Hong Kong Med J 2022 Feb;28(1):24–32 | Epub 5 Nov 2021
© Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Renal outcomes in Asian patients receiving oral
anticoagulants for non-valvular atrial fibrillation
Tayyab Salim Shahzada, Cosmos L Guo, Alex PW Lee, MD, FRCP
Department of Medicine and Therapeutics, The Chinese University of Hong Kong, Hong Kong
Corresponding author: Prof Alex PW Lee (alexpwlee@cuhk.edu.hk)
Abstract
Introduction: Patients with non-valvular atrial
fibrillation (NVAF) may be prescribed warfarin or
a non–vitamin K oral anticoagulant (NOAC). There
is increasing evidence that NOACs are superior to
warfarin in terms of renal function preservation. This
study aimed to compare renal outcomes in Chinese
patients with NVAF between patients receiving
NOACs and patients receiving warfarin.
Methods: In total, 600 Chinese patients with
NVAF receiving oral anticoagulant therapy were
retrospectively identified from an administrative
database. The renal outcomes (≥30% decline
in estimated glomerular filtration rate [eGFR],
doubling of serum creatinine, and kidney failure)
were compared among four propensity-weighted
treatment cohorts (warfarin, n=200; rivaroxaban,
n=200; dabigatran, n=100; and apixaban, n=100).
Results: The mean follow-up period across all
groups was 1000 ± 436 days. Compared with
warfarin, the three NOACs (pooled for consideration
as a single unit) had significantly lower risks of
≥30% decline in eGFR (hazard ratio [HR]=0.339; 95% confidence interval [CI]=0.276-0.417)
and doubling of serum creatinine (HR=0.550;
95% CI=0.387-0.782). Dabigatran and rivaroxaban
users both had lower risks of ≥30% decline in eGFR
(both P<0.001) and doubling of serum creatinine
(both P<0.05). Apixaban was only significantly
associated with a lower risk of ≥30% decline in eGFR
(P<0.001).
Conclusions: Compared with warfarin, NOACs may be associated with a significantly lower risk of
decline in renal function among Chinese patients
with NVAF.
New knowledge added by this study
- Decline in kidney function is common among Chinese patients who receive oral anticoagulant treatment for non-valvular atrial fibrillation.
- Warfarin usage is associated with significant long-term decline in renal function among patients treated for non-valvular atrial fibrillation.
- Compared with warfarin, non–vitamin K oral anticoagulant (NOAC) usage may be associated with a reduced risk of long-term decline in renal function among Chinese patients.
- Patients receiving oral anticoagulants, especially warfarin, should undergo close renal function monitoring during the course of treatment.
- Considering that the decline in renal function may be more accelerated in warfarin users than in NOAC users, clinicians may consider preferential use of NOACs for anticoagulant therapy, especially in patients with existing renal impairment or risk factors for future decline in renal function.
- The inconsistencies of NOAC prescribing patterns with drug labelling in routine clinical practice should receive greater attention because dose reduction in the absence of a renal indication may reduce treatment effectiveness without providing a greater safety benefit.
Introduction
Various randomised controlled trials have
demonstrated that non–vitamin K oral
anticoagulants (NOACs), including factor Xa and
direct thrombin inhibitors, are superior to warfarin, a
vitamin K antagonist, in terms of efficacy and safety for
preventing stroke and systemic thromboembolisms
in patients with non-valvular atrial fibrillation (NVAF).1 2 3 4 The superiority of NOACs compared
with warfarin appears to be consistent across ethnic
groups, including Asian populations.5 Furthermore,
data from two sub-studies of the NOAC trials6 7
and a real-world cohort study8 suggested that
NOACs may also be superior to warfarin in terms
of maintaining and preserving renal function.
A US-based cohort study demonstrated a lower risk of decline in renal function among patients
receiving NOACs than among patients receiving
warfarin.8 Moreover, findings from the ROCKET AF
(Rivaroxaban Once-Daily, Oral, Direct Factor Xa
Inhibition Compared With Vitamin K Antagonism
for Prevention of Stroke and Embolism Trial in Atrial
Fibrillation) and RE-LY (Randomized Evaluation of
Long Term Anti-coagulation Therapy) trials revealed
more rapid estimated glomerular filtration rate
(eGFR) decline in patients receiving warfarin than
in patients receiving rivaroxaban and dabigatran,
respectively.6 7 8 Further studies have demonstrated
that warfarin treatment may be associated with
more rapid progression of chronic kidney disease
and can cause acute kidney injury.8 9 10 This decline in
renal function has been attributed to a phenomenon
known as ‘warfarin-related nephropathy’, which is
associated with vitamin K antagonism and excessive
anticoagulation.8 9 11 In contrast, NOACs may offer
renovascular protection through pharmacological
mechanisms such as the inhibition of thrombin and
factor Xa.8 12 13
Differences in the pharmacological actions of
warfarin and NOACs are reflected in the growing
research that suggests NOACs are more effective
than warfarin for preserving renal function.8 14
Considering that Asian warfarin users tended to
have a lower time in therapeutic range (TTR)15 16
of the international normalised ratio (INR), which
is associated with decline in renal function,6 14 the renal effects of NOACs compared with warfarin
may differ from the effects in non-Asians. Dosage
prescription patterns, such as the frequency of low-dose
NOAC prescriptions, also vary between Asian
and non-Asian populations14 17 18; this may also affect
renal outcomes because the renal effects of NOACs
appear to be dose-dependent.14 19 Furthermore,
because of differences in NOAC-related bleeding
risk between Asian and non-Asian populations,
the renal protection effects of NOACs may also
vary; major bleeding can cause decline in renal
function.5 14 20 21 To our knowledge, there remain
limited data comparing NOACs to warfarin in terms
of decline in renal function among Asian patients.
In this study, we sought to assess the renal outcomes
of an ethnic Chinese patient population with NVAF
who received NOACs (ie, apixaban, dabigatran, and
rivaroxaban) compared with patients who received
warfarin.
Methods
Study design
This retrospective cohort study included four
study groups: warfarin, apixaban, dabigatran, and
rivaroxaban. Each NOAC was compared with warfarin.
Study population
Data were extracted from patients’ electronic
medical records in the Prince of Wales Hospital
of Hong Kong. In total, 2346 consecutive patients
with a prescription of warfarin or one of the three
NOACs (apixaban, rivaroxaban, and dabigatran)
in our hospital were screened for eligibility for this
analysis. Inclusion criteria were: first began to receive
an oral anticoagulant between 1 January 2012 and
31 December 2016, NVAF, age ≥18 years, minimum
on-treatment duration of 3 months, and availability
of laboratory data concerning serum creatinine
at baseline and during follow-up. We excluded
warfarin-experienced or NOAC-experienced
patients to minimise confounding bias.8 22 Other
exclusion criteria were previous kidney failure,
valvular atrial fibrillation,23 and/or other indications
for anticoagulation. A pre-study power analysis to
detect a 10% difference in the incidence of ≥30%
decline in eGFR between NOACs (pooled for
consideration as a single unit) and warfarin revealed
that the minimum sample size was 200 patients per
arm (all NOACs vs warfarin). The sample size in each
NOAC group was then matched to the approximate
proportion of patients that were prescribed each
of the three NOACs in actual clinical practice, in
accordance with the preferences of local physicians.
In our hospital during the study period, rivaroxaban
was available earlier locally and was more frequently
prescribed than the other two NOACs; apixaban was the last NOAC to receive local approval and
therefore exhibited a lower rate of prescription at the
time of the study. This paper adheres to the STROBE
reporting guidelines for observational studies.
Study endpoints
We studied the three renal outcome endpoints:
≥30% decline in eGFR, doubling of serum creatinine,
and kidney failure. Doubling of serum creatinine has
been used as a surrogate endpoint when studying
the progression of kidney disease in clinical trials.24
Based on the findings of clinical trials and meta-analyses,
the National Kidney Foundation and US
Food and Drug Administration proposed that with
at least 2 to 3 years of follow-up, a 30% to 40%
decline in eGFR may also be regarded as a surrogate
end point; thus, it has been used as a renal endpoint
in previous cohort analyses.8 24 Because doubling of
serum creatinine and kidney failure occur late in
kidney disease, ≥30% decline in eGFR serves as a
more sensitive renal decline endpoint; this change
is clinically significant regardless of a low follow-up
or event rate.8 24 Kidney failure is defined as eGFR
<15 mL/min/1.73 m2, long-term kidney dialysis, or
kidney transplantation.8 25 Efficacy outcomes were
stroke (ischaemic or haemorrhagic) or systemic
embolism (SE). Based on the initial dose prescribed,
the prevalence of dose reduction for each NOAC
(apixaban 2.5 mg twice daily, dabigatran 75 mg twice
daily, and rivaroxaban 15 mg once daily) without a
renal indication (eGFR <30 mL/min for apixaban
and dabigatran; eGFR <50 mL/min for rivaroxaban)
was assessed.26
Using the treatment initiation date as our index
date, we retrieved the pretreatment creatinine value
nearest to the index date as the baseline creatinine;
we used this value to calculate the baseline eGFR by
means of the Chronic Kidney Disease Epidemiology
Collaboration equation.8 27 Hospital electronic
records were used to identify co-morbidities
and specific drug prescriptions within 3 months
prior to the index date. Baseline HAS-BLED and
CHA2DS2-VASc scores were also recorded. The TTR
of patients in the warfarin cohort was calculated as
the number of INRs in therapeutic range (INR=2-3)
divided by the total INRs recorded for each patient
during the analysed period.28 29 Patients were
followed up until the end of treatment, death, or
when any efficacy or renal endpoint(s) were reached.
Statistical analysis
For minimisation of potential confounding, we
used inverse probability of treatment weighting
(IPTW) to balance identified covariates.8 14 17 30
Generalised boosted models were used to estimate
propensity scores and weights for optimal balance across treatment groups.31 32 Weights were obtained
to gather estimates representing the mean effects
of treatment among treated groups.8 14 Baseline
characteristics (eg, patient baseline medications
and pre-existing co-morbidities which may affect
outcomes) were included in our model (online supplementary Table).8 14 Both CHA2DS2-VASc
and HAS-BLED scores were not included in the
model because they are composite scores derived
from other covariates.14 The absolute standardised
mean difference was calculated for each NOAC
versus warfarin to ensure that the cohorts were
sufficiently balanced before comparison of each
NOAC to warfarin. An absolute standardised mean
difference of <0.2 is considered balanced for each
baseline covariate when comparing each NOAC to
warfarin.8 31 Fisher’s exact test was used to compare
the frequencies of dose reduction without renal
indication among NOACs.
Because of some extremely high or low weights
in our weighted population, we truncated weights
at the 1st and 99th percentiles before conducting
weighted analysis.8 33 We calculated hazard
ratios using weighted Cox proportional hazards
regression, then generated weighted Kaplan–Meier
curves that compared each NOAC to warfarin.
Cumulative incidences for the Kaplan–Meier curves
were presented as mean percentage incidences
with 95% confidence intervals. A P value of <0.05
was considered statistically significant. Predefined
subgroup analysis was performed for factors
potentially associated with renal outcome, including
age (≥75 or <75 years); sex; and baseline diabetes
mellitus, heart failure, and eGFR (≥60 mL/min/1.73 m2
or <60 mL/min/1.73 m2).
Results
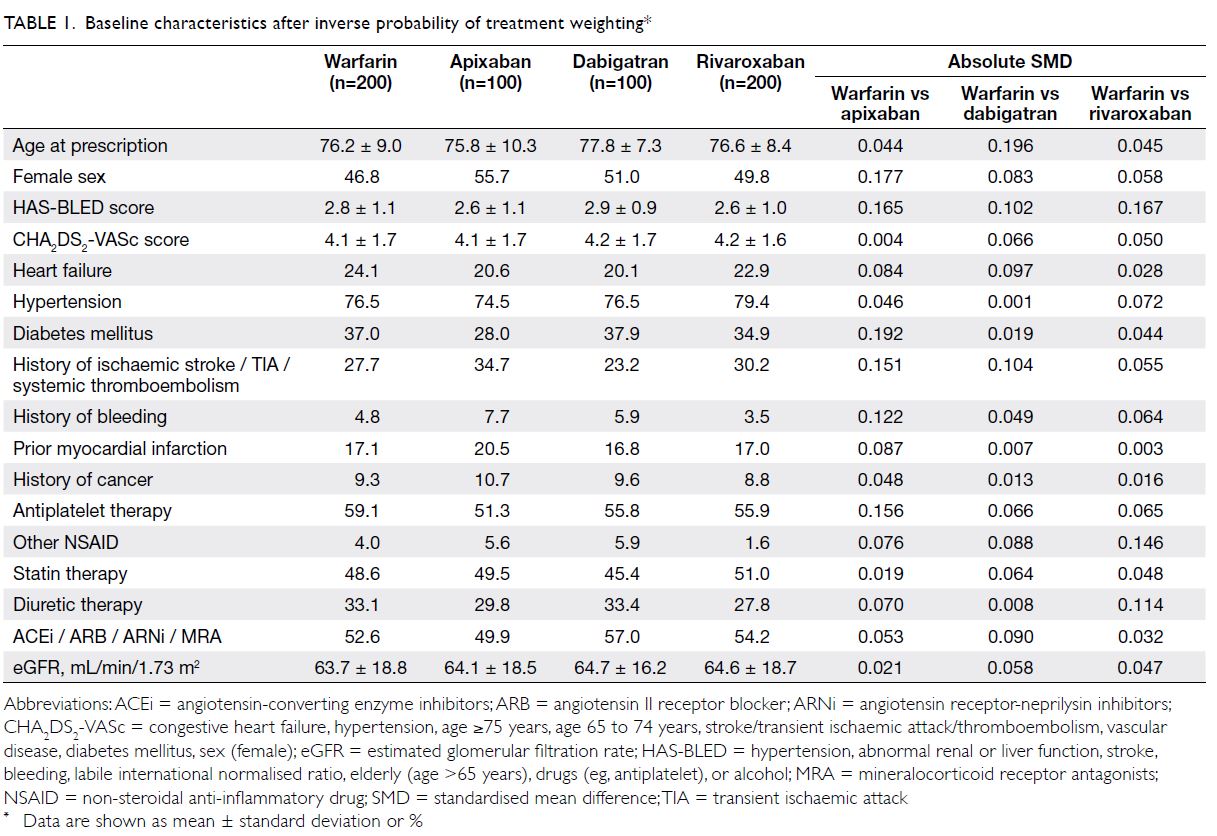
Cohort characteristics
We identified 600 patients with NVAF who were
receiving oral anticoagulants: 200, 100, 100, and
200 patients were receiving warfarin, apixaban,
dabigatran, and rivaroxaban, respectively. After
IPTW, all identified baseline characteristics were
balanced between warfarin and each NOAC group
(Table 1). The mean follow-up duration of the
overall study cohort was 1000 ± 436 days. The mean
follow-up durations for each NOAC group were
as follows: apixaban (790 ± 345 days), dabigatran
(1187 ± 322 days), and rivaroxaban (999 ± 430 days);
the median follow-up durations were 806, 1416,
and 1074 days, respectively. The mean TTR of the
warfarin cohort was 44.3%. The frequencies of dose
reduction without a renal indication for apixaban,
rivaroxaban and dabigatran were 46.9%, 35.7% and
2.0%, respectively (P<0.001 for dabigatran vs both
apixaban and rivaroxaban).
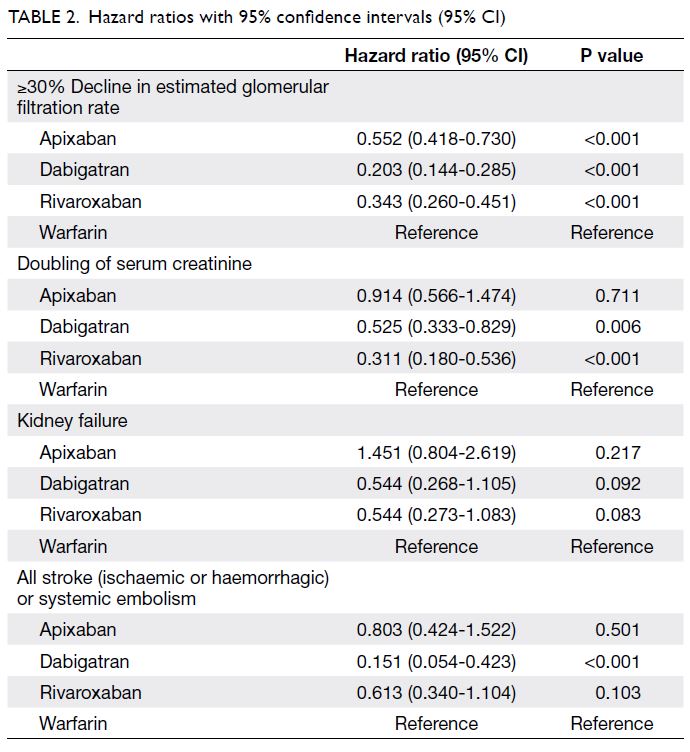
Renal and efficacy outcomes
When the three NOACs were pooled for
consideration as a single unit and compared with
warfarin, NOAC users exhibited lower risks of
≥30% decline in eGFR (hazard ratio [HR]=0.339;
95% confidence interval [CI]=0.276-0.417; P<0.001)
and doubling of serum creatinine (HR=0.550;
95% CI=0.387-0.782; P<0.001). Individual
comparisons of each NOAC to warfarin (Table 2)
revealed that dabigatran and rivaroxaban users
both had lower risks of ≥30% decline in eGFR
(both P<0.001) and doubling of serum creatinine
(both P<0.05). However, apixaban users only had
a lower risk of ≥30% decline in eGFR (P<0.001).
Despite trends suggestive of lower kidney failure
risk in patients receiving dabigatran or rivaroxaban,
the overall use of NOACs was not significantly
associated with lower kidney failure risk, compared
with the use of warfarin. Figure 1 shows the weighted
Kaplan–Meier curves for the renal endpoints. For
the efficacy outcome, dabigatran was associated
with a lower incidence of stroke/SE (HR=0.151;
95% CI=0.054-0.423); P<0.001 vs warfarin), whereas
the use of apixaban or rivaroxaban was not
significantly associated with a lower incidence of
stroke/SE, compared with the use of warfarin (Fig 2).
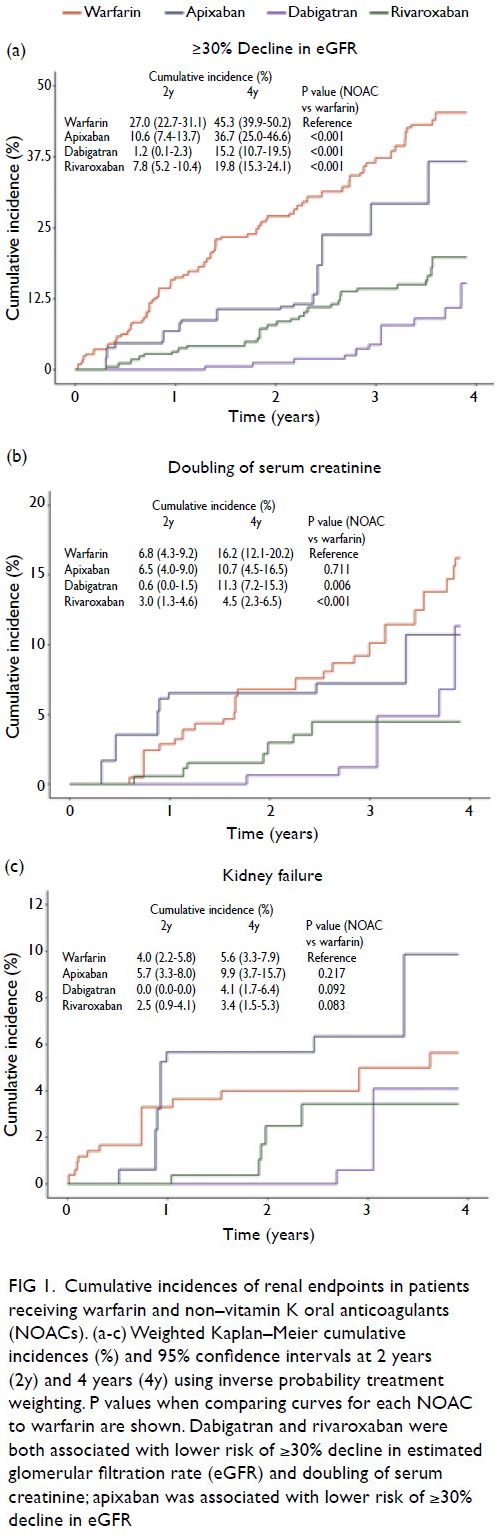
Figure 1. Cumulative incidences of renal endpoints in patients receiving warfarin and non–vitamin K oral anticoagulants (NOACs). (a-c) Weighted Kaplan–Meier cumulative incidences (%) and 95% confidence intervals at 2 years (2y) and 4 years (4y) using inverse probability treatment weighting. P values when comparing curves for each NOAC to warfarin are shown. Dabigatran and rivaroxaban were both associated with lower risk of ≥30% decline in estimated glomerular filtration rate (eGFR) and doubling of serum creatinine; apixaban was associated with lower risk of ≥30% decline in eGFR
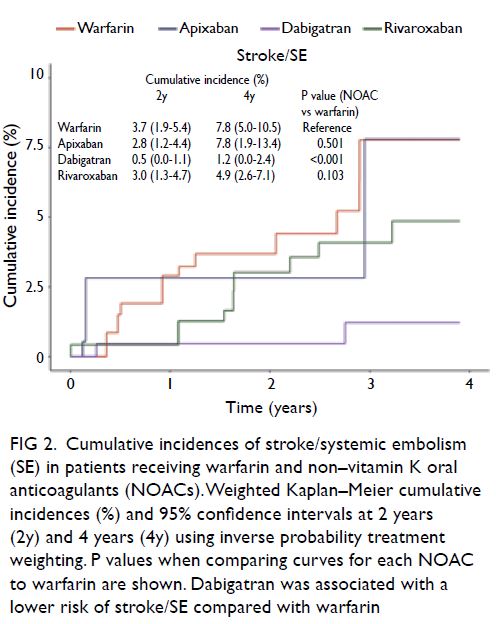
Figure 2. Cumulative incidences of stroke/systemic embolism (SE) in patients receiving warfarin and non–vitamin K oral anticoagulants (NOACs). Weighted Kaplan–Meier cumulative incidences (%) and 95% confidence intervals at 2 years (2y) and 4 years (4y) using inverse probability treatment weighting. P values when comparing curves for each NOAC to warfarin are shown. Dabigatran was associated with a lower risk of stroke/SE compared with warfarin
Subgroup analysis
Analysis of the main renal endpoint, ≥30% decline in eGFR, consistently favoured the use of dabigatran
or rivaroxaban, compared with warfarin, across all
subgroups (Fig 3). However, the use of apixaban was
not associated with a reduced risk of ≥30% decline
in eGFR in three subgroups: men (P=0.057), patients
with heart failure (P=0.835), and patients without
diabetes mellitus (P=0.090).
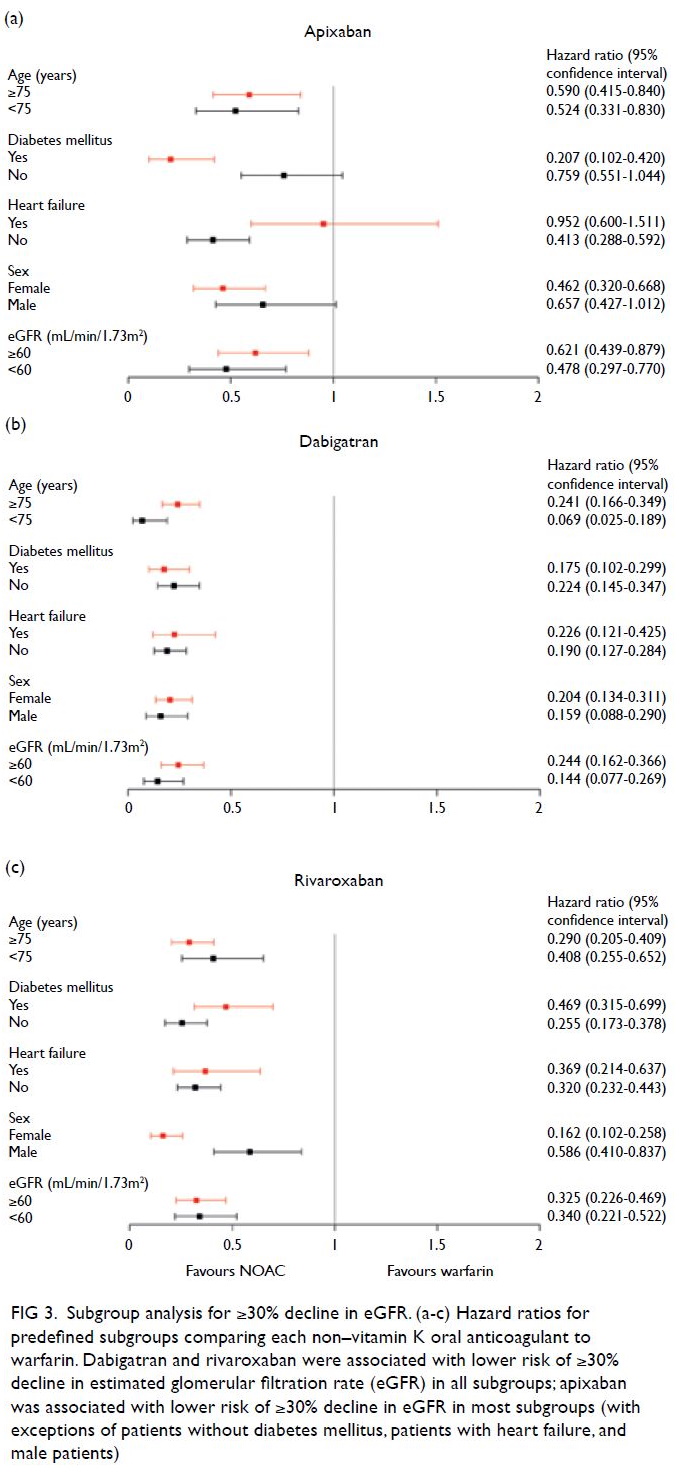
Figure 3. Subgroup analysis for ≥30% decline in eGFR. (a-c) Hazard ratios for predefined subgroups comparing each non–vitamin K oral anticoagulant to warfarin. Dabigatran and rivaroxaban were associated with lower risk of ≥30% decline in estimated glomerular filtration rate (eGFR) in all subgroups; apixaban was associated with lower risk of ≥30% decline in eGFR in most subgroups (with exceptions of patients without diabetes mellitus, patients with heart failure, and male patients)
Discussion
Summary and potential mechanisms
Our cohort study provides important insights into
the long-term renal impacts of NOACs versus
warfarin in an ethnic Chinese population. Decline
in renal function was evident among both warfarin
and NOAC users in our cohort. However, the use
of NOACs was generally associated with better
long-term renal outcomes, compared with the use
of warfarin, among Chinese patients. The general
superiority of NOACs compared with warfarin was
most evident for the ≥30% decline in eGFR surrogate
endpoint. The use of dabigatran or rivaroxaban was
associated with lower risks of ≥30% decline in eGFR
and doubling of creatinine in the overall population
and across predefined demographic and clinical
subgroups; in contrast, the use of apixaban was not
associated with a lower risk of doubling of serum
creatinine in the overall population, nor was it
associated with a lower risk of ≥30% decline in eGFR
among several subgroups (men, patients without
diabetes mellitus, and patients with heart failure).
Pharmacological mechanisms may explain our
findings concerning NOAC superiority. Because
warfarin is a vitamin K antagonist, it has inhibitory
effects on matrix gamma-carboxyglutamic acid,
a vitamin K–dependent protein which normally
protects against vascular calcification; thus,
warfarin administration potentially stimulates
and accelerates the calcification of renal vascular
tissue, which promotes nephropathy.8 34 35 The
mechanism of warfarin-related nephropathy has
various contributing factors, such as the occurrence
of glomerular haemorrhage and subsequent tubular
injury because of red blood cell casts and haem-related
free radical injury.10 36 37 Alternatively, NOACs
may offer renovascular protection through distinct
mechanisms such as the inhibition of thrombin and
factor Xa.8 12 13
Comparison with existing literature
Our data are generally consistent with previous
studies concerning the renal outcomes of NOACs
versus warfarin. A study by Yao et al8 regarding the
renal outcomes of NOACs showed that dabigatran
was associated with a lower risk of ≥30% decline
in eGFR, while rivaroxaban was associated with
lower risks of ≥30% decline in eGFR and doubling
of serum creatinine. Analysis of the RE-LY and
ROCKET AF trials similarly showed more rapid
decline in eGFR among warfarin users, compared
with dabigatran and rivaroxaban users.6 7 8
Hernandez et al38 demonstrated rivaroxaban
superiority for adverse renal events, compared with
warfarin, in patients with NVAF who had diabetes
mellitus. However, our results for apixaban were
inconsistent with the findings of the ARISTOTLE
trial, which did not show significant apixaban
superiority in terms of renal function preservation;
the analysis showed similar but slightly greater
decline in eGFR among apixaban users, compared
with warfarin users.8 39 Yao et al8 also showed no
clear benefits for apixaban, compared with warfarin,
in terms of renal protection. Nonetheless, a study
in Taiwan by Chan et al14 showed that, compared
with warfarin, all three NOACs were associated with
lower risk for acute kidney injury in both chronic
kidney disease-free and chronic kidney disease
cohorts.
The differences between our findings and the
results of previous studies—especially with respect
to apixaban in our cohort versus the ARISTOTLE
subanalysis39—may have several explanations. As
mentioned by Chan et al,14 Asian populations tended
to have lower TTR with warfarin usage, compared
with non-Asians15 16; our warfarin cohort had a mean
TTR of 44.3%, which was considerably lower than
findings in non-Asian populations.15 16 Combined
with findings that renal deterioration is greater when warfarin is poorly controlled—especially with INR
levels above the target range, as demonstrated in
the RE-LY trial6—indicates that Asian populations,
such as the Chinese, may have an elevated risk of
warfarin-related nephropathy.14 Because Asian
patients may be more susceptible to renal decline
associated with warfarin use, apixaban may appear
superior to warfarin in Asian populations, although
this superiority may not persist in non-Asian
populations.8 Additional apixaban superiority in
Asian populations, as discussed by Chan et al,14 may
also be explained by the superior efficacy and safety
of NOACs in Asians, compared with non-Asians.5 21
Because major bleeding can be associated with
renal function deterioration, the greater efficacy and
safety of NOACs in Asians may facilitate renal risk
reduction in such populations.5 14 21 Notably, there was
a high prevalence of non-guideline dose reduction
without a renal indication26 in the apixaban group,
compared with other NOACs, in our study; this dose
reduction has been associated with worse stroke
prevention effectiveness and provides no safety
benefit.40 Nonetheless, apixaban was not significantly
associated with risk reduction of the other two renal
outcomes in our study, compared with warfarin.
This may suggest uncertainty concerning its renal
risk reduction superiority compared with warfarin.
Overall, such inconsistencies across studies indicate
the need for additional research; they may also
reflect insufficient statistical power in our study to
generate more robust conclusions.
The aforementioned findings concerning
greater risk of warfarin-related nephropathy and
possible lower risk of renal decline with NOAC
usage in Asian populations are also potentially
reflected in the comparatively lower HRs for renal
endpoints in our study, compared with the US-based
cohort reported by Yao et al.8 When the
NOACs were pooled (for consideration as a single
unit) and compared with warfarin, HRs for ≥30%
decline in eGFR and doubling of serum creatinine
were both lower in our population, compared with
the pooled results described by Yao et al8 (HR=0.77;
95% CI=0.66-0.89 and HR=0.62; 95% CI=0.40-0.95).
Strengths and limitations
Notable strengths of our study were its long study
period and subsequent long mean follow-up
duration. The longer follow-up duration, compared
with previous cohort studies, indicates that previous
findings concerning NOAC superiority for renal
outcomes also persist during longer follow-up
periods. Furthermore, our database comprised
each patient’s complete laboratory data; this
allowed accurate recording of each renal outcome
through serum creatinine and eGFR values, thus
enhancing the consistency and preciseness of renal
measurement across all patients. We minimised potential confounding by only including patients
who were first-time users of oral anticoagulants; this
enabled us to balance numerous important baseline
characteristics.
Regarding limitations, although we utilised
IPTW to balance baseline covariates, confounding
bias may have persisted in the study.8 14 Nonetheless,
we achieved balance concerning the most important
identified baseline covariates that may impact
renal function across treatment groups. Moreover,
although the smaller number of events may have
limited the statistical power with respect to the less
sensitive endpoint of kidney failure, by including
≥30% decline in eGFR as a sensitive renal outcome,
we were able to sufficiently assess early renal decline.
Smaller declines in renal function (eg, ≥30% decline
in eGFR) serve as valuable and sensitive indicators
of renal decline8 that has been regarded as a useful
surrogate endpoint for progression to kidney
failure24; it is also reportedly associated with risks of
end-stage renal disease and mortality.41 Frequency
of testing, as mentioned in Yao et al,8 may also
affect results; the inclusion of patients with more
follow-up creatinine tests leads to greater sensitivity
concerning outcome incidence, compared with
patients who underwent fewer tests. To minimise
the potential impact of this sensitivity on the renal
endpoints, we only included patients for whom
creatinine tests were available throughout the entire
follow-up period; this was possible because all
patients were treated in a single centre.
Overall, the general consistency of our results
with the findings of previous cohort studies, as
well as the findings of the RE-LY and ROCKET
AF trials, enhances the reliability and robustness
of our results.6 7 8 14 Nonetheless, further studies are
needed to identify consistencies among the existing
discrepancies, especially concerning apixaban.
Greater certainty regarding renal outcomes of all
NOACs is also important because one previous
meta-analysis of various randomised controlled trials
concluded that the risk of kidney failure associated
with NOACs was similar to the risk associated with
other anticoagulants.42 Finally, although this study
only involved Hong Kong Chinese patients, whose
responses to NOACs and warfarin may differ from
the responses of their non-Asian counterparts, the
consistency of the results with findings from studies
in other regions suggests widespread applicability of
the findings.
Clinical implications
In patients with NVAF who are receiving oral
anticoagulants, gradual renal impairment is
associated with worse clinical outcomes.39 43 Our
results suggested that patients receiving oral
anticoagulant therapy, particularly warfarin, should
undergo close renal function monitoring. Decline in renal function during anticoagulant therapy
may be less likely to occur when receiving NOACs
than when receiving warfarin. The NOAC efficacy
findings in this study were generally consistent
with previously reported data in terms of stroke/SE
prevention non-inferiority or superiority, compared
with warfarin.44 45 46 47 In particular, the superior efficacy
of dabigatran compared with warfarin, in this local
study population is reassuring. The inconsistencies
of NOAC prescribing patterns with drug labelling
in routine clinical practice, particularly regarding
apixaban, should receive greater attention, because
dose reduction in the absence of a renal indication
has been associated with worse effectiveness and
no safety benefit in apixaban-treated patients with
normal or mildly impaired renal function.40
Conclusions
Compared with warfarin, NOAC treatment may be associated with a lower risk of renal decline in Chinese
populations; this should be considered by clinicians
during the selection of anticoagulant treatment.
Further studies are needed in Asian populations (eg,
Chinese) to better understand the renal superiority
or inferiority of NOACs compared with warfarin.
Besides, NOAC-to-NOAC comparisons are needed
to inform treatment selection. Additional research
is needed in specific populations, such as patients
with diabetes mellitus or heart failure, to better
understand the impacts of baseline co-morbidities
on renal risk reduction related to the use of NOACs,
compared with the use of warfarin. Large-scale
studies should also investigate how dosage patterns
may influence renal outcomes.
Author contributions
Concept or design: APW Lee.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: APW Lee.
Acquisition of data: All authors.
Analysis or interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: APW Lee.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Conflicts of interest
APW Lee has received research grants from Bayer, Pfizer, and Boehringer Ingelheim.
Funding/support
This work was funded by the Hong Kong SAR Government
Health and Medical Research Fund (05160976). The funder
had no role in study design, data collection/analysis/interpretation, or manuscript preparation.
Ethics approval
The study was approved by The Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research
Ethics Committee (Ref CREC 2019.405). Informed consent
was waived because of the retrospective nature of this study.
References
1. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients
who have nonvalvular atrial fibrillation. Ann Intern Med
2007;146:857-67. Crossref
2. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran
versus warfarin in patients with atrial fibrillation. N Engl J
Med 2009;361:1139-51. Crossref
3. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus
warfarin in nonvalvular atrial fibrillation. N Engl J Med
2011;365:883-91. Crossref
4. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban
versus warfarin in patients with atrial fibrillation. N Engl J
Med 2011;365:981-92. Crossref
5. Lip GY, Wang KL, Chiang CE. Non-vitamin K antagonist
oral anticoagulants (NOACs) for stroke prevention in
Asian patients with atrial fibrillation: time for a reappraisal.
Int J Cardiol 2015;180:246-54. Crossref
6. Böhm M, Ezekowitz MD, Connolly SJ, et al. Changes in
renal function in patients with atrial fibrillation: an analysis
from the RE-LY trial. J Am Coll Cardiol 2015;65:2481-93. Crossref
7. Fordyce CB, Hellkamp AS, Lokhnygina Y, et al. On-treatment
outcomes in patients with worsening renal
function with rivaroxaban compared with warfarin:
Insights from ROCKET AF. Circulation 2016;134:37-47. Crossref
8. Yao X, Tangri N, Gersh BJ, et al. Renal outcomes in
anticoagulated patients with atrial fibrillation. J Am Coll
Cardiol 2017;70:2621-32. Crossref
9. Brodsky SV, Nadasdy T, Rovin BH, et al. Warfarin-related
nephropathy occurs in patients with and without
chronic kidney disease and is associated with an increased
mortality rate. Kidney Int 2011;80:181-9. Crossref
10. Brodsky SV, Satoskar A, Chen J, et al. Acute kidney injury
during warfarin therapy associated with obstructive
tubular red blood cell casts: a report of 9 cases. Am J
Kidney Dis 2009;54:1121-6. Crossref
11. Brodsky SV, Collins M, Park E, et al. Warfarin therapy
that results in an international normalization ratio above
the therapeutic range is associated with accelerated
progression of chronic kidney disease. Nephron Clin Pract
2010;115:142-6. Crossref
12. Sparkenbaugh EM, Chantrathammachart P, Mickelson J,
et al. Differential contribution of FXa and thrombin to
vascular inflammation in a mouse model of sickle cell
disease. Blood 2014;123:1747-56. Crossref
13. Lee IO, Kratz MT, Schirmer SH, Baumhäkel M, Böhm M.
The effects of direct thrombin inhibition with dabigatran
on plaque formation and endothelial function in
apolipoprotein E-deficient mice. J Pharmacol Exp Ther
2012;343:253-7. Crossref
14. Chan YH, Yeh YH, Hsieh MY, et al. The risk of acute
kidney injury in Asians treated with apixaban, rivaroxaban,
dabigatran, or warfarin for non-valvular atrial fibrillation:
a nationwide cohort study in Taiwan. Int J Cardiol
2018;265:83-9. Crossref
15. Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and
safety of dabigatran compared with warfarin at different
levels of international normalised ratio control for stroke
prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010;376:975-83. Crossref
16. Singer DE, Hellkamp AS, Piccini JP, et al. Impact of global
geographic region on time in therapeutic range on warfarin
anticoagulant therapy: data from the ROCKET AF clinical
trial. J Am Heart Assoc 2013;2:e000067. Crossref
17. Nielsen PB, Skjøth F, Søgaard M, Kjældgaard JN, Lip GY,
Larsen TB. Effectiveness and safety of reduced dose non-
Vitamin K antagonist oral anticoagulants and warfarin
in patients with atrial fibrillation: propensity weighted
nationwide cohort study. BMJ 2017;356:j510. Crossref
18. Hori M, Matsumoto M, Tanahashi N, et al. Rivaroxaban
vs. warfarin in Japanese patients with atrial fibrillation–the
J-ROCKET AF study. Circ J 2012;76:2104-11. Crossref
19. Ryan M, Ware K, Qamri Z, et al. Warfarin-related
nephropathy is the tip of the iceberg: direct thrombin
inhibitor dabigatran induces glomerular hemorrhage
with acute kidney injury in rats. Nephrol Dial Transplant
2014;29:2228-34. Crossref
20. Makris K, Spanou L. Acute kidney injury: definition,
pathophysiology and clinical phenotypes. Clin Biochem
Rev 2016;37:85-98. Crossref
21. Wang KL, Lip GY, Lin SJ, Chiang CE. Non-vitamin K
antagonist oral anticoagulants for stroke prevention in
Asian patients with nonvalvular atrial fibrillation: meta-analysis.
Stroke 2015;46:2555-61. Crossref
22. Ray WA. Evaluating medication effects outside of clinical
trials: new-user designs. Am J Epidemiol 2003;158:915-20. Crossref
23. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline
for the management of patients with atrial fibrillation: a
report of the American College of Cardiology/American
Heart Association Task Force on Clinical Practice
Guidelines and the Heart Rhythm Society. J Am Coll
Cardiol 2019;74:104-32. Crossref
24. Levey AS, Inker LA, Matsushita K, et al. GFR decline as an
end point for clinical trials in CKD: a scientific workshop
sponsored by the national kidney foundation and the
US Food and Drug Administration. Am J Kidney Dis
2014;64:821-35. Crossref
25. Levin A, Stevens PE, Bilous RW, et al. Kidney disease:
improving global outcomes (KDIGO) CKD work group.
KDIGO 2012 clinical practice guideline for the evaluation
and management of chronic kidney disease. Kidney Int
Suppl 2013;3:1-150.
26. Lau YC, Proietti M, Guiducci E, Blann AD, Lip GY. Atrial
fibrillation and thromboembolism in patients with chronic
kidney disease. J Am Coll Cardiol 2016;68:1452-64. Crossref
27. Levey AS, Inker LA, Coresh J. GFR estimation: from
physiology to public health. Am J Kidney Dis 2014;63:820-34. Crossref
28. Lam AS, Lee IM, Mak SK, Yan BP, Lee VW. Warfarin
control in Hong Kong clinical practice: a single-centre
observational study. Hong Kong Med J 2020;26:294-303. Crossref
29. Schmitt L, Speckman J, Ansell J. Quality assessment of
anticoagulation dose management: comparative evaluation
of measures of time-in-therapeutic range. J Thromb
Thrombolysis 2003;15:213-6. Crossref
30. Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GY.
Comparative effectiveness and safety of non-vitamin K
antagonist oral anticoagulants and warfarin in patients
with atrial fibrillation: propensity weighted nationwide
cohort study. BMJ 2016;353:i3189. Crossref
31. McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized
boosted models. Stat Med 2013;32:3388-414. Crossref
32. Austin PC. An introduction to propensity score methods
for reducing the effects of confounding in observational
studies. Multivariate Behav Res 2011;46:399-424. Crossref
33. Austin PC, Stuart EA. Moving towards best practice when
using inverse probability of treatment weighting (IPTW)
using the propensity score to estimate causal treatment
effects in observational studies. Stat Med 2015;34:3661-79. Crossref
34. Chatrou ML, Winckers K, Hackeng TM, Reutelingsperger CP, Schurgers LJ. Vascular calcification: the price to pay for anticoagulation therapy with vitamin K-antagonists. Blood
Rev 2012;26:155-66. Crossref
35. Schurgers LJ, Joosen IA, Laufer EM, et al. Vitamin K-antagonists
accelerate atherosclerotic calcification
and induce a vulnerable plaque phenotype. PLoS One
2012;7:e43229. Crossref
36. Ozcan A, Ware K, Calomeni E, et al. 5/6 nephrectomy as
a validated rat model mimicking human warfarin-related
nephropathy. Am J Nephrol 2012;35:356-64. Crossref
37. Ware K, Brodsky P, Satoskar AA, et al. Warfarin-related
nephropathy modeled by nephron reduction and excessive
anticoagulation. J Am Soc Nephrol 2011;22:1856-62. Crossref
38. Hernandez AV, Bradley G, Khan M, et al. Rivaroxaban
vs. warfarin and renal outcomes in non-valvular atrial
fibrillation patients with diabetes. Eur Hear J Qual Care
Clin Outcomes 2020;6:301-7. Crossref
39. Hijazi Z, Hohnloser SH, Andersson U, et al. Efficacy and
safety of apixaban compared with warfarin in patients with
atrial fibrillation in relation to renal function over time:
insights from the ARISTOTLE randomized clinical trial.
JAMA Cardiol 2016;1:451-60. Crossref
40. Yao X, Shah ND, Sangaralingham LR, Gersh BJ,
Noseworthy PA. Non–vitamin K antagonist oral
anticoagulant dosing in patients with atrial fibrillation and
renal dysfunction. J Am Coll Cardiol 2017;69:2779-90. Crossref
41. Coresh J, Turin TC, Matsushita K, et al. Decline in estimated
glomerular filtration rate and subsequent risk of end-stage
renal disease and mortality. JAMA 2014;311:2518-31. Crossref
42. Caldeira D, Gonçalves N, Pinto FJ, Costa J, Ferreira JJ.
Risk of renal failure with the non-vitamin K antagonist
oral anticoagulants: systematic review and meta-analysis.
Pharmacoepidemiol Drug Saf 2015;24:757-64. Crossref
43. Roldán V, Marín F, Fernández H, et al. Renal impairment
in a ‘real-life’ cohort of anticoagulated patients with
atrial fibrillation (implications for thromboembolism and
bleeding). Am J Cardiol 2013;111:1159-64. Crossref
44. Li WH, Huang D, Chiang CE, et al. Efficacy and safety of
dabigatran, rivaroxaban, and warfarin for stroke prevention
in Chinese patients with atrial fibrillation: the Hong Kong
atrial fibrillation project. Clin Cardiol 2017;40:222-9. Crossref
45. Chan YH, See LC, Tu HT, et al. Efficacy and safety of
apixaban, dabigatran, rivaroxaban, and warfarin in Asians
with nonvalvular atrial fibrillation. J Am Heart Assoc
2018;7:e008150. Crossref
46. Zhang J, Tang J, Cui X, et al. Indirect comparison of novel
oral anticoagulants among Asians with non-valvular atrial
fibrillation in the real world setting: a network meta-analysis.
BMC Cardiovasc Disord 2019;19:182. Crossref
47. Liu X, Huang M, Ye C, Zeng J, Zeng C, Ma J. The role of
non-vitamin K antagonist oral anticoagulants in Asian
patients with atrial fibrillation: a PRISMA-compliant
article. Medicine 2020;99:e21025. Crossref