. Crossref
14. Kohmer N, Toptan T, Pallas C, et al. The comparative
clinical performance of four SARS-CoV-2 rapid antigen
tests and their correlation to infectivity in vitro. J Clin Med
2021;10:328. Crossref
15. Albert E, Torres I, Bueno F, et al. Field evaluation of a rapid
antigen test (Panbio™ COVID-19 Ag Rapid Test Device)
for COVID-19 diagnosis in primary healthcare centres.
Clin Microbiol Infect 2021;27:472.e7-10. Crossref

