Hong Kong Med J 2022 Apr;28(2):116–23 | Epub 20 Apr 2022
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Non-visualisation of fetal gallbladder in a Chinese
cohort
YH Ting, MB, BS, FRCOG1; PL So, MB, BS, MRCOG2; KW Cheung, MB, BS, MRCOG3; TK Lo, MB, BS, FRCOG4; Teresa WL Ma, MB, BS, FRCOG5; TY Leung, MD, FRCOG
1 Fetal Medicine Unit, Department of Obstetrics and Gynaecology, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong
2 Fetal Medicine Unit, Department of Obstetrics and Gynaecology, Tuen Mun Hospital, Hong Kong
3 Fetal Medicine Unit, Department of Obstetrics and Gynaecology, Queen Mary Hospital, Hong Kong
4 Fetal Medicine Unit, Department of Obstetrics and Gynaecology, Princess Margaret Hospital, Hong Kong
5 Fetal Medicine Unit, Department of Obstetrics and Gynaecology, Queen Elizabeth Hospital, Hong Kong
Corresponding author: Prof TY Leung (tyleung@cuhk.edu.hk)
Abstract
Introduction: Non-visualisation of fetal gallbladder
(NVFGB) is associated with chromosomal
abnormalities, biliary atresia, cystic fibrosis, and
gallbladder agenesis in Caucasian fetuses. We
investigated the outcomes of fetuses with NVFGB in
a Chinese cohort.
Methods: This retrospective analysis included
cases of NVFGB among Chinese pregnant women
at five public fetal medicine clinics in Hong Kong
from 2012 to 2019. We compared the incidences of
subsequent gallbladder visualisation, chromosomal
abnormalities, biliary atresia, cystic fibrosis, and
gallbladder agenesis between cases of isolated
NVFGB and cases of non-isolated NVFGB.
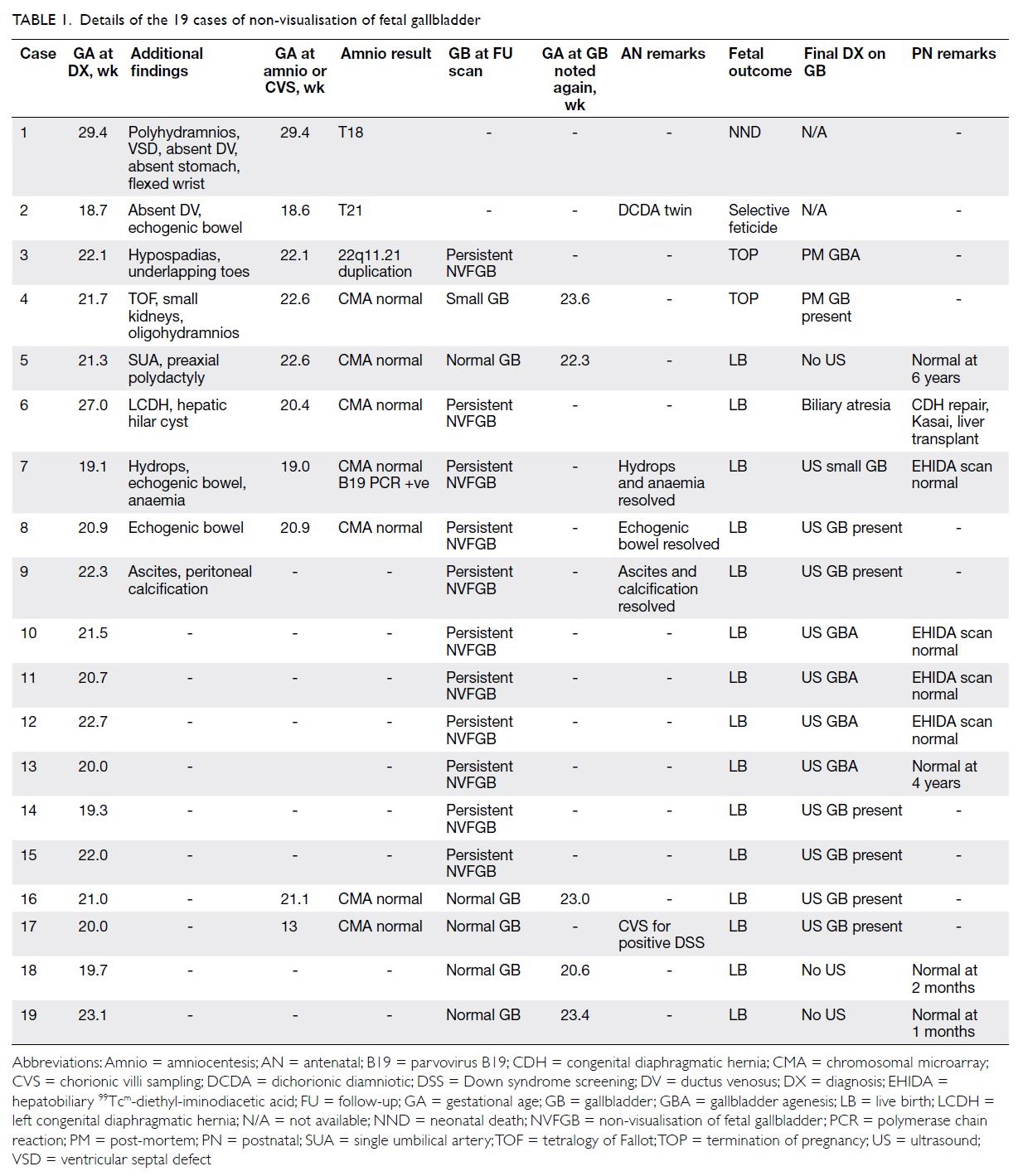
Results: Among 19 cases of NVFGB detected at a
median gestational age of 21.3 weeks (interquartile
range, 20.0-22.3 weeks), 10 (52.6%) were isolated and
nine (47.4%) were non-isolated. Eleven (58.0%) cases
had transient non-visualisation, four (21.0%) had
gallbladder agenesis, three (15.8%) had chromosomal
abnormalities (trisomy 18, trisomy 21, and 22q11.2
microduplication), one (5.2%) had biliary atresia,
and none had cystic fibrosis. The incidence of
serious conditions was significantly higher in the
non-isolated group than in the isolated group (44.4%
vs 0%; P=0.029); all three cases with chromosomal
abnormalities and the only case of biliary atresia
were in the non-isolated group, while all four cases
with gallbladder agenesis were in the isolated group. The incidences of transient non-visualisation were
similar (55.6% vs 60.0%; P=1.000).
Conclusion: Isolated NVFGB is often transient or
related to gallbladder agenesis. While investigations
for chromosomal abnormalities and biliary atresia
are reasonable in cases of NVFGB, testing for cystic
fibrosis may be unnecessary in Chinese fetuses unless
the NVFGB is associated with consistent ultrasound
features, significant family history, or consanguinity.
New knowledge added by this study
- Non-visualisation of fetal gallbladder (NVFGB) is associated with chromosomal abnormalities, biliary atresia, cystic fibrosis, and gallbladder agenesis in Caucasian fetuses. A similar pattern of associated conditions was observed in Chinese fetuses with NVFGB, but none had cystic fibrosis.
- While the incidences of chromosomal abnormalities and biliary atresia were significantly higher in cases of non-isolated NVFGB, isolated NVFGB was generally transient or related to gallbladder agenesis; the risks of chromosomal abnormalities and biliary atresia are presumably low in cases of isolated NVFGB.
- The chromosomal abnormalities associated with NVFGB include common aneuploidies, microdeletions, and microduplications.
- Considering the association with chromosomal abnormalities, amniocentesis is recommended in cases of NVFGB. Chromosomal microarray analysis is more appropriate than karyotyping for the detection of associated microdeletions and microduplications.
- Amniotic fluid gamma-glutamyl transpeptidase (AFGGT) assay may be useful because low AFGGT level is reportedly a marker for biliary atresia; it is sensitive but not specific, particularly after 22 weeks of gestation.
- Further testing for cystic fibrosis may be unnecessary in Chinese fetuses unless the NVFGB is associated with consistent ultrasound features, significant family history, or consanguinity.
Introduction
The fetal gallbladder can be observed by antenatal
ultrasound scan at 14 weeks of gestation.1 During
the morphology scan at approximately 20 weeks
of gestation, >99% of fetal gallbladders can be
observed; in 75% of cases of non-visualisation of
fetal gallbladder (NVFGB), a gallbladder is clearly
present during subsequent scans.2 However,
the visualisation rate drops to 75% to 85% after
32 weeks of gestation when the gallbladder becomes
contractile.3 4
Although sonographic examination of the
fetal gallbladder is not technically difficult, the
International Society of Ultrasound in Obstetrics
and Gynecology and other professional bodies
have not yet included the gallbladder as a routine
component of the mid-trimester anatomical
survey.5 6 7 8 The problem with routine examination of the fetal gallbladder is that non-visualisation of the
gallbladder can lead to challenging counselling and
antenatal diagnosis because NVFGB is related to a
wide spectrum of fetal conditions. While NVFGB
may be a transient phenomenon in a normal fetus or
the result of gallbladder agenesis (a benign congenital
anomaly), it can also be associated with more serious
underlying conditions such as biliary atresia, cystic
fibrosis, or chromosomal abnormalities. While
it is generally simple to identify chromosomal abnormalities and cystic fibrosis by amniocentesis,
the antenatal diagnosis of biliary atresia is challenging
because no diagnostic antenatal test is currently
available. Because biliary atresia can be fatal without
early postnatal intervention and may eventually
require liver transplantation, uncertainty regarding
the antenatal diagnosis of such a condition may
cause significant parental anxiety; some parents may
even consider termination of pregnancy to avoid the
risk of a severe abnormality in their child.9 10
A systematic review of isolated NVFGB in
Western populations revealed that the incidences
of transient non-visualisation, gallbladder agenesis,
biliary atresia, cystic fibrosis, and chromosomal
abnormalities were 69.4%, 24.7%, 3.5%, 2.4%, and
1.4%, respectively. The incidences of biliary atresia,
cystic fibrosis, and chromosomal abnormalities
were higher in cases of non-isolated NVFGB with
additional sonographic abnormalities: 18.2%, 23.1%,
and 20.4%, respectively.11 However, the incidences
may differ considerably among Chinese women with
NVFGB, as cystic fibrosis is uncommon in Asian
populations, while biliary atresia is more prevalent
in Chinese individuals.12 13 14 15 16 The aim of this study was
to investigate the outcomes of fetuses with NVFGB
in a cohort of Chinese women; the findings may
provide guidance for the management of NVFGB.
Methods
This retrospective review included cases of NVFGB
among Chinese pregnant women at five public fetal
medicine clinics in Hong Kong from 2012 to 2019.
In these clinics, fetal morphology scans were limited
to high-risk cases and fetal gallbladder assessment
was not routinely performed, in accordance
with guidelines from the International Society of
Ultrasound in Obstetrics and Gynecology.5 17 When
cases of NVFGB were detected incidentally or
referred from private clinics, the pregnant women
were provided counselling regarding possible
differential diagnoses and offered amniocentesis
for chromosomal analysis. In cases of serious
fetal abnormalities where parents decided for
legal termination of pregnancy before 24 weeks of
gestation, post-mortem examinations were arranged
with parental consent. After birth, babies with
NVFGB were referred to paediatricians for further
evaluation.
The following data were reviewed: demographic
information, gestational age at detection of NVFGB,
findings during the morphology scan and subsequent
scans, results of all amniotic fluid investigations
if amniocentesis had been performed, pregnancy
outcome, all neonatal imaging reports, operations
performed on the baby and intra-operative findings,
and autopsy findings in case of termination of
pregnancy. The cases were segregated into isolated
and non-isolated groups according to the absence or presence of additional sonographic findings. The
incidences of subsequent visualisation of gallbladder,
gallbladder agenesis, biliary atresia, cystic fibrosis,
and chromosomal abnormalities were compared
between the two groups.
The study protocol was approved by the Joint
Chinese University of Hong Kong–New Territories
East Cluster Clinical Research Ethics Committee
on 3 March 2020 (CREC Ref. No.: 2020.060). This
manuscript was written in accordance with STROBE
reporting guidelines.
Statistical analysis
Fisher’s exact test was used for comparisons between
the isolated and non-isolated groups. All statistical
analyses were performed using SPSS software
(Windows version 22.0; IBM Corp., Armonk [NY],
United States). P values <0.05 were considered
statistically significant.
Results
Among 19 cases of NVFGB detected at a median
gestational age of 21.3 weeks (interquartile range,
20.0-22.3 weeks), 10 (52.6%) were isolated and nine
(47.4%) were non-isolated. Eleven (58.0%) cases
had transient non-visualisation, four (21.0%) had
gallbladder agenesis, three (15.8%) had chromosomal
abnormalities (trisomy 18, trisomy 21, and 22q11.2
de novo microduplication), and one (5.2%) had
biliary atresia. There were no cases with features
suggestive of cystic fibrosis (Tables 1 and 2).
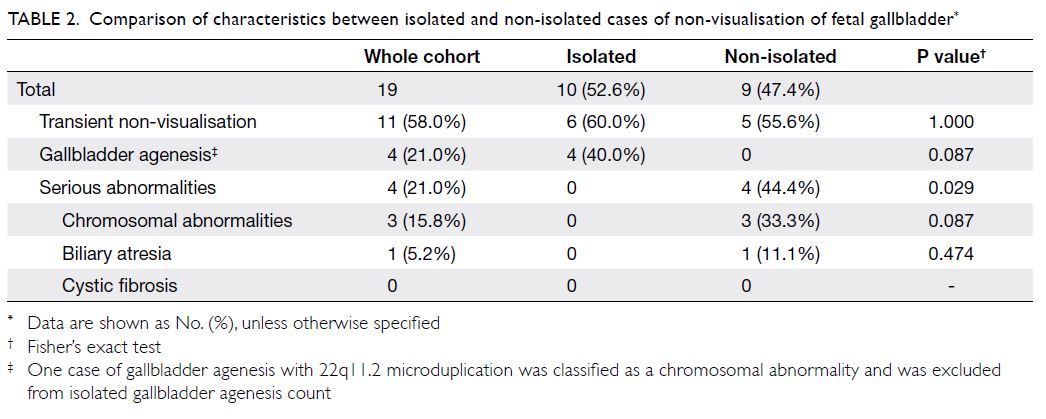
Table 2. Comparison of characteristics between isolated and non-isolated cases of non-visualisation of fetal gallbladder
Non-isolated non-visualisation of fetal
gallbladder
Amniocentesis was performed in eight of the
nine non-isolated cases; three chromosomal
abnormalities (33.3%) were found, including trisomy
18 (case 1), trisomy 21 (case 2), and 22q11.2 de
novo microduplication (case 3). Case 1 ended in
neonatal death and the parents declined post-mortem
investigation. Case 2 was a dichorionic twin
pregnancy; selective feticide was performed on the
fetus with trisomy 21, but post-mortem investigation
could not be performed because of the long interval
between selective feticide and delivery of the normal
co-twin. Termination of pregnancy was performed
in case 3 and gallbladder agenesis was confirmed
at autopsy. Termination of pregnancy was also
performed in case 4 because of multiple structural
abnormalities; chromosomal microarray (CMA)
findings were normal and post-mortem investigation
revealed normal gallbladder. Live birth occurred in
the remaining five cases; in one case, the gallbladder
was observed during a subsequent antenatal scan
and the postnatal outcome was normal, while
NVFGB persisted in the other four cases. Among
the four cases with persistent NVFGB, one (11.1%) had biliary atresia that required liver transplantation
(case 6); antenatal scans had shown a hepatic hilar
cyst (Fig 1), which was highly suggestive of cystic
biliary atresia.18 Postnatal examination showed a
normal gallbladder in the other three cases. None of
the five live births had features suggestive of cystic
fibrosis (Table 1 and Fig 2).
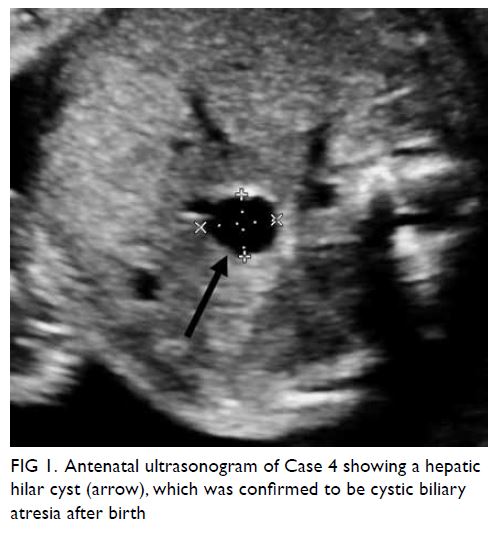
Figure 1. Antenatal ultrasonogram of Case 4 showing a hepatic hilar cyst (arrow), which was confirmed to be cystic biliary atresia after birth
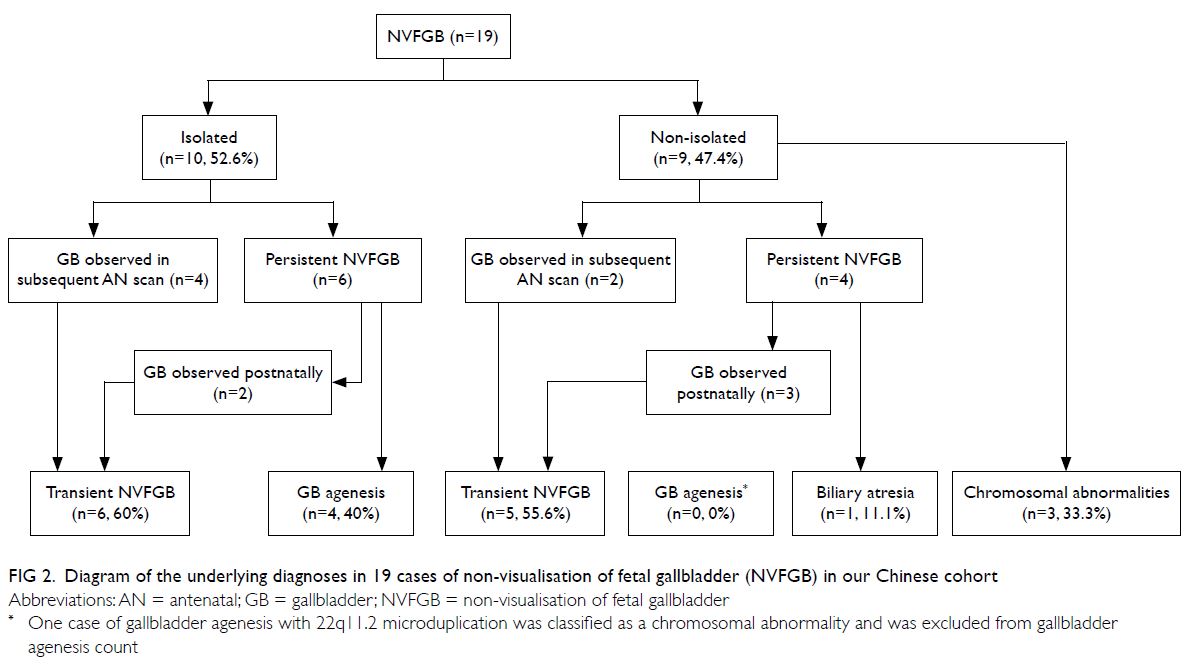
Figure 2. Diagram of the underlying diagnoses in 19 cases of non-visualisation of fetal gallbladder (NVFGB) in our Chinese cohort
Isolated non-visualisation of fetal gallbladder
Among the 10 cases of isolated NVFGB,
amniocentesis was performed in one, while chorionic
villi sampling was performed in the first trimester
because of positive Down syndrome screening
result in another; CMA findings were normal in
both cases. In four cases, gallbladders were observed
during subsequent antenatal scans at a mean follow-up
interval of 1.0 week (range, 0.3-2.0); NVFGB
persisted in the other six cases. Among the six cases
with persistent NVFGB, gallbladder agenesis was
confirmed in four (66.7%) and gallbladders were
observed after birth in two (33.3%). None of the 10
cases had features suggestive of cystic fibrosis after
birth (Table 1 and Fig 2).
Comparison between the isolated and non-isolated
groups
The characteristics of isolated and non-isolated
groups are compared in Table 2. The incidence of
serious abnormalities (chromosomal abnormalities,
biliary atresia) was significantly higher in the non-isolated
group than in the isolated group (44.4% vs
0%; P=0.029). Notably, all serious conditions in the
cohort (all three cases of chromosomal abnormalities
and the only case of biliary atresia) were observed in
the non-isolated group, while all benign conditions
(all four cases of isolated gallbladder agenesis) were
observed in the isolated group. The incidences of
transient non-visualisation did not significantly
differ between the isolated and non-isolated groups
(60.0% vs 55.6%; P=1.000).
Discussion
Isolated non-visualisation of fetal gallbladder
In our cohort, cases of isolated NVFGB had a good
overall prognosis, with a 60% probability that the
gallbladder would be observed in a subsequent
scan and a 40% probability of gallbladder agenesis.
This incidence of transient non-visualisation (60%)
is consistent with findings by Yayla and Bayik2
(75%) and Di Pasquo et al11 (69.4%). In our cases
of transient NVFGB, the gallbladder was observed
during a subsequent antenatal scan in 40%, and
during the postnatal period in the remaining 20%.
The mean interval between NVFGB and subsequent
antenatal detection of the gallbladder was 1 week.
Therefore, a second sonographic examination
within 1 week after NVFGB would help to alleviate parental anxiety and avoid the need for further
investigations in nearly half of such cases. Even in
cases with persistent isolated NVFGB, the prognosis
remains good, because the gallbladder is likely to
be observed after birth in one-third of cases; while gallbladder agenesis is likely in the remaining cases.
Our findings are similar to the results of a recent
systematic review of seven studies in Western
populations, including 217 cases of isolated NVFGB;
most cases had transient non-visualisation (69.4%) and gallbladder agenesis (24.7%), but some cases
had serious conditions (biliary atresia [3.5%], cystic
fibrosis [2.4%], and chromosomal abnormalities
[1.4%]).11 Therefore, further investigations to rule
out such serious abnormalities remain important in
cases of isolated NVFGB.
Non-isolated non-visualisation of fetal
gallbladder
In the aforementioned review, the incidences of
biliary atresia, cystic fibrosis, and chromosomal
abnormalities were much higher when NVFGB
occurred in combination with other ultrasound
abnormalities (18.2%, 23.1%, and 20.4%,
respectively).11 Copy number variants were
observed in one of three cases with chromosomal
abnormalities in our study and two of 11 such cases
in the study by Di Pasquo et al11; these findings support the recommendation for the use of CMA,
rather than karyotyping.19 20 21 However, our results
differ from the findings reported by Di Pasquo et al11
in that none of our cases had cystic fibrosis, which is
unsurprising because cystic fibrosis is rare in Chinese
individuals; moreover, our incidence of biliary atresia
(11.1%) was much lower than expected, considering
that biliary atresia is reportedly threefold more
common in Chinese individuals than in Caucasian
individuals.12 13 14 15 16
Non-visualisation of fetal gallbladder and
biliary atresia
Based on the data described above, in cases of non-isolated
NVFGB or persistent isolated NVFGB,
amniocentesis may help to rule out chromosomal
abnormalities; this approach is generally simple with
current CMA technology.19 20 However, the antenatal
diagnosis of biliary atresia is challenging because
fetal bile duct patency cannot be determined by
sonographic examination; NVFGB may be the only
suggestive sign of biliary atresia. When NVFGB is
associated with a hepatic hilar cyst or heterotaxy,
a diagnosis of biliary atresia is likely.18 However,
it is difficult to differentiate biliary atresia from
gallbladder agenesis in cases of isolated NVFGB.
Thus, a low amniotic fluid gamma-glutamyl
transpeptidase (AFGGT) level has been proposed as
an indicator of biliary atresia.22 23 24 Gamma-glutamyl
transpeptidase (GGT) is initially derived from the
fetal biliary tract, passed into the gastrointestinal
tract, and finally excreted into the amniotic fluid.
The AFGGT level decreases with increasing
gestational age because progressive maturation
of the anal sphincter impairs the passage of GGT
from the gastrointestinal tract into the amniotic
fluid.22 25 26 The anal sphincter muscles become fully
mature by 20 weeks of gestation, and the AFGGT
level becomes very low after 22 weeks of gestation.
Therefore, it may be difficult to distinguish between
a low level related to biliary atresia and a low level related to normal development after 22 weeks of
gestation.25 26 Using an AFGGT level below the 5th
centile, Bardin et al23 reported 100% sensitivity in
the detection of biliary atresia, with a false positive
rate of 4%, between 17 and 22 weeks of gestation
(Table 3). Using AFGGT level and/or intestinal
alkaline phosphatase <0.5 multiples of the median,
Dreux et al24 also reported 100% sensitivity before
22 weeks of gestation; however, their false positive rate was 20%. Notably, when the test was performed
after 22 weeks of gestation, the sensitivity decreased
to 20%. Therefore, gestational age at amniocentesis
is a critical consideration during the assessment
of biliary atresia; if NVFGB is first detected near
22 weeks of gestation, amniocentesis should be
performed immediately, rather than waiting for
sonographic examination to be repeated. Another
limitation of using the AFGGT level to identify biliary atresia is that it has a moderately low positive
predictive value: 43% to 75% before 22 weeks of
gestation, and 17% thereafter.23 24 Accordingly, a
positive AFGGT test result is not diagnostic of
biliary atresia, particularly in cases of isolated
NVFGB where the incidence of biliary atresia is
presumably low. Conversely, the negative predictive
value of AFGGT is near 100%; a negative test result
is very reassuring, which can help to alleviate
parental anxiety and avoid unwarranted termination
of pregnancy.23 24 When NVFGB is detected after
22 weeks of gestation, the fetal blood GGT level
may be useful for identification of biliary atresia.27 28
However, cordocentesis may be unwarranted, as
the procedure-related risk outweighs the possible
diagnostic benefit, particularly in cases of isolated
NVFGB where the risk of biliary atresia is presumably
low.
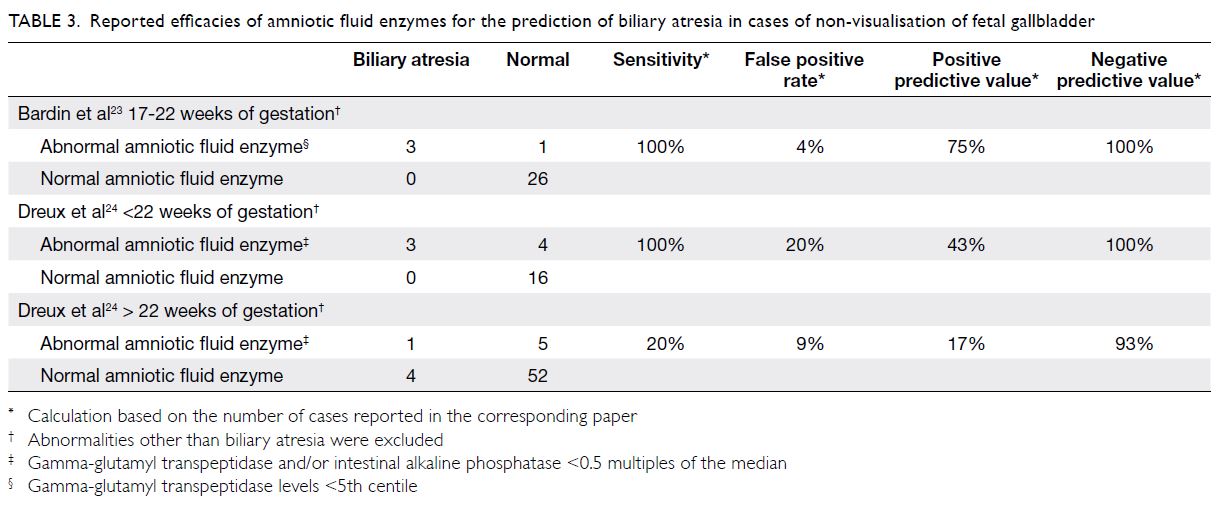
Table 3. Reported efficacies of amniotic fluid enzymes for the prediction of biliary atresia in cases of non-visualisation of fetal gallbladder
Non-visualisation of fetal gallbladder and
cystic fibrosis
In Caucasian populations, the incidence of cystic
fibrosis is 1:2500-3500 live births and the carrier
rate is 1:50; in contrast, this hereditary disease
is extremely rare among East Asian individuals
(1:350000 people in Japan and 1:300000 live births
in Hong Kong).15 16 29 Unsurprisingly, we did not
observe cystic fibrosis in either group of NVFGB
cases. Therefore, in the absence of significant family
history, consanguinity, or concurrent ultrasound
features suggestive of cystic fibrosis (eg, echogenic
or dilated bowel), amniocentesis for genetic testing
for cystic fibrosis is not recommended in cases of
NVFGB in Hong Kong. Assessment of the parental
CFTR gene mutation status may be a useful
alternative.
Management protocol for non-visualisation
of fetal gallbladder
Based on our findings and the results of previous
studies, we propose the following approach for the
management of NVFGB. When NVFGB is detected,
a detailed morphology scan should be performed to
identify associated abnormalities, such as hepatic
hilar cyst and heterotaxy (indicative of biliary
atresia) or echogenic and dilated bowel (suggestive
of cystic fibrosis). A sonographic examination
of the gallbladder should be repeated within
1 week. Considering the potential for chromosomal
abnormalities (even in cases of isolated NVFGB),
amniocentesis is recommended for CMA analysis
in cases of persistent NVFGB. The AFGGT assay
can also be performed before 22 weeks of gestation;
counselling prior to the test should involve an
explanation of the moderately low positive predictive
value for identification of biliary atresia. Beyond
22 weeks of gestation, the AFGGT level is not useful
for identifying biliary atresia, but cordocentesis for GGT level may be useful. However, cordocentesis is
generally not recommended because the procedure-related
risk outweighs the possible diagnostic
benefit, particularly in cases of isolated NVFGB
where the risk of biliary atresia is presumably low.
Further testing for cystic fibrosis may be unnecessary
in Chinese fetuses unless the NVFGB is associated
with other ultrasound features suggestive of cystic
fibrosis, significant family history, or consanguinity.
Further research is needed concerning AFGGT
reference values and the ability of the AFGGT level
to identify biliary atresia in Chinese fetuses with
NVFGB.
Limitations and strength
Similar to other reports regarding NVFGB, our study
was limited by its retrospective design and small
cohort size. Because fetal gallbladder examination
has not been a routine practice in Hong Kong, we
cannot calculate the prevalence of NVFGB. To our
knowledge, this is the first report of NVFGB in
a Chinese cohort. Moreover, our results differed
from findings in Caucasian populations in that
we did not observe cystic fibrosis in our cohort;
such information may be useful during antenatal
counselling in cases of NVFGB.
Conclusion
The prognosis of isolated NVFGB is generally good
because the non-visualisation is either transient or
related to gallbladder agenesis. While investigations
of chromosomal abnormalities and biliary atresia
are reasonable in cases of NVFGB, testing for cystic
fibrosis may be unnecessary in Chinese fetuses unless
the NVFGB is associated with consistent ultrasound
features, significant family history, or consanguinity.
Author contributions
Concept or design: YH Ting, TY Leung.
Acquisition of data: YH Ting, PL So, KW Cheung, TK Lo, TWL Ma.
Analysis or interpretation of data: YH Ting.
Drafting of the manuscript: YH Ting, TY Leung.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: YH Ting, PL So, KW Cheung, TK Lo, TWL Ma.
Analysis or interpretation of data: YH Ting.
Drafting of the manuscript: YH Ting, TY Leung.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Declaration
The research was presented as poster in the 30th World
Congress on Ultrasound in Obstetrics and Gynecology,
16-18 October 2020, and was published as an abstract in
Ultrasound in Obstetrics & Gynecology (Ting Y, Leung T, Law K, Lo T, Cheung K, So P. VP09.12: Non-visualisation of fetal
gall bladder in a Chinese cohort. Ultrasound Obstet Gynecol
2020;56(S1):84).
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study protocol was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster
Clinical Research Ethics Committee on 3 March 2020 (CREC
Ref. No.: 2020.060).
References
1. Goldstein I, Tamir A, Weisman A, Jakobi P, Copel JA.
Growth of the fetal gall bladder in normal pregnancies.
Ultrasound Obstet Gynecol 1994;4:289-93. Crossref
2. Yayla M, Bayik RN. Undetectable gall bladder in fetus: what to do? Perinat J 2016;24:11-9. Crossref
3. Hertzberg BS, Kliewer MA, Maynor C, et al. Nonvisualization of the fetal gallbladder: frequency and
prognostic importance. Radiology 1996;199:679-82. Crossref
4. Lehtonen L, Svedström E, Kero P, Korvenranta H. Gall
bladder contractility in preterm infants. Arch Dis Child
1993;68(1 Spec No):43-5. Crossref
5. Salomon LJ, Alfirevic Z, Berghella V, et al. Practice
guidelines for performance of the routine mid-trimester
fetal ultrasound scan. Ultrasound Obstet Gynecol
2011;37:116-26. Crossref
6. Australasian Society for Ultrasound in Medicine.
Guidelines for the performance of second (mid) trimester
ultrasound. 2018. Available from: https://www.asum.com.
au/files/public/SoP/curver/Obs-Gynae/Guidelines-for-the-Performance-of-Second-Mid-Trimester-Ultrasound.pdf. Accessed 24 Aug 2020.
7. NHS Mid and South Essex, University Hospitals Group.
NHS Fetal Anomaly Screening Programme: National
Standards and Guidance for England. Guidelines for
performing the 18 weeks and 0 days to 20 weeks and 6 days
gestation fetal anomaly scan. 2015. Available from: https://www.meht.nhs.uk/EasysiteWeb/getresource.axd?AssetID=17955&type=Full&servicetype=Attachment. Accessed
24 Aug 2020.
8. Cargill Y, Morin L. No. 223—Content of a complete routine
second trimester obstetrical ultrasound examination and
report. J Obstet Gynaecol Can 2017;39:e144-9. Crossref
9. Chan YM, Leung TN, Leung TY, Fung TY, Chan LW,
Lau TK. The utility assessment of Chinese pregnant
women towards the birth of a baby with Down syndrome
compared to a procedure-related miscarriage. Prenat
Diagn 2006;26:819-24. Crossref
10. Chan YM, Chan OK, Cheng YK, Leung TY, Lao TT,
Sahota DS. Acceptance towards giving birth to a child with
beta-thalassemia major—a prospective study. Taiwan J
Obstet Gynecol 2017;56:618-21. Crossref
11. Di Pasquo E, Kuleva M, Rousseau A, et al. Outcome of
non-visualization of fetal gallbladder on second-trimester
ultrasound: cohort study and systematic review of
literature. Ultrasound Obstet Gynecol 2019;54:582-8. Crossref
12. Hartley JL, Davenport M, Kelly DA. Biliary atresia. Lancet
2009;374:1704-13. Crossref
13. Hsiao CH, Chang MH, Chen HL, et al. Universal screening
for biliary atresia using an infant stool color card in Taiwan.
Hepatology 2008;47:1233-40. Crossref
14. Zhan J, Chen Y, Wong KK. How to evaluate diagnosis
and management of biliary atresia in the era of liver
transplantation in China. World Jnl Ped Surgery
2018;1:e000002. Crossref
15. Mirtajani SB, Farnia P, Hassanzad M, Ghanavi J, Farnia P,
Velayati AA. Geographical distribution of cystic fibrosis;
the past 70 years of data analysis. Biomed Biotechnol Res J
2017;1:105-12. Crossref
16. Leung GK, Ying D, Mak CC, et al. CFTR founder mutation
causes protein trafficking defects in Chinese patients with
cystic fibrosis. Mol Genet Genomic Med 2016;5:40-9. Crossref
17. Chen M, Leung TY, Sahota DS, et al. Ultrasound
screening for fetal structural abnormalities performed by
trained midwives in the second trimester in a low-risk
population—237-an appraisal. Acta Obstet Gynecol Scand
2009;88:713-9. Crossref
18. Chalouhi GE, Muller F, Dreux S, Ville Y, Chardot C.
Prenatal non-visualization of fetal gallbladder: beware of
biliary atresia! Ultrasound Obstet Gynecol 2011;38:237-40. Crossref
19. Chau MH, Cao Y, Kwok YK, et al. Characteristics and
mode of inheritance of pathogenic copy number variants
in prenatal diagnosis. Am J Obstet Gynecol 2019;221:493.
e1-11. Crossref
20. Zhu X, Chen M, Wang H, et al. Clinical utility of expanded
noninvasive prenatal screening and chromosomal
microarray analysis in high risk pregnancies. Ultrasound
Obstet Gynecol 2021;57:459-465. Crossref
21. Grati FR, Molina Gomes D, Ferreira JC, et al.
Prevalence of recurrent pathogenic microdeletions and
microduplications in over 9500 pregnancies. Prenat Diagn
2015;35:801-9. Crossref
22. Muller F, Gauthier F, Laurent J, Schmitt M, Boué J.
Amniotic fluid GGT and congenital extrahepatic biliary
damage. Lancet 1991;337:232-3. Crossref
23. Bardin R, Ashwal E, Davidov B, Danon D, Shohat M,
Meizner I. Nonvisualization of the fetal gallbladder: can
levels of gamma-glutamyl transpeptidase in amniotic fluid
predict fetal prognosis? Fetal Diagn Ther 2016;39:50-5. Crossref
24. Dreux S, Boughanim M, Lepinard C, et al. Relationship
of non-visualization of the fetal gallbladder and amniotic
fluid digestive enzymes analysis to outcome. Prenat Diagn
2012;32:423-6. Crossref
25. Burc L, Guibourdenche J, Luton D, et al. Establishment
of reference values of five amniotic fluid enzymes.
Analytical performances of the Hitachi 911. Application to
complicated pregnancies. Clin Biochem 2001;34:317-22. Crossref
26. Bardin R, Danon D, Tor R, Mashiach R, Vardimon D,
Meizner I. Reference values for gamma-glutamyl-transferase
in amniotic fluid in normal pregnancies. Prenat
Diagn 2009;29:703-6. Crossref
27. Muller F, Bernard P, Salomon LJ, et al. Role of fetal blood
sampling in cases of non-visualization of fetal gallbladder.
Ultrasound Obstet Gynecol 2015;46:743-4. Crossref
28. Ruiz A, Robles A, Salva F, et al. Prenatal nonvisualization of
the gallbladder: a diagnostic and prognostic dilemma. Fetal
Diagn Ther 2017;42:150-2. Crossref
29. Chan YM, Leung TY, Cao Y, et al. Expanded carrier
screening using next generation sequencing of 123 Hong
Kong Chinese families: a pilot study. Hong Kong Med J
2021;27:177-83. Crossref